Abstract
Aims
There is controversy regarding the potential antioxidant effect of captopril, therefore this study was performed to compare the in vitro antioxidant power of captopril with other angiotensin-converting enzyme (ACE) inhibitors.
Methods
Antioxidant power of captopril, enalapril, fosinopril, perindopril, quinapril and ramipril in aqueous solution was measured using the ferric reducing (antioxidant) power (FRAP) assay; captopril was also measured in ethanolic solution.
Results
Only captopril showed significant antioxidant power, demonstrating a stoichiometric factor of 1.0 in this assay. Concentration-related antioxidant power was seen in both aqueous and ethanolic solutions.
Conclusions
Captopril shows antioxidant activity in vitro. This property could be relevant in vivo if captopril is concentrated in membranes, lipoproteins or at other important sites.
Keywords: captopril, hypertension, ACE inhibitor, antioxidant, CHD, ROS, oxidative stress, FRAP assay
Introduction
Cardiovascular diseases, including hypertension, cardiac failure and post-ischaemic reperfusion injury, are associated with generation of increased amounts of reactive oxygen species (ROS) [1, 2]. Various antioxidant defences in vivo oppose ROS action, minimising oxidative tissue damage [1, 2]. Antioxidant power in a cardiovascular drug could augment its other established therapeutic benefits, conferring advantages over other drugs from the same class with no antioxidant properties. Captopril, a thiol group-containing ACE inhibitor, has been reported to be an efficient scavenger of ROS [3–6], particularly hypochlorous acid (HOCl) [3]. However, captopril's importance as an antioxidant has been disputed [6, 7]. In this study, in vitro antioxidant power of captopril and other ACE inhibitors was measured using a recently developed, automated test of ‘total antioxidant power’, the FRAP assay [1, 8].
Methods
Solutions were prepared from commercially available tablets or capsules of captopril (25 mg), enalapril (10 mg), fosinopril (10 mg), perindopril (4 mg), quinapril (10 mg) or ramipril (2.5 mg) by dissolving each in distilled water and, for captopril, additionally in ethanol. Antioxidant power of the filtered solutions was measured using the ferric reducing (antioxidant) power (FRAP*) assay and a 10 min reaction time. FRAP employs reduction of a ferric-tripyridyltriazine complex to the blue ferrous form by electron donating antioxidants, with measurement of the subsequent change in absorbance at 593 nm [8]. The FRAP assay has a limit of detection of <2.0 μmol l−1 antioxidant power, gives a linear response over a wide concentration range with various known antioxidants, including ascorbic acid (vitamin C) and uric acid, and results are highly reproducible; within run-and between run- CVs in the FRAP assay are <1% and <3% respectively [8].
Results
Captopril showed a linear concentration-related response in the FRAP assay for antioxidant power, with a ‘specific antioxidant activity’ of 1.0 μmol antioxidant power/μmol drug. That of fosinopril was ten times less than that of captopril, at 0.1 μmol antioxidant power/μmol drug. OtherACE inhibitors tested had negligible antioxidant activity, with FRAP values of ≤0.01 μmol antioxidant power/μmol drug for enalapril, perindopril, quinapril and ramipril.
The stoichiometric factor of captopril in the FRAP assay was 1.0 (mean 0.9; s.e. mean 0.04, n = 8). This factor was not concentration dependent, and was found in both aqueous and ethanolic solutions of the drug. Figure 1 shows the concentration-response line of captopril in the FRAP assay. The full concentration-response line of fosinopril was not determined because its antioxidant effect was so weak. However, an illustrative concentration-response line, based on the results obtained for 4.4 mmol l−l fosinopril is given in Figure 1. The FRAP assay concentration-response line of ascorbic acid, an antioxidant of established physiological importance with a specific antioxidant activity of 2.0 μmol antioxidant power/μmol ascorbic acid, i.e. a stoichiometric factor of 2.0, is also given for reference.
Figure 1.
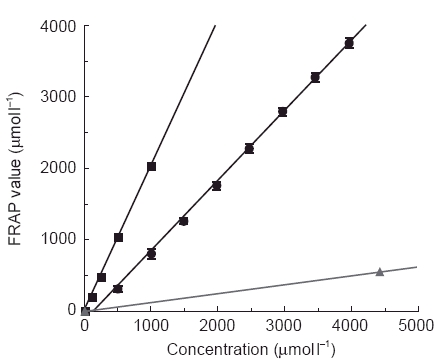
Concentration-dependent response of captopril (circles) in the FRAP assay for total ‘antioxidant’ (reducing) power; each point represents the mean of four readings, with ±1s.d. error bars plotted. A typical FRAP dose-response line of ascorbic acid, a known antioxidant of established physiological importance, is also shown (squares; each point represents a single reading), as is an illustrative concentration-response line for the poorly reacting fosinopril (triangles), based on its FRAP value at a fosinopril concentration of 4.4 mmol l−l.
Discussion
Captopril has significant antioxidant power in vitro, a property not shared by other ACE inhibitors. One thiol-containing molecule of captopril can donate one electron, thereby quenching one ‘free radical’. Furthermore, since captopril dissolved in ethanol retains its antioxidant power, captopril could augment lipophilic antioxidant defences.
The therapeutic plasma concentration of captopril is low (1–2 μmol l−1) [3–7], limiting captopril's contribution to the ‘total antioxidant power’ of plasma, which is around 1 mmol l−1 [1, 8, 9]. Lipid-soluble antioxidants have relatively low plasma concentrations however. Low-density lipoprotein (LDL) contains about 6–10 molecules of alpha tocopherol per particle, giving plasma concentrations of 11–30 μmol l−1; concentrations of other lipid soluble antioxidants, such as ubiquinol and lycopene, are <2 μmol l−1[1, 2, 10]. Thus, while treatment with captopril may increase total plasma or aqueous antioxidant power only slightly, captopril could, nevertheless, enhance antioxidant power of lipid compartments. In plasma, captopril is partially bound to proteins and thiols [11], but kinetic studies indicate extensive partitioning of captopril into tissues. Its disposition may best be described by a three-compartment model [12], with a steady state volume of distribution of 0.7 l kg−1. Captopril's contribution to tissue antioxidant defence, therefore, could become significant, especially if it is ‘redox recycled’ by ascorbate, a mechanism thought to be used in the recycling of lipid-bound alpha tocopherol [10]. Furthermore, binding of captopril to endothelial and cardiac ACE may result in a ‘site-specific’ increase in antioxidant defences. This could be important in atherosclerosis, as HOCl, produced by activated neutrophils and myeloperoxidase-expressing atherosclerotic tissue, is effectively scavenged by captopril [3].
While a significant captopril-induced increase in lipid antioxidant power in vivo is currently speculative, this study has shown that captopril has significant electron donating antioxidant power in vitro. Captopril may, therefore, have advantages over other ACE inhibitors, although we cannot exclude an antioxidant effect of the metabolites of these other agents in vivo. Further studies of in vivo antioxidant efficacy of captopril, in particular investigating LDL and membrane concentrations attained during treatment, would be useful.
Acknowledgments
We thank Ms I. Wu and Mr W.Y. Chung for their technical assistance, and the Hong Kong Polytechnic University for financially supporting this work.
References
- 1.Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimisation of nutritional antioxidant intake in humans. Free Rad Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- 2.Benzie IFF. Lipid peroxidation: a review of causes, consequences and methods of measurement. Int J Food Sci Nutr. 1996;47:233–262. doi: 10.3109/09637489609012586. [DOI] [PubMed] [Google Scholar]
- 3.Aruoma OI, Akanumu D, Cecchini R, Halliwell B. Evaluation of the ability of the angiotensin-converting enzyme inhibitor captopril to scavenge reactive oxygen species. Chem-Biol Interact. 1991;77:303–314. doi: 10.1016/0009-2797(91)90039-a. [DOI] [PubMed] [Google Scholar]
- 4.Bagchi D, Prasad R, Das DK. Direct scavenging of free radicals by captopril, an angiotensin converting enzyme inhibitor. Biochem Biophys Res Comm. 1989;158:52–57. doi: 10.1016/s0006-291x(89)80175-5. [DOI] [PubMed] [Google Scholar]
- 5.Chopra M, Scott N, McMurray J, et al. Captopril: a free radical scavenger. Br J Clin Pharmacol. 1989;27:396–399. doi: 10.1111/j.1365-2125.1989.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westlin W, Mullane K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation. 1988;77:30–39. [PubMed] [Google Scholar]
- 7.Lapenna D, De Gioia S, Ciofani G, Daniele F, Cuccurullo F. Captopril has no significant scavenging antioxidant activity in human plasma in vitro or in vivo. Br J Clin Pharmacol. 1996;42:451–456. doi: 10.1046/j.1365-2125.1996.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benzie IFF, Strain JJ. The reducing ability of plasma as a measure of ‘antioxidant’ power—the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 9.Benzie IFF, Tomlinson B, Critchley JAJH. Free radical scavenging activity of captopril in human plasma (letter) Br J Clin Pharmacol. 44:209–210. [PubMed] [Google Scholar]
- 10.Packer, L L. Interactions among antioxidants in health and disease: vitamin E and its redox cycle. Proc Soc Exp Biol. 1992;200:271–276. doi: 10.3181/00379727-200-43433. [DOI] [PubMed] [Google Scholar]
- 11.Ohman KP, Kagedal B, Larsson R, Karlberg BE. Pharmacokinetics of captopril and its effect on blood pressure during acute and chronic administration and in relation to food intake. J Cardiovasc Pharmacol. 1985;7(Suppl 1):S20–S24. doi: 10.1097/00005344-198507001-00005. [DOI] [PubMed] [Google Scholar]
- 12.Duchin KL, Singhvi SM, Willard DA, et al. Captopril kinetics. Clin Pharmacol Ther. 1982;31:452–458. doi: 10.1038/clpt.1982.59. [DOI] [PubMed] [Google Scholar]


