Abstract
In order to initially colonize a host, bacteria must avoid various components of the innate immune system, one of which is complement. The genus Bordetella includes three closely related species that differ in their ability to resist complement-mediated killing. Bordetella parapertussis and Bordetella bronchiseptica resist killing in naïve serum, a characteristic that may aid in efficient respiratory tract colonization and has been attributed to expression of O antigen. Bordetella pertussis lacks O antigen and is sensitive to naïve serum in vitro, yet it also efficiently colonizes the respiratory tract. Based on these observations, we hypothesized that B. pertussis may have an alternate mechanism to resist complement in vivo. While a number of reports on serum sensitivity of the bordetellae have been published, we show here that serum concentration and growth conditions can greatly alter the observed level of sensitivity to complement and that all but one strain of B. pertussis observed were sensitive to some level of naïve serum in vitro, particularly when there was excess complement. However, B. pertussis rapidly acquires increased resistance in vivo to naïve serum that is specific to the alternative pathway. Resistance is not efficiently acquired by B. parapertussis and B. bronchiseptica mutants lacking O antigen. This B. pertussis-specific mechanism of complement resistance does not appear to be dependent on either brkA or other genes expressed specifically in the Bvg+ phase. This in vivo acquisition of alternative pathway resistance suggests that there is a novel O antigen-independent method by which B. pertussis evades complement-mediated killing.
Since the discovery of complement as a heat-labile component of serum that is able to complement antibodies in killing bacteria (7, 30), over 30 plasma or membrane-bound proteins have been identified as components. These proteins can elicit a variety of effects, such as immune cell activation, chemotaxis, opsonization, and lysis of bacteria. They function as a catalytic cascade that can be activated by two major pathways. The classical pathway is primarily activated by antibodies bound to a cell surface, but it can also be initiated by C1q directly binding to bacterial surfaces, by mannose-binding lectin, or by C-reactive protein. The alternative pathway involves tickover of C3, where a small quantity of C3 to directly bind to bacterial surfaces, which initiates a catalytic cascade that specifically targets irregular or nonhost cells. These two pathways merge at a common amplification step involving C3 and proceed through a terminal pathway that results in the formation of a membrane attack complex, which can directly lyse cells. Healthy tissues are protected by molecules such as the classical pathway inhibitors C4 binding protein (C4BP) and C1 inhibitor, alternative pathway inhibitors such as factor H and factor I, and inhibitors of membrane attack complex completion such as S-protein, clusterin, and CD59.
To survive in a host environment, bacteria must be able to escape killing by numerous immune mechanisms, one of which is complement. Various bacteria have adapted different strategies to avoid complement-mediated lysis. Escherichia coli, Pseudomonas aeruginosa, Helicobacter pylori, Streptococcus pneumoniae, and some Neisseria species use molecular mimicry, modifying their outer membranes to resemble host tissues in order to avoid complement activation (19, 34, 36, 39, 42). In addition, many bacteria express proteins that are able to bind host-derived complement inhibitors, such as those mentioned above (10, 22, 28, 32, 34).
The gram-negative respiratory pathogens of the genus Bordetella have also developed means to resist the effects of complement, although there are conflicting reports concerning the levels of resistance of these closely related species that may in part be attributed to differences in experimental conditions (14, 16, 18). Under in vitro conditions where complement components are available in excess quantities, the O antigens of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharide (LPS) prevent activation of complement in the absence of Bordetella-specific antibodies (naïve serum) (8). When specific antibodies are present, both of these Bordetella species are effectively killed in vitro (immune serum) (8, 33). Bordetella pertussis lacks an O antigen due to an insertion sequence that replaces the wbm locus, and it is sensitive to rapid killing by naïve serum in vitro (8, 17, 18, 26, 33). However, B. pertussis appears to have other mechanisms to resist complement-mediated killing. The brkA locus has been reported to aid in inhibition of antibody-mediated classical pathway complement killing of this bacterium in vitro (3, 13). In addition, Berggard et al. have shown that B. pertussis can bind to the classical pathway regulator C4BP, retaining the ability to degrade C4b when it is bound in vitro, which may also inhibit the classical pathway of complement (5, 6). The value of resistance to antibody-mediated classical pathway killing for an organism that is killed by naïve serum in the absence of antibodies is paradoxical, underscoring an apparent discrepancy in the separately reported studies.
The purpose of this study was to better characterize the sensitivity of B. pertussis to complement in the absence of Bordetella-specific antibodies. We tested a number of different clinical and laboratory strains with different concentrations of naïve serum and found a broad range of sensitivity profiles. When excess complement was present, most B. pertussis strains grown in vitro were at least somewhat sensitive to naïve serum; however, when recovered from an infected mouse, B. pertussis displayed dramatically increased resistance to this type of killing. This resistance acquired in vivo appeared to be specifical against alternative pathway complement killing, and it was both BvgAS and BrkA independent. These results may help resolve the previously identified discrepancies and explain why B. pertussis does not express an O antigen. This organism seems to have a different means of resisting antibody-independent complement-mediated killing in vivo.
MATERIALS AND METHODS
Bacterial strains and growth.
The Tohama I strain of B. pertussis was originally isolated from a human patient with whooping cough and has been extensively studied (20). BP536 is a streptomycin- and nalidixic acid-resistant strain derived from Tohama I (35, 38). BP338, a nalidixic acid-resistant derivative of Tohama I, and RFBP 2152, a gentamicin-resistant BrkA mutant of BP338, have been described by Weiss and coworkers (3, 41). BP369 is a Bvg− derivative of Tohama I (27, 40). GMT1 (23, 24) and 6068, kind gifts from Jeff Miller, are both recent clinical isolates of B. pertussis. B. bronchiseptica RB50 (wild type) was obtained after a single passage from the original rabbit isolate (11). B. parapertussis wild-type strain CN2591 and Δwbm mutants of both B. bronchiseptica and B. parapertussis wild-type strains have been described previously (1, 33). All bordetellae were maintained on Bordet-Gengou (BG) agar (Difco) containing 7.5% defibrinated sheep blood (Hema Resources) and were grown to the mid-log phase in Stainer-Scholte (SS) broth containing heptakis(2,6-di-O-methyl)-β-cyclodextrin (0.1%; Sigma) and appropriate antibiotics.
Animal experiments.
Wild-type BALB/c, C57BL/6 (Jackson Laboratory or Taconic), or C3H/HEN (Charles River Laboratories) mice were used in this study. Mice were lightly sedated with isoflurane and inoculated with either a normal infective dose consisting of 5 × 105 CFU or a high dose consisting of 1 × 108 to 1 × 109 CFU, depending on the experiment, by pipetting the inoculum in 50 μl onto the tip of the external nares. For the BP536 time course experiments whose results are shown in Fig. 3A, groups of four BALB/c mice were inoculated with 5 × 105 CFU and sacrificed at zero time and on days 0, 1, 3, and 5 postinoculation. Four wild-type mice were inoculated with 1 × 109 CFU and sacrificed on day 1 postinoculation for the experiments whose results are shown in Fig. 3B. Groups of four BALB/c mice were given 5 × 105 CFU and sacrificed on day 5 postinoculation for the chelation experiments whose results are shown in Fig. 4, and the same inoculum and day of sacrifice were used for C57BL/6 mice in the B. bronchiseptica RB50 and RB50Δwbm and B. parapertussis CN2591 and CN2591Δwbm experiments whose results are shown in Fig. 5. Groups of four wild-type mice were given 1 × 108 CFU and sacrificed on day 1 postinoculation for the experiment with B. pertussis Bvg− strain BP369 and the ΔbrkA mutant RFBP 2152 whose results are shown in Fig. 6. The nasal cavity, a 1-cm piece of trachea, and the lungs were removed and homogenized in 1× phosphate-buffered saline (PBS) (500 μl for the nasal cavity and trachea and 1 ml for the lungs). Animals were handled in accordance with the proper institutional guidelines.
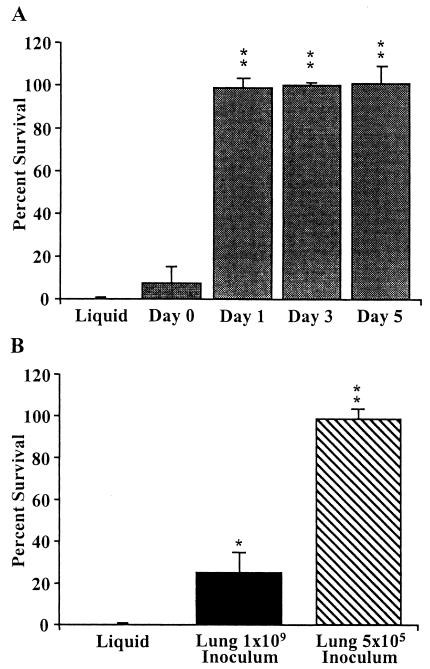
FIG. 3.
Resistance of BP536 to naïve serum acquired after mouse infection. (A) Groups of four BALB/c mice were infected intranasally with 5 × 105 CFU of BP536, and bacteria were recovered at zero time and 1, 3, and 5 days postinoculation from the lungs. BP536 liquid culture bacteria were grown to mid-log phase in SS broth. All bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with 90% naïve rabbit serum for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that survived; the error bars indicate standard deviations. All samples were run in triplicate, and the data are representative of the results of four experiments. Two asterisks indicate that the P value was <0.001 when the data were compared to the data for the in vitro-grown BP536 control. (B) Depletion in vivo of BP536 acquired naïve serum resistance. Four wild-type mice were infected intranasally with either 5 × 105 or 1 × 109 CFU of BP536 and recovered at 1 day postinoculation from the lungs. All other experimental details are identical to those described above for panel A. One asterisk indicates that the P value was 0.01 when the data were compared to the data for the in vitro-grown BP536 control, and two asterisks indicate that the P value was <0.001.
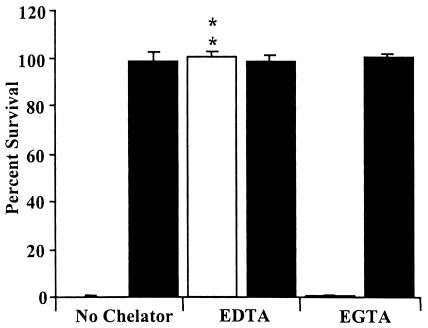
FIG. 4.
BP536 resistance to naïve serum after mouse infection with and without divalent cation chelation. Liquid culture BP536 samples were grown to mid-log phase in SS broth (open bars). BP536 samples were generated by intranasal inoculation of four BALB/c mice with 5 × 105 CFU, followed by recovery and homogenization of the lungs in PBS at 5 days postinoculation (solid bars). The bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with 90% naïve rabbit serum alone, serum with EDTA, or serum with EGTA plus MgCl2 for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that survived; the error bars indicate standard deviations. All samples were run in triplicate. Two asterisks indicate that the P value was <0.001 when the data were compared to the data for a control sample without a chelator present.
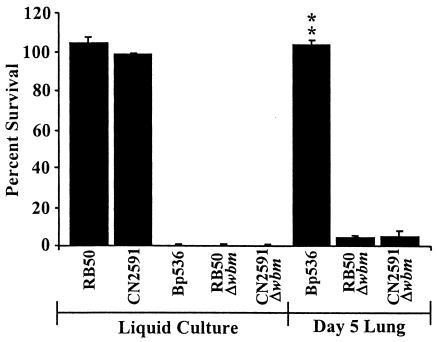
FIG. 5.
Naïve serum survival of B. bronchiseptica (RB50) and B. parapertussis (CN2591) wild-type strains and O antigen mutants (RB50Δwbm and CN2591Δwbm, respectively) compared to survival of BP536 B. pertussis. All five strains were grown to the mid-log phase in SS broth (liquid culture). Groups of four C57BL/6 mice were infected intranasally with 5 × 105 CFU of BP536, RB50Δwbm, or CN2591Δwbm and recovered from the lungs at 5 days postinoculation (Day 5 Lung). The bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with 90% naïve rabbit serum for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that survived; the error bars indicate standard deviations. All samples were run in triplicate. The data are representative of the results of three separate experiments. Two asterisks indicate that the P value was <0.001 when the data were compared to the data for the corresponding in vitro-grown controls.
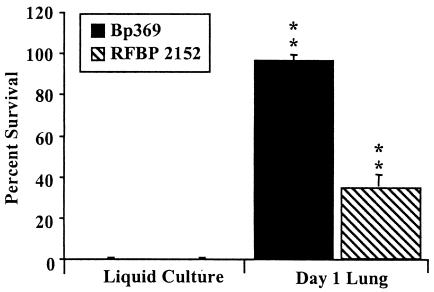
FIG. 6.
Naïve serum survival of Bvg− and ΔbrkA strains of B. pertussis. BP369 (Bvg−) and RFBP 2152 (ΔbrkA) in liquid culture were grown to mid-log phase in SS broth. Groups of four wild-type mice were infected intranasally with approximately 1 × 108 CFU of BP369 or RFBP 2152 and recovered at 1 day postinoculation to obtain day 1 lung data. The bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with 90% naïve rabbit serum for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that survived; the error bars indicate standard deviations. All samples were run in triplicate. The data are representative of the results of three separate experiments with BP369 and two separate experiments with RFBP 2152. Two asterisks indicate that the P value was <0.001 when the data were compared to the data for the in vitro-grown samples.
Serum resistance assays.
Naïve rabbit serum was obtained from Covance Research Products, Inc. Naïve rat and mouse sera were collected from uninfected specific-pathogen-free animals at The Pennsylvania State University. Human immune serum was collected from vaccinated volunteers. Serum aliquots were stored at −80°C. Bacteria were grown in SS broth to the mid-log phase and diluted in 1× PBS or SS broth to a final concentration of 100 CFU/μl. Ex vivo bacteria from various respiratory tract organs were used either undiluted or diluted in PBS to an appropriate concentration. Human immune, naïve rabbit, rat, and mouse sera were thawed on ice, and 45 μl of serum, either undiluted or diluted in 1× PBS to an appropriate concentration, was added on ice to 5 μl of bacteria or 5 μl of respiratory tract homogenate that was either undiluted or diluted. Controls were treated in the same manner except that 45 μl of heat-inactivated rabbit serum diluted in PBS to obtain a final assay concentration of 10% was used in place of the naïve complement active serum. A final concentration of approximately 500 CFU/50 μl of serum at the indicated concentration was used for all experiments. Assay mixtures were incubated at 37°C for 1 h and then were immediately placed on ice. All samples were plated on BG agar plates with appropriate antibiotics and incubated for 2 to 4 days, after which CFU were enumerated. The percentage of survival was expressed as a percentage of controls. In the chelation experiments 20 mM EDTA and 15 mM EGTA plus 5 mM MgCl2 (final concentrations) were used. The statistical significance of differences between values was calculated by using Student's t test.
RESULTS
B. pertussis strains grown in liquid culture exhibit different sensitivities to naïve serum complement.
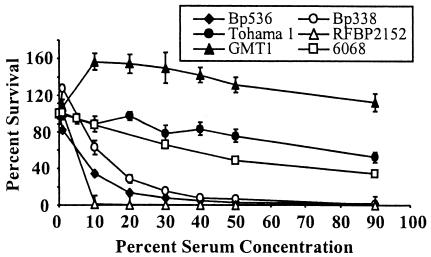
There have been a number of studies that have reported different serum sensitivities for B. pertussis (8, 9, 14, 16, 18). In order to put these reports in perspective with regard to the strain and serum concentration used, we assayed a number of laboratory and clinically isolated strains of B. pertussis over a wide range of serum concentrations. Bacteria were grown in SS broth overnight to mid-log phase and then diluted to a concentration of approximately 104 CFU/ml for use in naïve rabbit serum assays with serum concentrations ranging from 1 to 90%. This range covered the relatively low estimated concentrations of complement components in respiratory tract secretions up to the high concentrations in the bloodstream. Tohama I was fairly resistant to naïve serum killing, and about 40% of the bacteria survived for 1 h in the presence of serum concentrations as high as 90%, while the BP338 and BP536 strains, derived from Tohama I, were much more sensitive (Fig. 1). Both of the latter strains failed to survive (<1%) in the presence of serum concentrations greater than 50% (Fig. 1). The BrkA mutant RFBP 2152 was extremely sensitive to naïve serum killing, as previously reported (3), and there was no detectable survival in the presence of concentrations of serum greater than 1%. The recent clinical isolate GMT1 (23, 24) showed virtually no sensitivity to serum and was able to proliferate at lower concentrations, while another clinical isolate, 6068, had a resistance pattern similar to that of Tohama I (Fig. 1). These data illustrate the variability in serum sensitivity observed for different strains of B. pertussis and highlight what effects the concentration of serum used in an assay can have.
FIG. 1.
Naïve serum resistance of in vitro-grown B. pertussis strains in the presence of different concentrations of serum. B. pertussis liquid culture bacteria were grown to mid-log phase in SS broth. The bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with different concentrations of naïve rabbit serum for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that survived; the error bars indicate standard errors. All samples were run in quadruplicate, and each strain was tested at least twice.
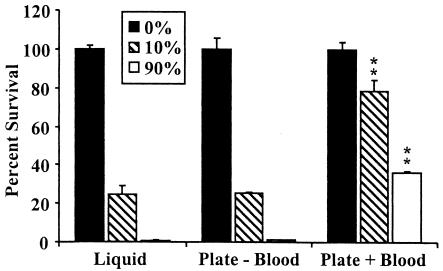
B. pertussis recovered from solid blood agar plates gains some resistance to the effects of naïve serum killing.
In contrast to the expected homogeneity of bacterial cells grown to mid-log phase in broth, bacteria recovered from plates may be more heterogeneous and/or may be altered by exposure to blood components on blood agar plates. We used a relatively sensitive strain of B. pertussis, BP536, to investigate whether the increased serum resistance observed in some studies could be related to growth conditions prior to the assays. Bacteria were harvested from solid agar plates in the absence and presence of blood and assayed for survival after 1 h of incubation in the presence of either a low concentration (10%) or a high concentration (90%) of naïve rabbit serum. Bacteria harvested from plates containing no blood were as sensitive to serum at both low and high concentrations as bacteria grown in liquid culture. However, the bacteria harvested from plates containing blood displayed significantly increased resistance to both 10 and 90% serum (Fig. 2). Increased resistance of bacteria harvested from blood agar plates was also observed with other B. pertussis strains (data not shown). It is possible that this increase in resistance reflects an interaction of B. pertussis with some factor found in mammalian blood.
FIG. 2.
Acquisition of naïve serum resistance by BP536 when the organism was grown in the presence of blood. BP536 grown to mid-log phase in SS broth (liquid) or grown on either BG agar alone (Plate − Blood) or BG agar containing 7.5% defibrinated sheep blood (Plate + Blood) and resuspended in SS broth to an optical density of approximately 0.1 was assayed. The bacteria were diluted to a concentration of 1 × 104 CFU/ml and treated with 90% naïve rabbit serum for 1 h at 37°C. Serum resistance is expressed as the percentage of bacteria that suvived; the error bars indicate standard errors. All samples were run in quadruplicate, and the data are representative of the results of two separate experiments. Two asterisks indicate that the P value was <0.001 when the data were compared to the data for the corresponding broth-grown (liquid) samples.
B. pertussis acquires resistance to naïve serum during growth in vivo.
Although mid-log-phase planktonic growth is the laboratory standard, it is possible that bacteria grown on blood agar plates more closely resemble bacteria growing in vivo. We therefore assessed the resistance of bacteria recovered from the respiratory tracts of infected mice at different times postinoculation and with two different inoculum concentrations. Again, the more serum-sensitive BP536 strain was used, and the assays were performed with 90% naïve rabbit serum, since this allowed us to observe the largest increase in resistance. Bacteria recovered from the lungs of mice on days 1, 3, and 5 postinoculation were highly resistant to naïve serum killing, exhibiting greater than 95% survival (Fig. 3A). Although B. pertussis appeared to exhibit a low level of naïve serum resistance as soon as 20 min postinoculation (Fig. 3A), the level of resistance was not found to be statistically significant. Tohama I had also gained a significant level of resistance when it was recovered from mouse lungs at 3 days postinoculation, exhibiting an increase in survival of more than 50% when it was assayed in 90% naïve rabbit serum (data not shown). Bacteria recovered from the nasal cavities and tracheae on days 1, 3, and 5 postinoculation were also more resistant than bacteria grown in vitro (data not shown). Similar results were obtained with naïve rat and mouse sera (data not shown). When wild-type mice were inoculated with a much larger dose (109 CFU), the acquired resistance was greatly reduced (Fig. 3B), suggesting that resistance may involve some host factor that is present in limited amounts.
B. pertussis acquires resistance primarily to alternative complement pathway killing.
In order to determine the mechanism by which naïve serum kills B. pertussis, we used chelating agents to verify that complement was responsible and to identify the specific pathway used for killing. EDTA has been shown to inhibit the classical and alternative complement pathways by removing free calcium and magnesium (37). EGTA-MgCl2 removes free calcium but leaves magnesium, thereby inhibiting the classical pathway but not the alternative pathway (15). Figure 4 shows that EDTA effectively inhibited complement killing, allowing both in vitro-grown and in vivo-grown B. pertussis to survive. Serum containing EGTA-MgCl2 successfully killed in vitro-grown B. pertussis but not in vivo-grown B. pertussis (Fig. 4). These results indicate that the alternative complement pathway is sufficient to kill in vitro-grown B. pertussis, but it is unable to kill B. pertussis recovered from an infected animal. Human immune serum, the serum typically used to determine the sensitivity of B. pertussis, successfully killed both in vitro-grown and in vivo-grown bacteria (data not shown), demonstrating that the resistance acquired during growth in mouse lungs is not effective against antibody-mediated complement killing.
Not all Bordetella species develop high levels of resistance to naïve serum after growth in vivo.
Wild-type B. bronchiseptica and B. parapertussis express a functional O antigen structure as part of the LPS component of their exterior cell membranes. This O antigen is necessary for protection against naïve serum killing, but it does not protect against immune serum complement killing (8, 18). Δwbm mutants of these two species, RB50Δwbm and CN2591Δwbm, do not express an O antigen due to deletion of a portion of the wbm locus. They produce a lipooligosaccharide (LOS) molecule that is structurally similar to the LOS of wild-type B. pertussis, and they are highly sensitive to naïve serum killing in vitro (8, 33). In order to determine whether B. bronchiseptica and B. parapertussis were capable of acquiring naïve serum resistance in a manner similar to that of B. pertussis, we inoculated mice with the Δwbm mutants and tested the naïve serum sensitivity of the bacteria recovered from the lungs of these mice on day 5 postinoculation as described above. The B. bronchiseptica and B. parapertussis Δwbm mutants recovered from mouse lungs on day 5 postinoculation exhibited approximately 5% survival in naïve serum, while the corresponding broth-grown controls exhibited less than 1% survival (Fig. 5). The low level of resistance exhibited by the Δwbm mutants was much lower than the level observed with B. pertussis recovered on day 5 postinoculation (Fig. 5). Although both the B. bronchiseptica Δwbm and B. parapertussis Δwbm strains exhibited a small increase in serum resistance during growth in vivo, these results indicate that, among the mammalian respiratory bordetellae, only B. pertussis efficiently acquires naïve serum resistance.
Naïve serum resistance is acquired by both Bvg− and ΔbrkA B. pertussis mutants in vivo.
BrkA has been reported to allow B. pertussis to resist complement-mediated killing, although it appears to affect the classical pathway (3, 13). To investigate the possible contribution of BrkA to the observed acquired resistance to the alternative pathway, we used the ΔbrkA mutant strain RFBP 2152 to inoculate wild-type mice for analysis (3). We used a larger-than-normal inoculum (1 × 108 CFU) because ΔbrkA mutants do not colonize the respiratory tracts of mice very well and are rapidly cleared (13; unpublished data). Bacteria were harvested from the lungs of these mice on day 1 postinoculation, early enough to recover an assayable number of bacteria. Although when this mutant was grown in broth it was the most sensitive strain assayed and all of the cells were killed by serum at levels as low as 10%, it acquired substantially more serum resistance when it was recovered from an animal, exhibiting approximately 35% survival in 90% naïve rabbit serum (Fig. 6), indicating that the acquired resistance is at least partially independent of BrkA.
The bvgAS two-component system has been shown to control the expression of many virulence factors, in addition to BrkA (12, 24, 43). The Bvg+ phase is both necessary and sufficient for infection and colonization of a host, while bacteria locked in the Bvg− phase are quickly eliminated from inoculated hosts (11, 23). To determine if the resistance to naïve serum killing acquired in vivo is a BvgAS-dependent trait, like other virulence characteristics, a Bvg− mutant of B. pertussis, BP369 (27, 40) was inoculated into mice by using a moderately high dose (1 × 108 CFU) to compensate for its rapid clearance in vivo (23). Approximately 96% of BP369 recovered from the lungs on day 1 postinoculation survived after 1 h in 90% naïve serum (Fig. 6), a level of survival similar to that seen with wild-type strains, suggesting that the resistance acquired in vivo is not due to genes exclusively expressed in the Bvg+ phase, such as brkA.
DISCUSSION
There are several conflicting reports on the ability of Bordetella to survive complement killing. Gueirard et al. reported that wild-type B. pertussis and B. bronchiseptica are sensitive to immune serum but resistant to naïve serum killing (16). Fernandez and Weiss reported that wild-type B. pertussis does not activate the alternative complement pathway and that resistance to the classical complement pathway is mediated through BrkAB (13, 14). These experiments were generally performed with lower concentrations of serum, usually 10%, and with relatively high numbers of bacteria using a procedure initially developed by Byrd et al. (9). It has recently been shown that bacterial concentrations greater than approximately 107 CFU/ml begin to deplete complement, resulting in decreased bacterial killing (8). At concentrations greater than 109 CFU/ml even the most sensitive strains are not affected by the limited amount of complement present in 10% serum (8; unpublished data). In this study we used a large excess of complement, 90% serum with carefully maintained complement activity, and relatively low numbers of bacteria, conditions under which high levels of sensitivity of B. pertussis to both naïve serum and immune serum have been demonstrated previously (8, 17, 18). Even under these conditions, we observed substantial variation in sensitivity between strains.
In addition to different assay conditions, it is possible that various growth conditions contributed to the differences in the reported sensitivities of B. pertussis strains to complement. Our data show that growth on BG plates containing blood, in contrast to growth in SS broth or on plates without blood, significantly increased the serum resistance of B. pertussis (Fig. 2). These results suggest that either there is a host factor in blood or B. pertussis turns on a defense system in response to blood, which protects the bacteria from subsequent complement exposure. B. pertussis recovered from various respiratory organs of a mouse as early as 1 day postinoculation is more than 95% resistant to various sources of complement, including rabbit, rat, and mouse. When mice were inoculated with a very high dose of B. pertussis (∼1 × 109 CFU), there was a significantly lower level of naïve serum resistance than the level seen with our normal infectious dose (Fig. 3B). Since very large numbers of bacteria appeared to decrease the effect, it appears that this resistance is not bacterially derived. It may therefore require some host factor that is rapidly recruited by B. pertussis but is present in limited quantities in the lungs.
Treatment of B. pertussis with EDTA and EGTA-MgCl2 indicated that the acquired resistance observed in this study with B. pertussis in naïve serum is sufficient to protect the bacteria from the alternative complement pathway (Fig. 4). However, when both in vitro-grown B. pertussis and in vivo-grown B. pertussis were treated with immune serum, these bacteria were sensitive to classical complement pathway killing (data not shown). During the normal course of infection, B. pertussis manages to survive and increase in the numbers even in the presence of an active innate immune response and is then cleared in the presence of an adaptive immune response (17, 23, 24). The acquired resistance to alternative pathway complement killing observed here is one possible mechanism that B. pertussis uses to combat the effects of innate immunity.
The in vitro sensitivity of B. pertussis has been attributed to the absence of O antigen on its LOS, since both wild-type B. bronchiseptica and B. parapertussis make the longer LPS due to expression of O antigen and are completely resistant to naïve serum complement, while mutants of these species unable to express O antigen are very sensitive to it (8, 18, 33). O antigen mutants of B. bronchiseptica and B. parapertussis developed only very low levels of naïve serum resistance by day 5 postinoculation, compared to the >95% survival seen with B. pertussis, indicating that a high level of acquired complement resistance is specific to B. pertussis. Wild-type B. bronchiseptica and B. parapertussis are normally resistant to the alternative complement pathway and may not have developed this acquired resistance mechanism to the same extent as B. pertussis, which must avoid complement killing in a manner independent of O antigen in order to successfully colonize a host. Once the host or bacterial factors involved in this process are identified, it should be possible to distinguish if this system is truly exclusive or just more refined in B. pertussis.
We tested the ΔbrkA mutant RFBP 2152 in order to determine whether contributions to the acquired resistance which we observed are made by BrkA, a BvgAS-regulated protein already known to act in classical pathway inhibition in the presence of antibodies (3, 4, 13). We found that this mutant does acquire a significant amount of resistance in vivo (Fig. 6), but the level is not as high as the level seen in wild-type strains. We also tested BP369, a mutant lacking a functional BvgAS two-component system which regulates the expression of many Bordetella virulence genes other than BrkA (11, 24), and we found that BP369 recovered from the lungs exhibited levels of survival near 100% (Fig. 6), similar to the level seen with wild-type B. pertussis. The fact that both mutants acquired resistance to naïve serum killing indicates that neither the BvgAS system nor any of its regulated genes are required for the observed acquisition of resistance to alternative pathway killing in vivo.
Since the complement system is typically one of the first lines of defense in the innate immune response to invading pathogens, many bacteria have developed ways to use proteins, such as C1q binding protein, C4BP, factor H, FHL-1/reconectin, and serum amyloid P, that they acquire in the host to circumvent the effects of complement (6, 22, 29, 39). Mutants that are unable to bind these complement regulators are often defective in survival in the host (2, 25, 28, 31). B. pertussis can bind C4BP in a manner shown to down regulate complement activity, but this may not be the mechanism that we investigated in this study because C4BP is primarily a regulator of classical pathway complement killing (5, 6). It is possible that B. pertussis has more than one mechanism for complement evasion. The mechanism reported here appears to enable B. pertussis to evade innate immunity through disruption of alternative pathway killing. Other mechanisms, either C4BP dependent or BrkA dependent, may be more effective against antibody-mediated classical pathway killing during the adaptive immune response. Some evidence for the presence of these types of mechanisms can be found in the fact that adoptive transfer of immune serum to mice infected with B. pertussis at the time of transfer fail to rapidly clear the infection, indicating that something prevents complement from being effective in vivo in the presence of antibodies, even though immune serum alone is sufficient to kill B. pertussis in vitro (21). We are currently working on identifying proteins that enable B. pertussis to circumvent particular elements of both the innate and adaptive immune responses.
Acknowledgments
This work was funded by a grant from Neose Corp, by Pennsylvania Department of Agriculture grant ME440678, and by USDA grant 2002-35204-11684.
We acknowledge Alison Weiss for critical reading of the manuscript and helpful discussions.
E.J.P. and D.J.B. contributed equally to this work.
Editor: D. L Burns
REFERENCES
- 1.Allen, A., and D. Maskell. 1996. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 19:37-52. [DOI] [PubMed] [Google Scholar]
- 2.Barba, G. M., T. J. Kaufmann, P. M. Schneider, C. Rittner, and M. Brai. 1994. Polymorphism of the complement C8A and -B genes in two families with C8 beta deficiency and neisserial infections. Clin. Immunol. Immunopathol. 72:83-89. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, M. G., and A. A. Weiss. 2002. Growth phase influences complement resistance of Bordetella pertussis. Infect. Immun. 70:403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berggard, K., E. Johnsson, F. R. Mooi, and G. Lindahl. 1997. Bordetella pertussis binds the human complement regulator C4BP: role of filamentous hemagglutinin. Infect. Immun. 65:3638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berggard, K., G. Lindahl, B. Dahlback, and A. M. Blom. 2001. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur. J. Immunol. 31:2771-2780. [DOI] [PubMed] [Google Scholar]
- 7.Buchner, H. 1889. Ueber die nahere Natur der bekterientodtenden Substanz im Blutserum. Central Bakteriol. Parasitol. 6:561-565. [Google Scholar]
- 8.Burns, V. C., E. J. Pishko, A. Preston, D. J. Maskell, and E. T. Harvill. 2003. Role of Bordetella O antigen in respiratory tract infection. Infect. Immun. 71:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, D. W., R. M. Roop, H. P. Veit, and G. G. Schurig. 1991. Serum sensitivity and lipopolysaccharide characteristics in Bordetella bronchiseptica, B. pertussis and B. parapertussis. J. Med. Microbiol. 34:159-165. [DOI] [PubMed] [Google Scholar]
- 10.China, B., M. P. Sory, B. T. N’Guyen, M. De Bruyere, and G. R. Cornelis. 1993. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect. Immun. 61:3129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter, P. A., and J. F. Miller. 1998. In vivo and ex vivo regulation of bacterial virulence gene expression. Curr. Opin. Microbiol. 1:17-26. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, R. C., and A. A. Weiss. 1994. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect. Immun. 62:4727-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez, R. C., and A. A. Weiss. 1998. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol. Lett. 163:57-63. [DOI] [PubMed] [Google Scholar]
- 15.Fine, D. P., S. R. Marney, Jr., D. G. Colley, J. S. Sergent, and R. M. Des Prez. 1972. C3 shunt activation in human serum chelated with EGTA. J. Immunol. 109:807-809. [PubMed] [Google Scholar]
- 16.Gueirard, P., K. Le Blay, A. Le Coustumier, R. Chaby, and N. Guiso. 1998. Variation in Bordetella bronchiseptica lipopolysaccharide during human infection. FEMS Microbiol. Lett. 162:331-337. [DOI] [PubMed] [Google Scholar]
- 17.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janulczyk, R., F. Iannelli, A. G. Sjoholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 20.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping cough. Kitasato Arch. Exp. Med. 27:57-62. [PubMed] [Google Scholar]
- 21.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 23.Martinez de Tejada, G., P. A. Cotter, U. Heininger, A. Camilli, B. J. Akerley, J. J. Mekalanos, and J. F. Miller. 1998. Neither the Bvg- phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895-908. [DOI] [PubMed] [Google Scholar]
- 25.Masson, L., and B. E. Holbein. 1985. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect. Immun. 47:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLafferty, M. A., D. R. Harcus, and E. L. Hewlett. 1988. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J. Gen. Microbiol. 134:2297-2306. [DOI] [PubMed] [Google Scholar]
- 27.Miller, J. F., S. A. Johnson, W. J. Black, D. T. Beattie, J. J. Mekalanos, and S. Falkow. 1992. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J. Bacteriol. 174:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeleman, C., S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noursadeghi, M., M. C. Bickerstaff, J. R. Gallimore, J. Herbert, J. Cohen, and M. B. Pepys. 2000. Role of serum amyloid P component in bacterial infection: protection of the host or protection of the pathogen. Proc. Natl. Acad. Sci. USA 97:14584-14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuttal, G. 1888. Experimente uber die bakterienfeidlichen Einflusse des thierischen Korpers. Z. Hyg. Infektionskr. 4:353-395. [Google Scholar]
- 31.Orren, A., D. A. Caugant, C. A. Fijen, J. Dankert, E. J. van Schalkwyk, J. T. Poolman, and G. J. Coetzee. 1994. Characterization of strains of Neisseria meningitidis recovered from complement-sufficient and complement-deficient patients in the western Cape Province, South Africa. J. Clin. Microbiol. 32:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Caballero, D., S. Alberti, F. Vivanco, P. Sanchez-Corral, and S. Rodriguez de Cordoba. 2000. Assessment of the interaction of human complement regulatory proteins with group A Streptococcus. Identification of a high-affinity group A Streptococcus binding site in FHL-1. Eur. J. Immunol 30:1243-1253. [DOI] [PubMed] [Google Scholar]
- 33.Preston, A., A. G. Allen, J. Cadisch, R. Thomas, K. Stevens, C. M. Churcher, K. L. Badcock, J. Parkhill, B. Barrell, and D. J. Maskell. 1999. Genetic basis for lipopolysaccharide O-antigen biosynthesis in bordetellae. Infect. Immun. 67:3763-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 35.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 36.Rokita, E., A. Makristathis, E. Presterl, M. L. Rotter, and A. M. Hirschl. 1998. Helicobacter pylori urease significantly reduces opsonization by human complement. J. Infect. Dis. 178:1521-1525. [DOI] [PubMed] [Google Scholar]
- 37.Sandberg, A. L., and A. G. Osler. 1971. Dual pathways of complement interaction with guinea pig immunoglobulins. J. Immunol. 107:1268-1273. [PubMed] [Google Scholar]
- 38.Stibitz, S., and M. S. Yang. 1991. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J. Bacteriol. 173:4288-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Berg, R. H., M. C. Faber-Krol, J. A. van de Klundert, L. A. van Es, and M. R. Daha. 1996. Inhibition of the hemolytic activity of the first component of complement C1 by an Escherichia coli C1q binding protein. J. Immunol. 156:4466-4473. [PubMed] [Google Scholar]
- 40.Weiss, A. A., and S. Falkow. 1984. Genetic analysis of phase change in Bordetella pertussis. Infect. Immun. 43:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wurzner, R. 1999. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol. Immunol. 36:249-260. [DOI] [PubMed] [Google Scholar]
- 43.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]