Abstract
Cytotoxic necrotizing factor 1 (CNF-1) is an exotoxin of Escherichia coli that constitutively activates the GTPases Rho, Rac, and CDC42. Stimulation of Rho was shown to enhance myosin light-chain (MLC) phosphorylation via Rho kinase-mediated inhibition of MLC phosphatase in endothelial cells. Here we report that 3 h after CNF stimulation of endothelial cells, RhoA was activated and MLC phosphorylation was increased in a Rho/Rho-kinase-dependent manner, but no decrease in MLC phosphatase activity could be detected. Despite continuous RhoA activation, MLC phosphatase activity was doubled after 24 h of CNF stimulation, and this coincided with decreased MLC phosphorylation and cell spreading. Rac was also activated at 3 to 24 h but did not contribute to MLC phosphorylation, and its amount gradually decreased in the CNF-stimulated cells. CDC42Hs was not activated above control values by CNF. These results suggest that CNF can induce specific decoupling (Rho kinase from MLC phosphatase) and deactivation events in Rho GTPase signaling, potentially reflecting cellular protection mechanisms against permanently active Rho GTPases.
It was reported recently that various bacterial toxins and atherogenic mediators induce contraction of endothelial cells via the Rho/Rho-kinase pathway (1, 8, 9, 21). Upon activation by upstream signals, the GTPase Rho stimulates one of two Rho kinase isoforms, which in turn phosphorylate and thereby inactivate myosin light-chain (MLC) phosphatase (3, 11, 12, 16). In combination with basal or enhanced Ca2+-calmodulin-dependent MLC kinase activity, the diminished MLC phosphatase activity propagates MLC phosphorylation. Phosphorylated MLC interacts with actin to form contractile actomyosin structures including stress fibers (5).
The exotoxin cytotoxic necrotizing factor 1 (CNF-1) of Escherichia coli is taken up by endocytosis into host cells and deamidates Gln-63 of Rho and Gln-61 of Rac and CDC42Hs (2, 4, 6, 10, 13, 14, 18, 20). Deamidation of the Gln residues leads to inhibition of the intrinsic GTPase activity and therefore to constitutive activation of Rho, Rac, and CDC42Hs (10, 14, 20). In fibroblasts, CNF stimulates stress fiber formation, consistent with Rho activation. However, in endothelial cells CNF was shown to promote spreading (10, 20, 23). CNF-induced cell spreading cannot easily be explained, as spreading is caused by inactivation of Rho and activation of Rho induces contraction of endothelial cells (1, 8, 9, 21, 22, 24).
Here we report that within 3 h, CNF induces activation of Rho and Rac as well as MLC phosphorylation and contraction in endothelial cells. This CNF effect was dependent on Rho and Rho kinase but independent of Rac and CDC42. Surprisingly, CNF-induced MLC phosphorylation was not associated with inactivation of MLC phosphatase. Furthermore, after 24 h CNF-stimulated endothelial cells displayed an increase in MLC phosphatase and cell spreading despite activated RhoA. The effect of CNF on endothelial cells therefore is dynamic and time dependent and involves downregulation of Rho GTPase effector mechanisms.
MATERIALS AND METHODS
Cell culture.
Human umbilical vein endothelial cells (HUVEC) were obtained and cultured as described previously (8). Briefly, cells harvested from umbilical cords were plated onto collagen-coated (24 h, 100-μg/ml collagen G; Biochrom, Berlin, Germany) plastic culture flasks and cultured in endothelial growth medium (Promo Cell, Heidelberg, Germany), containing ECGS/H2 (endothelial cell growth supplement/heparin) and 10% FCS.
Inhibitors and recombinant proteins.
Y27632 was from Calbiochem, Bad Soden, Germany. CNF, PAK-CRIB domain, C21-Rho-binding domain (RBD) of Rho kinase and N17Rac were expressed as glutathione S-transferase (GST) fusion proteins in E. coli and purified on glutathione-Sepharose beads as described (8, 19). The expression vectors for GST-PAK-CRIB and GST-C21 were generous gifts of John Collard (Amsterdam, The Netherlands), the vector for GST-N17Rac1 was a generous gift of Alan Hall (MRC Laboratory, London, United Kingdom). The gene encoding CNF-1 from E. coli was amplified using PCR primers 5′CACAGAGGAGTTAAAGGATCCATGGGTAACCAATGGC3′ and 5′GGCCAATAAATAATTTGAATTCTCAAAATTTTTTTG3′ and cloned into the BamHI and EcoRI restriction sites of vector pGEX-2T (Pharmacia, Freiburg, Germany). The vector was then transformed into E. coli strain DH5α. Protein expression was induced with 20 μM IPTG (isopropyl-β-d-thiogalactopyranoside).
Microinjection.
Microinjection was performed using transjector 5246 (Eppendorf) and a Compic Inject micromanipulator (Cell Biology Trading, Hamburg, Germany). All proteins were microinjected at a concentration of 1 to 2 μg/μl. Cells were incubated for 30 min postinjection for recovery, with an additional incubation for 3 h in the presence of CNF (2 μg/μl) where indicated. Injected cells were identified by detection of coinjected rat immunoglobulin G (IgG) (5 mg/ml) with fluorescein isothiocyanate-labeled goat anti-rat IgG antibody (Dianova, Hamburg, Germany).
Immunofluorescence.
For fluorescence staining, HUVEC were plated (2 × 104 cells/cm2) on Eppendorf Cellocate glass coverslips (Eppendorf, Hamburg, Germany) coated with collagen G (100 μg/ml) and grown to confluency for 10 days. For staining of F-actin, cells were fixed for 10 min with 3.7% formaldehyde in phosphate-buffered saline (PBS) containing 1 mM Ca2+ and permeabilized in ice-cold acetone for 5 min. Coverslips were incubated with rhodamine phalloidin for 20 min and washed with PBS. For phospho-MLC staining, formaldehyde-fixed HUVEC were permeabilized for 5 min with 0.1% Triton X-100 and then incubated with polyclonal rabbit anti-phosphorylated MLC antibody (diluted 1/10 in PBS-0.5% bovine serum albumin) for 1 h, followed by a 1-h incubation with Alexa 568-conjugated goat anti-rabbit IgG antibody. Coverslips were mounted in Mowiol containing 0.2% p-phenylenediamine as antifading reagent, and fluorescence was recorded with a Leica RBM 3 epifluorescence microscope.
MLC phosphorylation and Western blot.
HUVEC were grown for 10 days in Costar 6-well culture dishes and treated with CNF as indicated. Cells were lysed with boiling Laemmli buffer. MLC phosphorylation was analyzed by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE) and Western blotting. Proteins (50 μg per lane; protein concentration determined with BCA protein assay kit; Pierce) were electroblotted onto PVDF membranes using Mini Protean II electrophoresis cells (Bio-Rad). Membranes were incubated overnight with the anti-phospho MLC antibody (1/500) in Tris-buffered saline containing 0.05% Tween 20, washed three times, incubated for 1 h with horseradish peroxidase-labeled goat anti-rabbit antibody (Amersham; 1/7,500 in Tris-buffered saline), washed three times, and then developed with Luminol solution (Pierce) and exposed to Kodak X-Omat films.
Preparation of myosin-enriched cell fractions.
Myosin-enriched fractions of HUVEC were prepared as described (8). Briefly, confluent HUVEC monolayers on 100-mm-diameter dishes were washed two times with ice-cold PBS (Sigma) and 200 μl of homogenization buffer (50 mM Tris-aminomethane [pH 7.5], 0.1 mM EDTA, 28 mM β-mercaptoethanol, a 1-μg/ml concentration each of leupeptin, pepstatin, Pefabloc, and aprotinin) was added. Cells were frozen at −80°C, scraped and homogenized. Homogenates were treated with high-salt buffer (0.6 M NaCl, 0.1% Tween 20, containing 1-μg/ml concentrations of leupeptin and pepstatin, aprotinin, and Pefabloc) for 1 h at 4°C and centrifuged at 4,500 × g for 30 min at 4°C. The supernatant was diluted 10-fold with assay buffer (50 mM Tris, 0.1 mM EDTA, 28 mM β-mercaptoethanol [pH 7.0]) and centrifuged for 30 min at 10,000 × g at 4°C. The resulting pellet was resolved in 10 μl of high-salt buffer.
Measurement of MLC phosphatase activity.
Phosphatase activity was determined in myosin-enriched fractions by using the protein phosphatase assay system (GIBCO) according to the instructions of the manufacturer, as described previously (8). Briefly, phosphorylase b was in vitro phosphorylated in the presence of [32P]ATP by phosphorylase kinase to obtain a substrate for protein phosphatase 1 and protein phosphatase 2. Samples of myosin-enriched fractions were diluted with 30 μl of assay buffer (50 mM Tris, 0.1 mM EDTA, 28 mM β-mercaptoethanol, 6.25 mM caffeine [pH 7.0]) and mixed with 20 μl of radioactive phosphatase substrate. Parallel samples contained okadaic acid (1 nM) to inhibit protein phosphatase 2. Reaction was stopped after 10 min at 30°C with ice-cold 20% trichloroacetic acid. Samples were then incubated on ice for 10 min and centrifuged at 12,000 × g for 3 min. The radioactivity released in the supernatant was measured using a Wallac 1410 liquid scintillation counter. The difference of phosphatase activity in the presence and absence of okadaic acid was attributed to MLC phosphatase (mPP1).
Rho, Rac, and CDC42 pull down.
Rho, Rac and CDC42 pull down assays were performed as described in detail elsewhere (19). Briefly, HUVEC (5 × 106 to 10 × 106 cells) treated with or without CNF were lysed for 5 min at 4°C in GST-Fish buffer containing 10% glycerol, 50 mM Tris (pH 7.4), 100 mM NaCl, 1% NP-40 and 2 mM MgCl2. Glutathione-Sepharose GST-C21-RBD or -PAK-CRIB beads were added for 30 min at 4°C, washed three times in GST-Fish buffer, and then run on SDS-PAGE gel followed by incubation with anti-RhoA (Santa Cruz, Heidelberg, Germany), anti-Rac1 or anti-CDC42 (Transduction Laboratories, Heidelberg, Germany) antibodies.
RESULTS
CNF induces MLC phosphorylation in human endothelial cells.
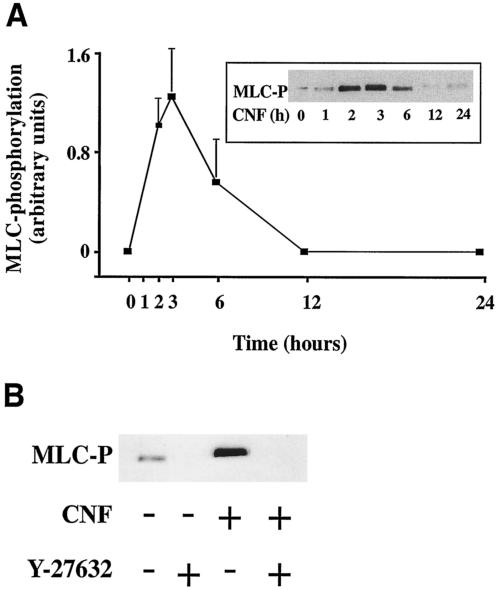
The effect of CNF on MLC phosphorylation in HUVEC was investigated by immunoblotting using specific antibodies to phosphorylated MLC. The phospho-MLC bands were quantitated by densitometry. Figure 1A shows that CNF (2 μg/ml) induced maximal MLC phosphorylation in HUVEC within 3 h. Thereafter, MLC phosphorylation continuously declined and reached baseline values at 12 to 24 h. To test whether the CNF-induced MLC phosphorylation depends on Rho kinase, we pretreated cells with the Rho kinase inhibitor Y-27632 (20 min, 10 μM) and then stimulated them with CNF (3 h, 2 μg/ml) in the continuous presence of Y-27632. Figure 1B shows that CNF-induced MLC phosphorylation could be completely blocked by pretreatment of HUVEC with Y-27632.
FIG. 1.
CNF induces MLC phosphorylation in human endothelial cells. (A) HUVEC were stimulated with CNF (2 μg/ml), and at the indicated time points, MLC phosphorylation was determined by Western blot using a specific antibody to phosphorylated MLC (MLC-P). Phospho-MLC bands were quantified by densitometry. Results are the mean of three experiments ± standard error of the mean (error bars). (Inset) Representative Western blot depicting the time course of CNF-induced MLC phosphorylation. (B) HUVEC were not treated or pretreated with Rho kinase inhibitor Y-27632 (20 min, 10 μM) and were then stimulated or not with CNF (3 h, 2 μg/ml). MLC phosphorylation (MLC-P) was completely blocked by Y-27632.
CNF induces time-dependent actomyosin rearrangements in human endothelial cells.
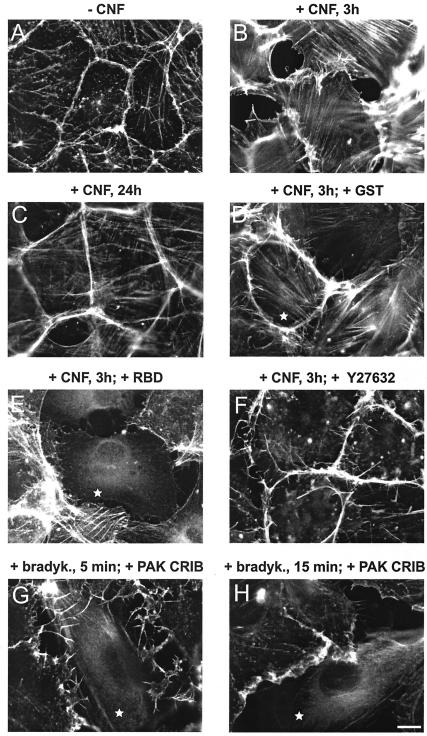
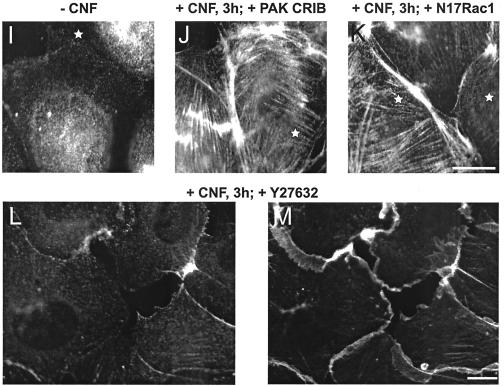
MLC phosphorylation promotes interaction of myosin with filamentous (F)-actin and induces formation of contractile actomyosin filaments. Consistent with this, CNF caused formation of actin stress fibers and cell contraction at 3 h (Fig. 2A and B). To test involvement of Rho and Rho kinase in the 3-h CNF effect, endothelial cells were microinjected with the isolated Rho-binding domain (RBD) of Rho kinase or pretreated with the Rho kinase inhibitor Y-27632 before stimulation with CNF. Both inhibitors blocked CNF-induced stress fiber formation (Fig. 2E and F). As reported, the specific Rho-inhibitor C3-transferase of Clostridium botulinum was unable to inhibit the CNF effect (4) (data not shown). Consistent with decreased MLC phosphorylation after 6 h, CNF-stimulated cells lost stress fibers and displayed a spread phenotype most prominent at 24 h (Fig. 2C). To test a potential role of Rac or CDC42 in CNF-stimulated MLC phosphorylation at 3 h, cells were microinjected with recombinant CRIB domain of PAK, which binds to and inactivates Rac and CDC42 (Fig. 2G and H) or with dominant inhibitory N17Rac and then stained with anti-phospho MLC antibody. As demonstrated in Fig. 2I-K, CNF induced the assembly of phospho-myosin along stress fibers and this effect could not be prevented by microinjection of PAK-CRIB or N17Rac. Together these data indicate that Rho and Rho kinase, but not Rac or CDC42 control MLC phosphorylation and contraction in CNF-stimulated HUVEC.
FIG. 2.
CNF-induced actin/myosin rearrangements in human endothelial cells are dependent on Rho/Rho kinase and independent of Rac and CDC42. (A to F) HUVEC were not stimulated (A) or were stimulated with 2-μg/ml CNF for 3 h (B, D, E, and F) or 24 h (C) and were then stained for F-actin using rhodamine phalloidin. Cells were microinjected with GST (D) or Rho-binding domain of Rho kinase (E), as indicated with a star, or were pretreated for 20 min with 10 μM Rho kinase inhibitor Y-27632 (F). (G and H) HUVEC indicated with a star were microinjected with CRIB domain of PAK and then stimulated with bradykinin (100 ng/ml) for 5 min to induce microspikes and for 15 min to induce ruffles. Microinjected cells showed no microspikes or ruffles, indicating Rac and CDC42 inhibiting activity of the PAK CRIB domain. The scale bar in H equals 10 μm. (I to M) HUVEC were not stimulated (I) or were stimulated with 2-μg/ml CNF for 3 h (J, K, L, and M) and then stained for or phospho-MLC using a specific antibody. Before CNF stimulation, cells were microinjected with CRIB domain of PAK (J) or N17Rac1 (K), as indicated by a star, or were treated with 10 μM Rho kinase inhibitor Y-27632 (L). (M) F-actin staining of the cells in L to demonstrate the presence of membrane ruffles. Results are representative of three independent experiments. The scale bar in K and M equals 10 μm.
CNF does not decrease MLC phosphatase in human endothelial cells.
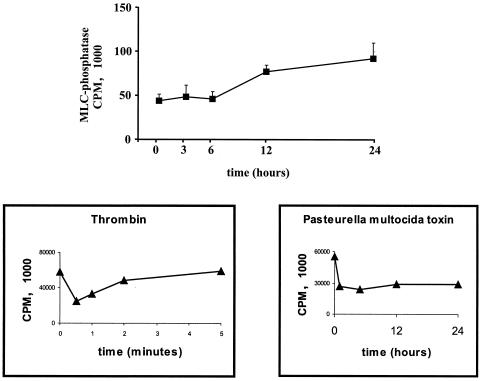
We next addressed the question whether the CNF-induced increase in MLC phosphorylation is due to inactivation of MLC phosphatase, a mechanism employed by a variety of bacterial and human Rho/Rho kinase activators in endothelial cells (8, 9, 1, 21, 20, 24). Surprisingly, although the peak of MLC phosphorylation and the most dramatic rearrangements of actin occurred within the first 6 h of CNF treatment, MLC phosphatase activity was not altered (Fig. 3). However, within 6 to 24 h of CNF treatment MLC phosphatase activity increased almost twice. As indicated by a shift in SDS-PAGE (10, 20), Rho was modified by CNF after 1 to 3 h and remained so during the entire stimulation period (Essler et al., unpublished data). These findings suggest that CNF induces Rho/Rho-kinase-mediated MLC phosphorylation in HUVEC without decreasing MLC phosphatase. Under the same conditions thrombin (1 U/ml) or Pasteurella multocida toxin (40 ng/ml) were able to induce transient or long-lasting inhibition of MLC phosphatase, respectively (boxes below Fig. 3).
FIG. 3.
CNF does not decrease MLC phosphatase activity in human endothelial cells. HUVEC were stimulated with CNF (2 μg/ml), and at indicated time points, phosphorylase b phosphatase activity (MLC phosphatase) was determined as described in Materials and Methods. In the absence of HUVEC lysate, the value for phosphorylase b phosphatase activity was zero. Values represent mean ± standard error of the mean (error bars) of three experiments performed in duplicate. The two boxes below are controls showing that under the conditions used by us, thrombin (1 U/ml) and Pasteurella multocida toxin (40 ng/ml) were able to induce a transient and long-lasting inhibition of MLC phosphatase, respectively.
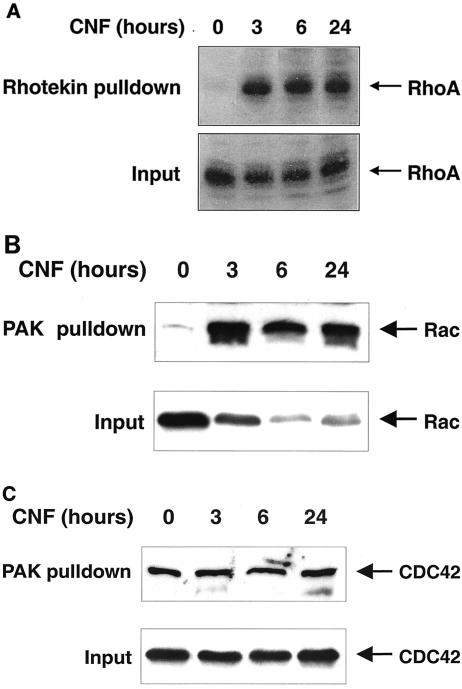
CNF activates Rho, Rac, and CDC42 in endothelial cells.
To relate the time-dependent changes of MLC phosphorylation, MLC phosphatase activity and actin rearrangement to activation of Rho, Rac, and CDC42 we performed pull down experiments using recombinant Rho-binding domain of Rhotekin and Rac/CDC42-binding domain of PAK, as previously described (19).
The results indicate that Rho was activated at 3 h and remained activated up to 24 h with CNF stimulation (Fig. 4A). Similarly, GTP-loaded active Rac was detected from 3 to 24 h (Fig. 4B). Although Rac was degraded considerably during the CNF stimulation period as described previously (14), the absolute amount of GTP-loaded active Rac remained constant (Fig. 4B). In contrast to Rho and Rac, CDC42Hs was already active in resting cells and could not be further stimulated by CNF (Fig. 4C). The seeming paradox that Rho is activated at 24 h and MLC phosphatase activity is increased almost twice at the same time further supports the notion that signaling downstream of Rho/Rho kinase, which normally leads to inactivation of MLC phosphatase, is downregulated by CNF. We conclude that after 24 h of CNF treatment, endothelial cells reach a phenotype very similar to the phenotype of unstimulated cells, mainly due to decoupling of Rho from its normal downstream signaling.
FIG. 4.
Time courses of Rho, Rac and CDC42 activation in CNF-stimulated endothelial cells. HUVEC were incubated with CNF (2 μg/ml) for the indicated time periods and then subjected to Rhotekin RBD pull down to detect active, GTP-loaded RhoA (A) or PAK-CRIB pull down to detect active, GTP-loaded Rac1 (B) or CDC42Hs (C). The input in A, B, and C represents approximately 5% of the cell lysate used for the respective pull down at the indicated time point. Immunoblots of pull down assays and inputs were investigated with the same antibodies. RhoA and Rac 1 were activated at 3, 6, and 24 h, whereas CDC42Hs was already active at 0 h. The level of Rac protein but not of Rho or CDC42Hs decreased during CNF stimulation. One representative experiment out of three similar ones is shown.
DISCUSSION
We report here that stimulation of endothelial cells with the Rho-activating toxin CNF results in increased MLC phosphorylation and cell contraction which could be blocked by inhibition of Rho and Rho kinase but not that of Rac or CDC42. Surprisingly, MLC phosphatase was not decreased by the CNF stimulation. This is noteworthy because all stimuli reported so far to activate Rho/Rho kinase in endothelial cells lead to a decrease in MLC phosphatase activity, and this was thought to be mainly responsible for the observed increase in MLC phosphorylation. Because Rho kinase can principally be activated by CNF-modified Rho in vitro (14), and because the Rho kinase inhibitor Y-27632 and Rho-binding domain of Rho kinase inhibited CNF-induced MLC phosphorylation, one can conclude that Rho kinase is activated but becomes decoupled from MLC phosphatase after 3 h of CNF stimulation. One possibility is that Rho kinase under these circumstances directly phosphorylates MLC, as was demonstrated in vitro (21). We found that CNF-induced activation of Rac whereas there was no difference in pull down of active CDC42 with and without CNF treatment. Rac and CDC42 have been reported to regulate myotonic dystrophy kinase-related kinases, which are able to phosphorylate MLC on Ser19 and are characterized by catalytic subunits highly homologous to that of Rho kinase (16). However, the lack of an effect of Rac and CDC42 inhibitors on CNF- stimulated MLC phosphorylation is inconsistent with a role for myotonic dystrophy kinase-related kinases in our system.
In CNF-treated HUVEC, MLC phosphatase was unchanged after 3 h and increased twofold after 24 h in the presence of activated Rho. This suggests that Rho kinase is disconnected from MLC phosphatase up to a point where even the basal inhibitory effect exerted by Rho kinase on MLC phosphatase is completely released (1). This is consistent with previous work showing that complete inhibition of the Rho/Rho kinase pathway by using C3-transferase from C. botulinum and Rho kinase inhibitor Y-27632 enhances MLC phosphatase activity twofold (1). Increased MLC phosphatase may explain the observed dephosphorylation of MLC and increased cell spreading after 24 h of CNF treatment. Alternatively, Rac could be responsible for the observed spreading at 24 h (17). It was shown previously that pretreatment of HUVEC with CNF inhibited thrombin-induced endothelial cell contraction and prevented the thrombin-induced increase in endothelial permeability (23). In that study, the CNF effect was investigated after a prolonged stimulation period of HUVEC, and therefore an increased MLC phosphatase, as demonstrated here, could be responsible for the blocking effect. Consistently, we found that in endothelial cells stimulated with CNF for 12 h, thrombin-induced MLC phosphorylation was blocked (Essler et al., unpublished data).
Recently, it was reported that CNF induces degradation of Rho GTPases via the ubiquitin-proteasome machinery (7, 15). Although degradation of Rho or Rac cannot play a role in the CNF effects described by us, it is conceivable that Rho GTPase effectors or regulators are also degraded by the proteasome when they are continuously activated/engaged by CNF stimulation. Thus, to look for degradation of signaling proteins downstream of Rho GTPases may be a worthwhile future effort.
In conclusion, CNF induces time-dependent activation, effector decoupling, and inactivation-degradation events of Rho GTPases in endothelial cells. Because the activity of Rho GTPases must be strictly controlled in cells, the use of CNF reveals the diverse mechanisms by which cells can protect themselves from continuous Rho GTPase activity.
Acknowledgments
M.E. and S.L. contributed equally to this study.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) Ae11, GRK 438, and by August-Lenz-Stiftung.
We thank Barbara Böhlig and Claudia Trasak for expert technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Aepfelbacher, M., and M. Essler. 2001. Disturbance of endothelial barrier function by bacterial toxins and atherogenic mediators: a role for Rho/Rho-kinase. Cell. Microbiol. 3:649-658. [DOI] [PubMed] [Google Scholar]
- 2.Aktories, K. 1997. Bacterial toxins that target Rho proteins. J. Clin. Investig. 99:827-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246-20249. [DOI] [PubMed] [Google Scholar]
- 4.Barth, H., C. Olenik, P. Sehr, G. Schmidt, and D. K. Meyer. 1999. Neosynthesis and activation of Rho by Escherichia coli cytotoxic necrotizing factor (CNF-1) reverse cytopathic effects of ADP-ribosylated Rho. J. Biol. Chem. 274:27407-27414. [DOI] [PubMed] [Google Scholar]
- 5.Chrzanowska-Wodnicka, M., and K. Burridge. 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 134:1403-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contamin, S., A. Galmiche, A. Doye, G. Flatau, A. Benmerah, and P. Boquet. 2000. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 11:1775-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doye, A., A. Mettouchi, G. Bossis, R. Clement, C. Buisson-Touati, G. Flatau, L. Gagnoux, M. Piechaczyk, P. Boquet, and E. Lemichez. 2002. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111:553-564. [DOI] [PubMed] [Google Scholar]
- 8.Essler, M., M. Amano, H.-J. Kruse, K. Kaibuchi, P. C. Weber, and M. Aepfelbacher. 1998. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho-kinase in human endothelial cells. J. Biol. Chem. 273:21867-21874. [DOI] [PubMed] [Google Scholar]
- 9.Essler, M., K. Hermann, M. Amano, K. Kaibuchi, J. Heesemann, P. C. Weber, and M. Aepfelbacher. 1998. Pasteurella multocida toxin increases endothelial permeability via Rho-kinase and myosin light chain phosphatase. J. Immunol. 161:5640-5646. [PubMed] [Google Scholar]
- 10.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G-protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 11.Ishizaki, T., M. Maekawa, K. Fujisawa, K. Okawa, A. Iwamatsu, A. Fujita, N. Watanabe, Y. Saito, A. Kakizuka, N. Morii, and S. Narumiya. 1996. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 15:1885-1893. [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, Y. Feng, T. Nakano, K. Okawa, A. Iwamatsu, and K. Kaibuchi. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245-248. [DOI] [PubMed] [Google Scholar]
- 13.Lerm, M., G. Schmidt, U. M. Goering, J. Schirmer, and K. Aktories. 1999. Identification of the region of Rho involved in substrate recognition by Escherichia coli cytotoxic necrotizing factor-1 (CNF-1). J. Biol. Chem. 274:28999-29004. [DOI] [PubMed] [Google Scholar]
- 14.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerm, M., M. Pop, G. Fritz, K. Aktories, and G. Schmidt. 2002. Proteasomal degradation of cytotoxic necrotizing factor 1-activated Rac. Infect. Immun. 70:4053-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung, T., X. Chen, E. Manser, and L. Lim. 1996. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal organization. Mol. Cell. Biol. 16:5313-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobes, C., and A. Hall. 1999. Rho-GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol. 147:831-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oswald, E., M. Sugai, A. Labigne, H. C. Wu, C. Fiorentini, P. Boquet, and A. D. O'Brien. 1994. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding protein Rho involved in assembly of actin stress fibers. Proc. Natl. Acad. Sci. USA 91:3814-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander, E. E., J. P. ten Klooster, S. van Delft, R. A. van der Kammen, and J. G. Collard. 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behaviour. J. Cell Biol. 147:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 21.van Nieuw Amerongen, G. P., R. Drajer, M. A. Vermeer, and V. W. M. van Hinsberg. 1998. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium and RhoA. Circ. Res. 83:1115-1123. [DOI] [PubMed] [Google Scholar]
- 22.van Nieuw Amerongen, G. P., and V. W. M. van Hinsbergh. 2001. Cytoskeletal effects of Rho-like small guanine-nucleotide-binding proteins in the vascular system. Arterioscler. Thromb. Vasc. Biol. 21:300-311. [DOI] [PubMed] [Google Scholar]
- 23.Vouret-Craviari, V., D. Grall, G. Flatau, J. Pouysségur, P. Boquet, and E. van Obberghen-Schilling. 1999. Effects of cytotoxic necrotizing factor 1 and lethal toxin on actin cytoskeleton and VE-cadherin localization in human endothelial cell monolayers. Infect. Immun. 67:3002-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wojciak-Stothard, B., A. Entwistle, R. Garg, and A. J. Ridley. 1998. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell Physiol. 176:150-165. [DOI] [PubMed] [Google Scholar]