Abstract
Aims
Carbamazepine is a known enzyme inducer. The aim of this study was to determine whether carbamazepine induces the metabolism of caffeine in children.
Methods
Children due to receive carbamazepine for epilepsy were recruited into the study. The caffeine breath test was carried out prior to the administration of carbamazepine and after a minimum of 2–3 weeks therapy. Five children were studied and they received 200–600 mg carbamazepine daily.
Results
The mean values of the 2 h cumulative labelled carbon dioxide were 3.47% before and 7.65% during carbamazepine. There was a significant increase in the percentage labelled caffeine exhaled as carbon dioxide during the administration of carbamazepine (Student's paired t-test, P < 0.05).
Conclusions
The results suggest that carbamazepine induces the metabolism of caffeine by the CYP1A2 pathway in the children studied.
Keywords: caffeine, carbamazepine, children, caffeine breath test, enzyme induction, CYP1A2
Introduction
Carbamazepine has been shown to have an enzyme inducing effect on the metabolism of several drugs [1–3]. We decided to study the drug interaction between caffeine and carbamazepine in children. Studies of drug interactions usually require the collection of blood or urine samples, neither of which is easy in children.
We, and others, have used the caffeine breath test to study both drug interactions and the effect of disease on drug metabolism [4–7]. The test involves the use of a non-radioactive stable isotope ([13C] on the 3-methyl group of caffeine). The caffeine is given orally and undergoes 3-N-demethylation which is a cytochrome P450 dependent reaction (CYP1A2). After N-demethylation the labelled methyl group enters the one carbon pool as it is converted to formaldehyde, formate, bicarbonate and then exhaled as carbon dioxide. We studied the effect of carbamazepine on caffeine metabolism in children utilising the caffeine breath test.
Methods
Plan of study
The study was approved by the local Ethics Committee. Informed consent was obtained from the children and their parents, prior to commencement into the study. Five children (aged 6–17 years) were studied both prior to and 2 to 3 weeks after commencing carbamazepine. All the children were patients with epilepsy at Alder Hey Children's Hospital and were prescribed carbamazepine as monotherapy.
Caffeine breath test procedure
All subjects abstained from caffeinated products for 20 h and fasted for at least 4 h prior to the caffeine breath test. All breath tests were commenced between 09.00 and 10.00 h. Carbamazepine was administered during the 2 h prior to commencement of the test. The subjects remained seated for 10 min prior to the collection of the first breath sample. They were kept occupied by the Research Nurse throughout the whole test in order to minimise physical activity which can affect carbon dioxide production and result in dilution of the labelled carbon dioxide [8]. The labelled caffeine (Cambridge Isotope Laboratories, Massachusetts, USA; chemical purity >98%) was given at a dose of 3 mg kg−1 dissolved in water and mixed with sugar-free squash to disguise the bitter caffeine taste. The solution was taken by mouth followed by a 20 ml water rinse of the container.
Breath samples were collected by getting the child to blow into a sample balloon (Alveosampler; Quintron, Menomonee Falls, U.S.A). Samples were collected at −20, −10, −1, 15, 30, 45, 60, 75, 90, 105 and 120 min after ingestion of caffeine. Expired air (10 ml) was removed from the balloon and transferred by syringe into a headspace analyser vial (Chromacol; London, U.K.) that had been pre-evacuated. Samples were unaffected by 8 weeks of storage in these vessels. The samples were sent by registered mail to The Scottish Universities Research and Reactor Centre for analysis.
Analytical methods
[13C] enrichment of breath carbon dioxide was determined by continuous flow isotope ratio mass spectrometry [9]. Breath samples (10 ml) were injected automatically into the gas preparation device (Roboprep-G; Europa Scientific Ltd, Crewe, U.K.) where they were in turn, dried, resolved from interfering components by gas chromatography and passed, using helium as carrier, into the electron impact ion source of an isotope ratio mass spectrometer (Tracermass; Europa Scientific Ltd). The ion beams m/z 44, m/z 45 and m/z 46 were monitored continuously and used to calculate the partial pressure and [13C] enrichment of carbon dioxide, with reference to a 2% CO2/98% N2 gas supply which had been calibrated against a bicarbonate standard of known [13C] enrichment. Duplicates of each breath sample were analysed.
Calculations and statistical analysis
The [13C] enrichment of exhaled carbon dioxide was converted from delta units to atom % using the accepted atom fraction of the international bicarbonate standard [10]. [13C] enrichment was expressed as atom % [13C] excess, by subtracting the average pre-dose enrichment from each post-dose measurement. Cumulative 13CO2 output was calculated from the measured [13C] enrichment of the eight breath samples taken during the first 2 h following administration of the [13C] caffeine dose and multiplying this by the average output of CO2 over this period (assumed to be 24 mmol CO2 kg−1 body weight; [11]). This was expressed as a percentage of the caffeine dose.
There was no significant change in CO2 partial pressure either in breath samples analysed during the breath test for each patient or in the group overall. The mean (±s.d.) CO2 partial pressure was 3.06 (±0.52)%.
The precision of a [13C] enrichment, derived from the average of 40 replicated breath analyses, was 0.00104 atom %13C excess. This equates to 1.11% recovered of a typical caffeine dose.
Student's paired t-test was used to compare the data before and during carbamazepine. A sample size of 10 patients has a power of 90%, assuming a difference of 3 in the mean score (of the 2 h percentage dose recovered) to be significant at the 5% level [7]. The study was terminated when interim analysis of the data showed a significant effect.
Results
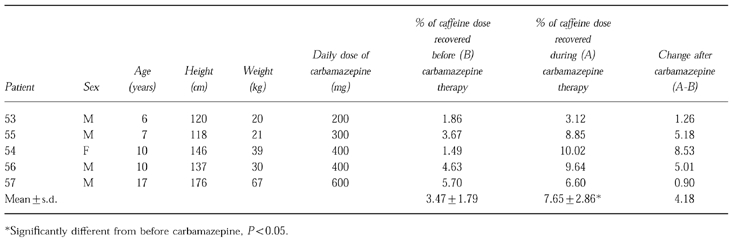
Table 1 shows the labelled cumulative 2 h CO2 output for each patient both before and during carbamazepine expressed as a percentage of the oral caffeine dose administered. The mean values of the caffeine dose exhaled as carbon dioxide in 2 h were 3.47% before and 7.65% during carbamazepine. There was a significant increase in the percentage labelled caffeine exhaled as carbon dioxide during the administration of carbamazepine (Student's paired t-test P < 0.05).
Table 1.
Clinical details of patients and cumulative 2 h CO2 output.
Discussion
Carbamazepine has an enzyme inducing effect on many different drugs including cyclosporin, warfarin, oral contraceptives, doxycycline, tricyclic antidepressants and anti-fungals such as itraconazole, as well as an inducing effect on its own metabolism [1–3]. The metabolism of cyclosporin, oral contraceptives and carbamazepine involve the CYP3A4 pathway. The induction of this pathway has been confirmed in human hepatocyte cultures [12].
The effect of carbamazepine on CYP1A2 activity has not been extensively studied. A previous study suggested that carbamazepine does not have an inducing effect on caffeine metabolism [13]. This study estimated caffeine clearance rather than partial metabolic clearance by CYP1A2, but as CYP1A2 is the major pathway of caffeine metabolism, [14] significant induction of CYP1A2 should have resulted in an increase in clearance. This study, however, used only two time points to determine the plasma clearance and half-life of caffeine and, therefore, its conclusions are open to question. Caffeine is accepted as the current gold standard for the assessment of CYP1A2 activity [14, 15].
The caffeine breath test and, in particular, the 2 h cumulative exhalation of labelled carbon dioxide is a measure of 3-N-demethylation which is catalysed by CYP1A2. The use of the 2 h cumulative exhalation of labelled carbon dioxide as an appropriate measure in the caffeine breath test has been well established in both adults and children [16, 17]. Our findings suggest that carbamazepine has an inducing effect on CYP1A2 as well as CYP3A4. This may result in clinically significant drug interactions involving drugs such as theophylline, imipramine and propranolol which undergo metabolism by CYP1A2 [15].
Acknowledgments
We thank The Wellcome Trust (Grant No. 040145) for financial support. We thank Gill Croxton for her help in preparing the caffeine and Mrs Alexandra Longworth for typing the manuscript.
References
- 1.Levy RH, Wurden CJ. Carbamazepine interactions with other drugs. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic Drugs. 4. New York: Raven Press; 1995. pp. 543–554. [Google Scholar]
- 2.Crawford P, Chadwick DJ, Martin C, Tjia J, Back DJ, Orme M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br J Clin Pharmacol. 1990;30:892–896. doi: 10.1111/j.1365-2125.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penttila O, Neuvonen PJ, Aho K, Lehtovaara R. Interaction between doxycycline and some antiepileptic drugs. Br Med J. 1974;2:470–472. doi: 10.1136/bmj.2.5917.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitsky LL, Schoeller DA, Lambert GH, Edidin DV. Effect of growth hormone therapy in growth hormone-deficient children on cytochrome P-450-dependent 3-N-demethylation of caffeine as measured by the caffeine 13CO2 Breath Test. Dev Pharmacol Ther. 1989;12:90–95. [PubMed] [Google Scholar]
- 5.Rost KL, Brösicke H, Brockmöller J, Scheffler M, Helge H, Roots I. Increase of cytochrome P450IA2 activity by omeprazole: Evidence by the 13C-[N-3-methyl]-caffeine breath test in poor and extensive metabolizers of S-mephenytoin. Clin Pharmacol Ther. 1992;52:170–180. doi: 10.1038/clpt.1992.126. [DOI] [PubMed] [Google Scholar]
- 6.Parker AC, Preston T, Heaf D, Kitteringham NR, Choonara I. Inhibition of caffeine metabolism by ciprofloxacin in children with cystic fibrosis as measured by the caffeine breath test. Br J Clin Pharmacol. 1994;38:573–576. doi: 10.1111/j.1365-2125.1994.tb04399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker AC, Pritchard P, Preston T, Dalzell AM, Choonara I. Lack of inhibitory effect of cimetidine on caffeine metabolism in children using the caffeine breath test. Br J Clin Pharmacol. 1997;43:467–470. doi: 10.1046/j.1365-2125.1997.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoeller DA, Schneider JF, Solomons NW, Watkins JB, Klein PD. Clinical diagnosis with the stable isotope 13C in CO2 breath tests: methodology and fundamental considerations. J Lab Clin Med. 1977;90:412–421. [PubMed] [Google Scholar]
- 9.Preston T, McMillan DC. Rapid sample throughput for biomedical stable isotope tracer studies. Biomedical and Environmental Mass Spectrometry. 1988;16:229–235. doi: 10.1002/bms.1200160142. [DOI] [PubMed] [Google Scholar]
- 10.Craig H. The geochemistry of stable carbon isotopes. Geochimica Cosmochimica Acta. 1957;3:53–92. [Google Scholar]
- 11.Lambert GH, Kotake AN, Schoeller DA. The CO2 breath tests as monitors of cytochrome P-450 dependent mixed function mono-oxygenase system in metabolism. In: Macleod SM, Okey L, Spielberg SP, editors. Developmental Pharmacology. New York: Liss; 1983. pp. 119–145. [Google Scholar]
- 12.Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions. Drug Metab Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 13.Wietholtz H, Zysset TH, Kreiten K, Dohl D, Büchsel R, Matern S. Effect of phenytoin, carbamazepine and valproic acid on caffeine metabolism. Eur J Clin Pharmacol. 1989;36:401–406. doi: 10.1007/BF00558303. [DOI] [PubMed] [Google Scholar]
- 14.Kalow W, Tang BK. The use of caffeine for enzyme assays: A critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen BB, Brosen K. Theophylline has no advantage over caffeine as a putative model drug for assessing CYP1A2 activity in humans. Br J Clin Pharmacol. 1997;43:253–258. doi: 10.1111/j.1365-2125.1997.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450 1A2), the phenacetin 0-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert GH, Schoeller DA, Kotake AN, Flores C, Hay D. The effect of age, gender and sexual maturation on the caffeine breath test. Dev Pharmacol Ther. 1986;9:375–368. doi: 10.1159/000457262. [DOI] [PubMed] [Google Scholar]


