Abstract
Rhoptry-associated protein 1 (RAP-1) is a targeted vaccine antigen for Babesia bovis and Babesia bigemina infections of cattle. The 60-kDa B. bovis RAP-1 is recognized by antibodies and T lymphocytes from cattle that recovered from infection and were immune to subsequent challenge. Immunization with native or recombinant protein was reported to reduce parasitemias in challenged animals. We recently reported that the NT domain of B. bovis RAP-1 contained immunodominant T-cell epitopes, whereas the repeat-rich CT domain was less immunostimulatory for T lymphocytes from cattle immune to B. bovis. The present study was therefore designed to test the hypothesis that the NT region of RAP-1, used as a vaccine with interleukin-12 and RIBI (catalog no. R-730; RIBI Immunochem Research, Inc., Hamilton, Mont. [now Corixa, Seattle, Wash.]) adjuvant to induce a type 1 response, would prime calves for antibody and T-helper cell responses comparable to or greater than those induced by full-length RAP-1 containing the C-terminal repeats. Furthermore, a type 1 immune response to RAP-1 was hypothesized to induce protection against challenge. Following four inoculations of either recombinant full-length RAP-1 or RAP-1 NT protein, RAP-1-specific immunoglobulin G (IgG) titers, T-lymphocyte proliferation, and gamma interferon production were similar. Similar numbers of NT region peptides were recognized. However, in spite of the presence of strong RAP-1-specific IgG and CD4+-T-lymphocyte responses that were recalled upon challenge, neither antigen stimulated a protective immune response. We conclude that successful priming of calves with recombinant RAP-1 and adjuvants that elicit strong Th1 cell and IgG responses is insufficient to protect calves against virulent B. bovis challenge.
Tick-transmitted intraerythrocytic babesial parasites cause significant morbidity in humans and in domestic animals, characterized predominantly by anemia (20, 21). The cattle parasite, Babesia bovis, also causes an acute and often fatal infection in immunologically naïve animals, a consequence of the ability of the parasite to be sequestered in microcapillaries of the kidneys, lungs, and brain, resulting in organ failure, cerebral anoxia, and systemic shock (44). Cattle that do recover from B. bovis infection remain persistently infected and are resistant to developing clinical disease upon reinfection with a homologous strain. Protective immunity can be achieved by vaccinating animals with B. bovis strains attenuated through repeated passage in splenectomized calves, although these vaccines pose the obvious risks of transmitting other blood-borne pathogens and evoking hemolytic anemia. Subunit vaccines for B. bovis are not commercially available; however, several studies have indicated the feasibility of stimulating protective immunity by immunization with individual or combined recombinant protein antigens (8, 43).
Rhoptry-associated protein 1 (RAP-1) is one antigen that has been targeted as a vaccine candidate (4, 8, 43). B. bovis RAP-1 is the product of a member of a multigene family encoding 58- to 60-kDa proteins identified in Babesia bigemina, Babesia canis, Babesia divergens, Babesia ovis, and Babesia caballi parasites (13, 14, 22, 23, 33, 34, 37). RAP-1 is highly conserved among otherwise antigenically variant strains of B. bovis. For example, there is complete amino acid sequence identity among the RAP-1 proteins of Mexico, Texas, and Argentina R1A strains (36) and nearly complete identity among the Mexico Mo7 and Australian S and L strains (12). In B. bovis, RAP-1 is associated with the parasite membrane (16, 45) and cytosolic merozoite preparations (6) and was recently shown to be expressed on sporozoites as well (25). Immunization with native B. bigemina RAP-1 (23, 29), B. bovis RAP-1 (11), or a truncated recombinant B. bovis RAP-1-glutathione S-transferase fusion protein (43) resulted in substantially reduced parasitemias in cattle following challenge. Although not abundant (43), RAP-1 is an immunogenic protein, as it was consistently recognized by sera and T lymphocytes from B. bovis-infected cattle that were immune to challenge (6, 26, 35).
The mechanism of protective immunity to B. bovis is postulated to involve both CD4+-T-helper 1 (Th1) lymphocyte and antibody responses (4, 8, 43). Documented immune responses to RAP-1 are consistent with these types of response. Th cells specific for RAP-1 secrete large amounts of gamma interferon (IFN-γ), which is important for activating macrophages to produce nitric oxide and other babesiacidal molecules and for stimulating increased IgG2 production (5, 8, 26, 32). It was recently demonstrated that RAP-1-specific immune rabbit sera effectively neutralized binding of B. bovis sporozoites to erythrocytes (25) and that a RAP-1-specific monoclonal antibody (MAb), 1C1, blocked binding of soluble RAP-1 to merozoites and inhibited merozoite growth in vitro (45).
The structure of the B. bovis RAP-1 molecule consists of a unique N-terminal (NT) region (amino acids [aa] 1 to 316) and a C-terminal (CT) region (aa 317 to 565) consisting of seven tandem repeats of a degenerate 23-aa sequence (37). The NT region contains four cysteine residues and additional amino acid motifs that are highly conserved among RAP-1 orthologs from the different species of Babesia (12, 13, 33, 38). The presence of such conserved amino acid motifs in the NT region of RAP-1 indicates that the region is functionally important and may therefore be useful as a target of immune intervention (38).
An effective recombinant-protein or DNA vaccine against B. bovis will likely consist of multiple proteins, or combinations of T- and B-cell epitopes from multiple proteins, to enable T-cell recognition by populations of cattle that express a large repertoire of major histocompatibility complex class II molecules (8, 30, 43). Therefore, it is critical to identify those immunostimulatory regions of the molecule involved in Th cell recognition, as well as antibody binding. In a recent study, it was determined that the immunodominant T-lymphocyte epitopes in RAP-1 recognized by B. bovis-immune cattle were in the NT region, whereas there was only weak recognition by a single animal in the study to the CT repeat region (26). In contrast, B-cell epitopes recognized by immune bovine sera are located in both NT and CT domains, although the CT repeat domain appeared to be serologically dominant (35). Interestingly, MAb BABB75, specific for a B. bovis RAP-1 CT repeat epitope (35), did not neutralize infectivity for merozoites in vitro (S. Hines, personal communication). In a review article, Wright et al. stated that the repetitive region of RAP-1 (formerly called 21B4) was not protective, but no data were presented (43). Thus, the relative importance of the NT and CT regions of RAP-1 for stimulating protective immunity remains unsubstantiated. It has been proposed that repetitive epitopes in protozoan antigens, which are often serologically immunodominant and nonprotective, can cause suppression of a response to other potentially immunogenic regions of the protein (28, 31). By removing such repeat regions from an immunogen, additional epitopes present in the nonrepeat domain could become more antigenic. The present study was designed to test the hypothesis that the Th cell-stimulatory NT region of RAP-1, used as a vaccine with interleukin-12 (IL-12) and RIBI adjuvant to induce a type 1 cytokine and IgG response, would prime calves for antibody and Th cell responses comparable to or greater than those induced by full-length RAP-1 containing the CT repeats. Furthermore, we hypothesized that these type 1 immune responses to RAP-1 would be sufficient to provide protective immunity against B. bovis challenge infection.
MATERIALS AND METHODS
B. bovis strains and antigen preparation.
For use in all in vitro assays, B. bovis merozoite antigen was prepared from the cultured Mexico strain by homogenization of merozoites with a French pressure cell (SLM Instruments, Inc., Urbana, Ill.) and ultracentrifugation to yield a fraction enriched in membranes (CM) (3). Antigen from uninfected erythrocytes (URBC) was similarly prepared for use as a negative control. Recombinant proteins (Table 1), including full-length RAP-1 protein (aa 1 to 565), RAP-1 NT (aa 1 to 316), RAP-1 N2 (aa 1 to 134), and RAP-1 CT (aa 317 to 565), were expressed as histidine-tagged thioredoxin fusion proteins as described previously (26). Nickel affinity column-purified recombinant RAP-1 proteins and similarly purified negative control B. bovis 20-kDa heat shock protein (Hsp20) and Anaplasma marginale MSP-5 protein expressed in the same vector (9, 26) were dialyzed extensively. These contained trace amounts of endotoxin (0.06 or 0.12 endotoxin units/25 μg of protein) as determined by the Limulus amebocyte lysate assay (BioWhittaker, Inc., Walkersville, Md.). Synthetic 23- to 35-mer peptides spanning the RAP1 NT region from aa 109 to 316 and the entire CT region (Table 1) were synthesized by Gerhardt Munske, Laboratory for Biotechnology and Bioanalysis I, Washington State University, Pullman, Wash. A 30-mer peptide derived from A. marginale MSP-2 (designated MSP-2 P1) was used as a negative control peptide (7). Peptides were dissolved in phosphate-buffered saline (PBS). All proteins and peptides were stored at −20 or −80°C.
TABLE 1.
Recombinant B. bovis RAP-1 proteins and peptides used in this study
| Protein or peptide | Amino acid positions | Amino acid sequencea or reference |
|---|---|---|
| RAP-1 | 1-565 | 37 |
| RAP-1 N2 | 1-134 | 37 |
| RAP-1 NT | 114-226 | 37 |
| RAP-1 CT | 317-565 | 37 |
| NT peptides | ||
| P0 | 109-139 | DNVKYPLYQEYQPLSLPNPYQLDAAFRLFKE |
| 60-0 | 121-134 | PLSLPNPYQLDAAF |
| P1 | 134-163 | FRLFKESASNPAKNSVKREWLRFRNGANHG |
| P2 | 154-183 | LRFRNGANHGDYHYFVTGLLNNNVVHEEGT |
| P3 | 174-203 | NNNVVHEEGTTDVEYLVNKVLYMATMNYKT |
| P4 | 194-223 | LYMATMNYKTYLTVNSMNAKFFNRFSFTTK |
| P5 | 214-243 | FFNRFSFTTKIFSRRIRQTLSDIIRWNVPE |
| P6 | 234-263 | SDIIRWNVPEDFEERSIERITQLTSSYEDY |
| P7 | 254-283 | TQLTSSYEDYMLTQIPTLSKFARRYADMVK |
| P8 | 274-303 | FARRYADMVKKVLLGSLTSYVEAPWYKRWI |
| P9 | 294-316 | VEAPWYKRWIKKFRDFFSKNVTQ |
| CT peptides | ||
| CT1 | 307-341 | RDFFSKNVTQPTKKFIEDTNEVTKNYLKANVAEPT |
| CT2 | 332-361 | YLKANVAEPTKKFMQDTHEKTKGYLKENVA |
| CT3 | 352-381 | TKGYLKENVAEPTKTFFKEAPQVTKHFFDE |
| CT4b | 372-394 | PQVTKHFFDENIGQPTKEFFREA |
| CT5c,d | 386-408 | PTKEFFREAPQATKHFLDENIGQ |
| CT6d | 399-428 | KHFLDENIGQPTKEFFREAPQATKHFLGEN |
| CT7 | 419-448 | QATKHFLGENIAQPTKEFFKDVPQVTKKVI |
| CT8 | 439-468 | DVPQVTKKVITENIAQPTKEFRREVPHATM |
| CT9 | 459-488 | FRREVPHATMKVLNENIAQPAKEIIHEFGT |
| CT10 | 479-508 | AKEIIHEFGTGAKNFISAAHEGTKQFLNET |
| CT11 | 499-528 | EGTKQFLNETVGQPTKEFLNGALETTKDAL |
| CT12 | 519-548 | GALETTKDALHHLGKSSEEANLYDATENTT |
| CT13 | 539-565 | NLYDATENTTQANDSTTSNGEDTAGYL |
Calf immunization and B. bovis challenge.
Twelve Holstein steers, aged 5 to 6 months and weighing ∼200 to 250 kg at the start of the experiment, were used. The major histocompatibility complex class II DRB3 alleles of all the calves were defined by PCR-restriction fragment length polymorphism analysis of exon 2 (42). The nomenclature of the alleles is described on the bovine leukocyte antigen (BoLA) nomenclature website (http://www2.ri.bbscr.ac.uk.bola/). The BoLA class II haplotypes are shown in Tables 2 and 3.
TABLE 2.
Response of CD4+-T-cell lines from RAP-1 and RAP-1 NT vaccinees to NT region peptides
| Antigen or peptide | Proliferation (SI) by T-cell lines from calvesa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| RAP-1 vaccinees
|
RAP-1 NT vaccinees
|
|||||||
| 45 (27/23b) | 46 (16/14) | 51 (27/24) | 55 (8/24) | 47 (27/14) | 48 (16/22/26) | 52 (16/24) | 53 (23/11) | |
| RAP-1 | 22.5 | 52.3 | 275.8 | 69.3 | 77.3 | 9.3 | 169.3 | 69.2 |
| RAP-1 NT | 4.3 | 45.0 | 164.3 | 64.5 | 75.6 | 9.7 | 174.2 | 84.4 |
| N2 | 5.6 | 35.9 | 4.6 | 3.6 | 77.3 | 2.9 | 12.0 | 4.7 |
| P0 | 1.0 | 29.2 | 109.1 | 71.9 | 365.0 | 1.2 | 177.0 | 7.6 |
| 60-0 | 1.1 | 1.6 | 0.5 | 0.7 | 1.3 | 0.8 | 0.8 | 1.6 |
| P1 | 1.6 | 6.3 | 0.6 | 28.6 | 107.6 | 1.0 | 21.9 | 24.7 |
| P2 | 1.7 | 34.4 | 0.2 | 55.1 | 347.3 | 0.9 | 1.0 | 2.5 |
| P3 | 1.9 | 1.7 | 28.1 | 43.9 | 325.0 | 5.4 | 18.1 | 140.4 |
| P4 | 2.0 | 17.3 | 18.9 | 41.7 | 414.7 | 1.5 | 3.2 | 147.6 |
| P5 | 1.7 | 41.1 | 0.9 | 2.2 | 2.8 | 4.9 | 172.7 | 156.8 |
| P6 | 1.8 | 23.7 | 1.5 | 1.6 | 301.9 | 10.2 | 111.7 | 53.2 |
| P7 | 9.6 | 45.1 | 18.3 | 5.1 | 211.0 | 1.1 | 154.0 | 143.9 |
| P8 | 1.0 | 11.2 | 0.9 | 18.3 | 356.5 | 7.4 | 31.6 | 49.9 |
| P9 | 1.4 | 30.0 | 2.8 | 23.1 | 238.1 | 0.9 | 1.9 | 33.5 |
Cell lines were stimulated for 1 week with B. bovis CM and rested for 1 week before being tested. The results are reported for the optimal response to 1 or 10 μg of recombinant protein or peptide/ml. SIs were calculated by dividing the mean counts per minute of the response to RAP-1 or RAP-1 NT by the mean counts per minute of the response to MSP-5 or by dividing the mean counts per minute of the response to NT peptides by the mean counts per minute of the response to the control MSP2 peptide, P1. Significant responses (SI ≥ 3.0) are indicated in boldface type. These responses were also significant when individual counts per minute were compared by the one-tailed Student t test (P < 0.05).
DRB3 haplotypes for the indicated calves. Calf 48 was apparently a chimeric twin.
TABLE 3.
Response of CD4+-T-cell lines from RAP-1-immunized calves to CT region peptides
| Antigen or peptide | Proliferation (SI) by T-cell lines from calvesa
|
|||
|---|---|---|---|---|
| 45 (27/23b) | 46 (16/14) | 51 (27/24) | 55 (8/24) | |
| RAP-1 CT | 103.2 | 66.5 | 124.1 | 6.7 |
| CT-1 | 1.5 | 3.9 | 1.0 | 1.6 |
| CT-2 | 1.4 | 1.3 | 1.2 | 1.4 |
| CT-3 | 21.3 | 0.5 | 0.9 | 1.2 |
| CT-4 | 4.7 | 1.2 | 1.6 | 1.4 |
| CT-5 | 47.4 | 1.4 | 1.6 | 1.4 |
| CT-6 | 19.6 | 0.6 | 0.6 | 1.2 |
| CT-7 | 20.5 | 22.2 | 0.8 | 1.4 |
| CT-8 | 10.6 | 27.6 | 0.5 | 1.2 |
| CT-9 | 25.1 | 36.8 | 53.4 | 1.1 |
| CT-10 | 2.7 | 70.9 | 19.3 | 5.3 |
| CT-11 | 1.0 | 2.1 | 1.1 | 1.4 |
| CT-12 | 1.4 | 1.9 | 1.0 | 2.3 |
| CT-13 | 30.9 | 1.7 | 1.0 | 1.5 |
Cell lines were stimulated for 1 week with B. bovis CM and rested for 1 week before being tested. The results are reported for the response to 10 μg of recombinant protein or peptide/ml. SIs were calculated by dividing the mean counts per minute of the response to RAP-1 NT or RAP-1 CT by the mean counts per minute of the response to MSP-5 or by dividing the mean counts per minute of the response to CT peptides by the mean counts per minute of the response to the control MSP2 peptide, P1. Significant responses (SI ≥ 3.0) are indicated in boldface type. These responses were also significant when individual counts per minute were compared by the one-tailed Student t test (P < 0.05).
DRB3 haplotypes for the indicated calves are in parentheses.
Lymphocyte proliferation assays (described below) were performed on peripheral blood mononuclear cells (PBMC) from all animals by using B. bovis merozoite antigens and recombinant RAP-1 proteins, and none of the calves responded prior to immunization (data not shown). The calves were divided into three groups with four calves per group. The calves received four subcutaneous inoculations, at 3-week intervals, of 20 μg of RAP-1 (group I) or RAP-1 NT (group II) protein or PBS (group III) emulsified in 1 ml of RIBI adjuvant consisting of monophosphoryl lipid A, trehalose dimycolate, and cell wall skeleton. An additional 10 μg (0.5 ml) of human IL-12 (kindly provided by Genetics Institute, Cambridge, Mass.) was inoculated immediately into the same injection site for the first inoculation only. The calves were closely monitored after each immunization, and no localized inflammatory responses at the injection site or generalized signs of discomfort were observed throughout the immunization series. Serum samples and PBMC were collected before immunization and 2 to 3 weeks after each inoculation for analysis of antigen-specific antibody production and lymphocyte proliferation. The calves were challenged 5 months later by intravenous inoculation of B. bovis-infected bovine blood diluted in 3 ml of RPMI 1640 medium (GIBCO BRL, Gaithersburg, Md.) to yield ∼5 × 103 viable B. bovis merozoites of the virulent Texas T2Bo strain per inoculation (17). The parasites were obtained from a splenectomized calf that had been infected with cryopreserved stabilate (S1-T2Bo) and monitored for the development of parasitemia. The challenged calves were monitored daily for inappetence, lethargy, body temperature, packed erythrocyte cell volume (PCV), and parasitemia on Giemsa-stained blood smears. The calves were treated with 3.0 mg of berenil (diaminazine aceturate; Sigma, St. Louis, Mo.)/kg of body weight on day 11 (four calves) or 12 (eight calves) postchallenge if they displayed signs of inappetence and recumbency or when the PCVs fell to 50% of starting levels. Calf no. 48 died on day 11, whereas the others survived following treatment. Analysis of variance and the Fisher's least-significant-difference method were used to perform pairwise comparisons among the different groups for parasitemia and percentage reduction in PCV (a P value of <0.05 was considered significant).
Lymphocyte proliferation assays.
Two weeks after the second and third antigen inoculations, PBMC were tested for antigen-specific proliferation. Briefly, 2 × 105 PBMC cultured for 6 days in triplicate 100-μl volumes in 96-well round-bottom plates (Costar, Cambridge, Mass.) with 25 μg of antigen/ml were radiolabeled for the last 18 h of culture with 0.25 μCi of [3H]thymidine (Dupont-New England Nuclear, Boston, Mass.), and radiolabeled nucleic acids were harvested onto glass filters and counted in a beta counter. A stimulation index (SI) of ≥3.0 was considered statistically significant (1). Because several of the calves repeatedly had high background responses of PBMC cultured in medium alone, short-term T-cell lines were established thereafter and tested for specific responses. Briefly, 4 × 106 PBMC depleted of CD8+ T cells and γδ T cells (7) were cultured in 24-well plates (Costar) in 1.5 ml of complete RPMI 1640 medium (3) with 10 μg of B. bovis CM antigen/ml for 1 week (26). CD4+-T-cell-enriched lymphocytes (7 × 105) were either restimulated with B. bovis CM antigen and 2 × 106 irradiated (3,000 rad) autologous PBMC per well as antigen-presenting cells (APC) or cultured with APC in the absence of antigen for an additional week to avoid high background proliferation. The 2-week T-cell lines were tested in proliferation and IFN-γ assays. Briefly, 3 × 104 T cells and 2 × 105 autologous APC were cultured with 0.1 to 10 μg of antigen or peptide/ml in triplicate wells containing a total volume of 100 μl of complete RPMI 1640 medium for 3 or 4 days and were radiolabeled during the last 18 h of culture (26). Data are reported as mean counts per minute plus 1 standard deviation or as SI, calculated by dividing the mean counts per minute of the response to the test antigen or peptide by the mean counts per minute of the response to the appropriate negative control antigen or peptide.
Detection of IFN-γ in supernatants of T-cell lines.
To measure IFN-γ production, short-term T-cell lines were cultured with specific antigen in 96-well plates under the same conditions as for proliferation assays, and 50-μl supernatants were harvested from each well before the wells were pulsed with [3H]thymidine. The bovine IFN-γ assay was performed using an enzyme-linked immunosorbent assay kit (BOVIGAM; CSL Ltd., Parkville, Victoria, Australia) according to the manufacturer's protocol. The IFN-γ levels in culture supernatants from cells stimulated with 0.1 or 1 μg of antigen/ml and diluted 1:4 to 1:500 were determined by comparison with a standard curve obtained with a supernatant from a Mycobacterium bovis purified protein derivative-specific Th lymphocyte clone that contained 440 U of IFN-γ/ml (previously determined by the neutralization of vesicular stomatitis virus). In our assay, 1 U corresponds to 1.7 ng of IFN-γ (2), and the results are presented as nanograms of IFN-γ per milliliter.
The Fisher least-significant-difference method was used to perform comparisons between RAP-1- and RAP-1-NT-immunized groups for T-lymphocyte proliferation and IFN-γ production (a P value of <0.05 was considered statistically significant).
Determination of RAP-1-specific IgG1 and IgG2 titers by immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting were performed with MAbs specific for bovine IgG1 and IgG2 to determine the subclass of the specific IgG response essentially as described previously (10) with the following modifications. B. bovis CM (100 μg of protein per lane), recombinant RAP-1 NT, or RAP-1 CT (3 μg per lane) protein was applied to a 4 to 12% polyacrylamide gradient gel, electrophoresed, and transferred at 4°C to nitrocellulose membranes. The membranes were air dried and immersed for 1 h in blocking buffer consisting of PBS (pH 7.4) with 0.5% Tween 20 and 0.2% I-Block (TROPIX, Bedford, Mass.) with gentle agitation. The membranes were placed in a Mini-Protein II Multi Screen apparatus (Bio-Rad), and 600-μl volumes of bovine sera serially diluted (1:100 to 1:62,500) in the blocking solution were added per slot according to the manufacturer's protocol. The bovine sera were absorbed with 50 μg of recombinant MSP-5 antigen/ml in the blocking buffer prior to use. The membranes were incubated for 1 h at room temperature, washed twice with the blocking buffer, and incubated for an additional 1 h at room temperature with murine anti-bovine IgG1 or IgG2 MAb (Serotec Ltd., Oxford, United Kingdom) diluted 1:100 in the blocking buffer. The membranes were washed three times with the blocking buffer and were then labeled for 1 h at room temperature with peroxidase-conjugated affinity-purified goat anti-mouse IgG (H and L chains) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:4,000 in the blocking buffer. The membranes were washed repeatedly with the blocking buffer, and the chemiluminescence was developed with ECL Western Blotting Detection Reagents (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, United Kingdom) according to the manufacturer's instructions. A nonparametric Kruskal-Wallis pairwise comparison test was used to determine statistical differences in antibody titers among the groups (P < 0.05).
Merozoite neutralization assays.
Sera were obtained from immunized or control calves 2 weeks after the last immunization for testing merozoite neutralization as described previously (24). Briefly, merozoites were obtained from in vitro cultures of the Mo7 biological clone, which was derived from a Mexican strain by limiting dilution, as previously described in detail (18). The number of live merozoites was determined using the 6-carboxyfluorescein diacetate staining method (32). Sera were heat-inactivated, diluted 1:5 in complete M199 medium (Sigma), and incubated with 106 live merozoites for 30 min at 4°C. Antibody-exposed merozoites were added to an equal volume of 1.5% bovine erythrocytes in complete medium, and the cultures were incubated in 96-well plates at 37°C in a 5% CO2 atmosphere. Microscopic examination of Giemsa-stained smears prepared from each well at 5 and 48 h were used to determine the number of infected erythrocytes from a total of 2,000 cells. A positive control bovine antiserum (no. 682) specific for B. bovis major surface antigen 1 was included (24). The results were analyzed by one-way analysis of variance and Fisher's pairwise comparisons.
RESULTS
RAP-1-specific proliferative and IFN-γ responses following immunization.
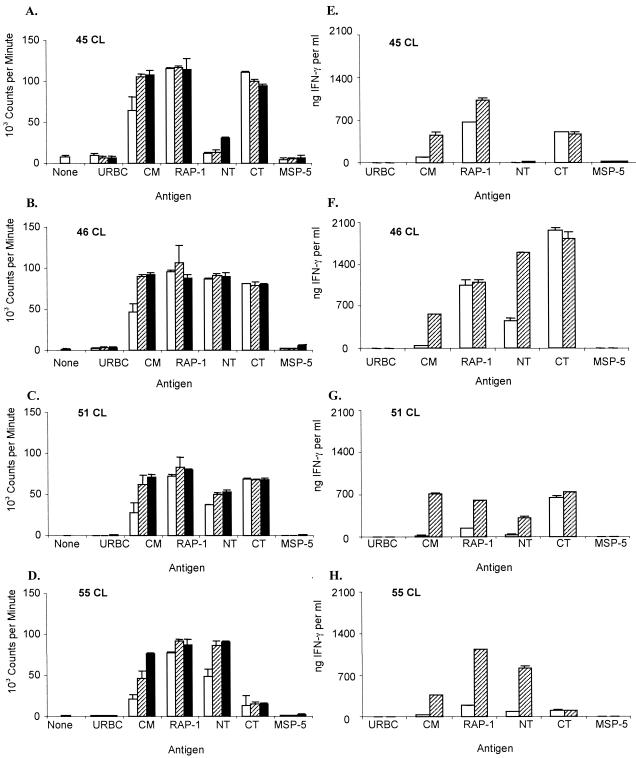
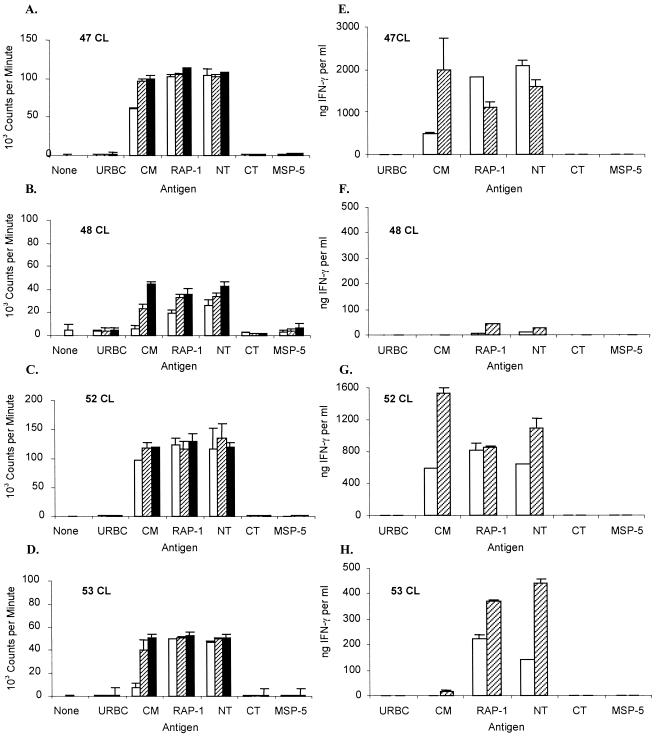
B. bovis-specific proliferation by PBMC was observed in both RAP-1- and RAP-1 NT-immunized cattle. After the third immunization, SIs ranged from 3.3 to 12.2 in the RAP-1-immunized group, from 6.9 to 94.9 in the RAP-1 NT-immunized group, and from 0.6 to 2.4 in the control group when stimulations with B. bovis CM and URBC antigens were compared (data not shown). The responses by immunized, but not control, calves were considered statistically significant (1). However, because several animals had high levels of background proliferation, short-term CD4+-T-cell lines were established and used in subsequent assays to compare responses to the NT and CT regions of RAP-1. When tested repeatedly after the last immunization to just before challenge 5 months later, cell lines from all immunized calves responded strongly to B. bovis CM and recombinant RAP-1 proteins but not to control URBC or recombinant A. marginale MSP-5 or B. bovis Hsp20 protein (Fig. 1 and 2 and data not shown). No significant differences between the responses of the two immunized groups to either recombinant RAP-1 or B. bovis CM were identified. Furthermore, in the RAP-1-immunized group, all calves responded to both NT and CT proteins (Fig. 1A to D). However, the response by calf 45 to RAP-1 NT was relatively weak (SIs, 4.3 and 13.4 with 10 μg of NT and CT/ml, respectively), and the response by calf 55 to RAP-1 CT was also relatively weak (SIs, 44.6 and 5.6 to 10 μg of NT and CT/ml, respectively). In the RAP-1 NT-immunized group, the response to RAP-1 NT was uniformly strong, and the response to RAP-1 CT was negative, as expected (Fig. 2A to D). These data are representative of experiments repeated three or more times, with the exception of the response of RAP-1-immunized calf 55 to RAP-1 CT, which was weak in three experiments, as shown in Fig. 1, but negative in two assays. There were no significant differences between the groups in the level of response to either RAP-1 or RAP-1 NT protein when SIs were compared using any antigen concentration (P ≥ 0.18). These data demonstrate that immunization with the whole RAP-1 protein stimulated significant proliferative responses to both the NT and CT regions and that removal of the CT region did not stimulate stronger responses to the RAP-1 NT region.
FIG. 1.
Proliferation and IFN-γ production by short-term CD4+-T-cell lines from RAP-1 vaccinees. Short-term T-cell lines were established after the fourth immunization by depleting PBMC of CD8+ cells and γδ T cells, culturing the remaining cells with B. bovis CM for 1 week, and culturing the CD4+ T cells with APC alone for 1 week. T-cell lines (CL) from calves 45 (A and E), 46 (B and F), 51 (C and G) and 55 (D and H) were stimulated for 72 h with 0.1 (open bars), 1.0 (hatched bars), or 10.0 (solid bars) μg of the indicated antigens/ml. The cells were radiolabeled for the last 18 h of culture, harvested, and counted in a beta counter, and the results are presented as the mean counts per minute plus 1 standard deviation (A to D). Supernatants collected prior to radiolabeling the cells were assayed for IFN-γ by enzyme-linked immunosorbent assay, and the results are presented as the mean number of nanograms of IFN-γ/ml of supernatant (E to H).
FIG. 2.
Proliferation and IFN-γ production by short-term CD4+-T-cell lines from RAP-1 NT vaccinees. The experiments were performed as described for Fig. 1. Results of proliferation and IFN-γ production are presented for T-cell lines from RAP-1 NT-vaccinated calves 47 (A and E), 48 (B and F), 52 (C and G), and 53 (D and H).
IFN-γ levels in the supernatants of T-cell lines from calves immunized with RAP-1 and stimulated with 1 μg of B. bovis CM or RAP-1 protein/ml were high, ranging from 372 to 706 and 595 to 1,135 ng/ml, respectively (Fig. 1E to H). T-cell lines from RAP-1 NT-immunized calves 47 and 52 also produced high levels of IFN-γ in response to both antigens (Fig. 2E and G). In contrast, T-cell lines from RAP-1 NT-immunized calves 48 and 53 (Fig. 2F and H) made relatively low levels of IFN-γ in response to B. bovis (undetectable and 20 ng of IFN-γ/ml, respectively) and RAP-1 (43 and 369 ng of IFN-γ/ml, respectively). However, there were no significant differences between groups (P ≥ 0.1) in the IFN-γ response to B. bovis CM, RAP-1, or RAP-1 NT protein. Three of the four RAP-1-immunized calves also produced high levels of IFN-γ when stimulated with RAP-1 CT (Fig. 1E to G).
Epitope mapping on RAP-1 NT using overlapping peptides.
Previous studies using three B. bovis-immune cattle determined that in these animals, the dominant response to RAP-1 mapped to the NT region, consisting of aa 154 to 316 (26), and the RAP-1 N2 protein (aa 1 to 134) was not recognized. In the present study, N2 protein and peptides overlapping the immunostimulatory NT region spanning aa 109 to 316 (Table 1) were tested with short-term T-cell lines from the immunized calves to determine whether the absence of the CT region in the immunogen resulted in increased recognition of epitopes in the RAP-1 NT region. In experiments repeated at least three times, consistent peptide-specific responses were observed for individual calves. However, the spectrum of peptides recognized differed among individuals (Table 2). The majority of calves in each group responded to RAP-1 N2 protein (aa 1 to 134), and RAP-1 vaccinees 45, 46, 51, and 55 had significant responses to a total of one, nine, five, and eight peptides, respectively, compared with medium alone or negative control peptide derived from A. marginale MSP-2 (Table 2). RAP-1 NT vaccinees 47, 48, 52, and 53 recognized a total of nine, four, eight, and nine peptides. There were no significant differences between the numbers of NT peptides recognized by the two groups. When the numbers of calves responding to individual peptides were compared, the only notable difference was in the responses to peptides P5 and P6, which were recognized by a single RAP-1 vaccinee (calf 46) but were seen by three of four RAP-1 NT vaccinees. This difference could be explained by expression of different class II haplotypes. Overall, these data indicate that removing the repetitive CT region from the immunogen did not significantly enhance priming for additional epitopes in the NT region. It was also determined that the N2 region does contain Th cell epitopes.
Epitope mapping on RAP-1 CT.
Studies using B. bovis-immune cattle indicated that the CT region was only weakly stimulatory for one of three calves examined (26). However, in the present study, immunization with recombinant RAP-1 elicited strong RAP-1 CT-specific responses in three of four individuals, whereas the fourth (calf 55) demonstrated either relatively weak (Fig. 1 and Table 3) or undetectable (data not shown) CD4+-T-lymphocyte-proliferative and IFN-γ responses to this protein. The CT region consists of seven degenerate 23-aa repeats arranged in tandem in B. bovis (37). One repeat epitope (FFREAPQATKHFL) present in peptides CT-5 and CT-6 was previously shown to stimulate T cells from the single B. bovis-immune responder animal (26). To determine if this and additional epitopes were recognized by RAP-1-immunized calves, peptides spanning the entire RAP-1 CT region were tested in proliferation assays with short-term T-cell lines (Table 3). Calves 45, 46, and 51 recognized a total of seven, five, and two CT region peptides, whereas calf 55 responded to a single RAP-1 CT peptide (CT-10). These studies show that, in contrast to what was previously observed with experimentally infected cattle, the RAP-1 CT region does contain highly immunogenic Th cell epitopes when RAP-1 is used as an immunogen.
RAP-1-specific IgG1 and IgG2 responses following immunization.
RAP-1-specific antibody titers were measured by immunoblotting after the fourth immunization to detect IgG1 and IgG2 antibodies. There were no significant differences in either IgG1 or IgG2 titers specific for RAP-1 or RAP-1 NT when RAP-1 vaccinees and RAP-1 NT vaccinees were compared (Table 4). One RAP-1 vaccinee and two RAP-1 NT-vaccinees did not make detectable IgG2 antibody to either native or recombinant proteins. These data indicate that the presence of the repeat-rich RAP-1 CT region in the immunogen did not diminish the RAP-1 NT-specific antibody response.
TABLE 4.
B. bovis RAP-1-specific IgG1 and IgG2 responses in calves following immunization
| Immunogen and calf no. | Antibody titera
|
|||||
|---|---|---|---|---|---|---|
|
B. bovis CM
|
RAP-1 NT
|
RAP-1 CT
|
||||
| IgG1 | IgG2 | IgG1 | IgG2 | IgG1 | IgG2 | |
| RAP-1 | ||||||
| 45 | 12,500 | Negb | 2,500 | Neg | 12,500 | Neg |
| 46 | 12,500 | 12,500 | 12,500 | 12,500 | 12,500 | 12,500 |
| 51 | 12,500 | 12,500 | 12,500 | 12,500 | 2,500 | 2,500 |
| 55 | 2,500 | 2,500 | 2,500 | 2,500 | 12,500 | 2,500 |
| RAP-1 NT | ||||||
| 47 | 12,500 | Neg | 2,500 | Neg | NDc | ND |
| 48 | 12,500 | 12,500 | 12,500 | 12,500 | ND | ND |
| 52 | 12,500 | 12,500 | 12,500 | 12,500 | ND | ND |
| 53 | 2,500 | Neg | 2,500 | Neg | ND | ND |
Antibody titer in sera collected after the fourth immunization is expressed as the reciprocal of the highest dilution that gave a positive signal on immunoblots against specific antigen.
Neg, titer of <100, the lowest serum dilution tested.
ND, not determined.
Clinical response to challenge with virulent B. bovis (Texas strain).
Five months after the final immunization, all calves were challenged with B. bovis freshly isolated from a splenectomized calf with ascending parasitemia. In the RAP-1 NT vaccinees and PBS control groups, the group mean temperatures peaked on day 9 at 106.3 and 106.4°F, respectively. The mean temperature for RAP-1 vaccinees was 106.1°F on day 9, and it peaked on day 10 at 106.3°F. The mean group temperatures on a given day were not significantly different. On day 6, three control calves, two RAP-1 vaccinees, and none of the RAP-1 NT vaccinees had a decrease in PCV compared with prechallenge levels (Table 5). The maximum decrease in PCV occurred on day 11 or 12, the days on which berenil was administered. The percentage decrease in PCV was not significant in RAP-1 vaccinees or controls on days 6 to 11 (Table 5). However, the percentage decrease in PCV was significantly less in RAP-1 NT vaccinees than in controls on days 6 and 9 (P < 0.05) and than RAP-1 vaccinees on days 9 to 11 (P ≤ 0.02). Nevertheless, the clinical signs in all calves were severe enough to warrant treatment with berenil, which was administered on day 11 or 12. Despite treatment, one calf (RAP-1 NT vaccinee no. 48) died on day 11 of clinical babesiosis, characterized by pulmonary edema and intravascular hemolysis, with cerebral sequestration of parasitized cells. Parasitemias, determined on days 11 and 12, were <0.5% and were not significantly different among groups.
TABLE 5.
Decrease in PCVs in B. bovis-challenged calves
| Immunogen and calf no. | PCV (% of prechallenge level) on day after challenge:
|
|||||
|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | |
| RAP-1 | ||||||
| 45 | 100.0 | 93.3 | 86.7 | 80.0 | 63.3 | 50.0a |
| 46 | 94.1 | 94.1 | 86.7 | 78.5 | 67.6 | 55.9 |
| 51 | 94.1 | 85.3 | 70.6 | 64.7 | 70.6 | 50.0 |
| 55 | 105.9 | 97.0 | 85.3 | 64.7 | 58.8 | 41.2a |
| RAP-1 NT | ||||||
| 47 | 103.1 | 87.5 | 84.3 | 92.0 | 71.9 | 59.4 |
| 48 | 110.7 | 107.1 | 96.4 | 96.0 | 85.7 | 75.0 |
| 52 | 100.0 | 97.0 | 87.9 | 78.8 | 81.8 | 69.7 |
| 53 | 100.0 | 88.2 | 79.4 | 76.5 | 67.6 | 55.9 |
| Control | ||||||
| 49 | 103.2 | 90.3 | 77.4 | 77.4 | 67.7 | 45.2a |
| 54 | 94.1 | 97.1 | 76.5 | 73.5 | 58.8 | 50.0 |
| 60 | 85.7 | 82.9 | 77.1 | 65.7 | 51.4 | 40.0a |
| 61 | 93.5 | 90.3 | 74.2 | 74.2 | 80.6 | 74.2 |
Calf was treated with berenil on day 11. All unmarked calves were treated on day 12.
Merozoite neutralization assay using immune bovine sera.
Studies using rabbit antisera specific for B. bovis RAP-1 demonstrated significant blocking of sporozoite attachment to erythrocytes (25), and a RAP-1-specific MAb was also reportedly able to inhibit both erythrocyte binding by RAP-1 and in vitro replication of B. bovis merozoites (45). It was therefore of interest to determine whether RAP-1- and RAP-1-NT-immune bovine sera would similarly neutralize merozoites in vitro, considering the failure to demonstrate any protective response from vaccination. A positive control bovine anti-MSA-1 immune serum from calf B682 (24) blocked merozoite growth by 70.6 and 47.0% in two experiments when the parasites were cultured for 48 h. This antiserum also inhibited merozoite growth by 79.6% in a third experiment when parasites were cultured for 5 h. In contrast, in the same experiments, no significant differences in merozoite growth inhibition were observed at either time point when sera from RAP-1 or RAP-1 NT vaccinees were compared with sera from adjuvant control calves (data not shown). Thus, in spite of relatively high IgG titers against native RAP-1 protein in bovine sera collected 2 weeks after the fourth immunization, these sera did not inhibit merozoite replication in vitro.
Anamnestic antibody responses following B. bovis challenge.
It was of interest to determine whether virulent challenge infection stimulated an anamnestic antibody response in RAP-1 and RAP-1 NT vaccinees compared with control calves. Sera were obtained 10 days prior to challenge and 11 days postchallenge from all cattle, and IgG1 and IgG2 titers against B. bovis CM protein were determined by immunoblotting, as described for postimmunization sera. Prechallenge IgG1 titers were negative in control calves, as expected, and generally low in the vaccinees, ranging from undetectable to 500 (Table 6). Prechallenge IgG2 titers were also undetectable in control calves and low in vaccinees, with the exception of calf 52. More RAP-1-immunized calves had detectable IgG1 and IgG2 antibody than RAP-1-NT-immunized calves. By day 11 postchallenge, when the calves were clinically ill and treatment was begun, RAP-1 vaccinees did mount significant B. bovis-specific anamnestic IgG1 responses that were at least 25-fold higher than prechallenge levels, and three of four calves made anamnestic IgG2 responses. IgG1 responses were at least fivefold higher in RAP-1 vaccinees than in RAP-1 NT vaccinees, and IgG2 responses were also generally higher, but the differences were not significant. The higher titers in RAP-1 vaccinees may reflect responses to the repeat-rich CT region. Only one control calf made detectable antibody (IgG1) by day 11 postchallenge. However, a month later, all control calves had IgG1 titers of 2,500 and three had IgG2 titers of 2,500 (data not shown). These results demonstrate that the development of anamnestic IgG responses to B. bovis in immunized calves following challenge was insufficient to prevent severe clinical disease.
TABLE 6.
B. bovis RAP-1-specific IgG1 and IgG2 responses in calves following challenge
| Immunogen and calf no. | Antibody titers against B. bovis CMa
|
|||
|---|---|---|---|---|
| Before challenge
|
After challenge
|
|||
| IgG1 | IgG2 | IgG1 | IgG2 | |
| RAP-1 | ||||
| 45 | 500 | Negb | 12,500 | Neg |
| 46 | Neg | 500 | 2,500 | 2,500 |
| 51 | 100 | 100 | 2,500 | 2,500 |
| 55 | 100 | 100 | 2,500 | 2,500 |
| RAP-1 NT | ||||
| 47 | 100 | Neg | 500 | Neg |
| 48 | Neg | Neg | 500 | 500 |
| 52 | Neg | 2,500 | 500 | 2,500 |
| 53 | 500 | Neg | 500 | Neg |
| PBS | ||||
| 49 | Neg | Neg | 500 | Neg |
| 54 | Neg | Neg | Neg | Neg |
| 60 | Neg | Neg | Neg | Neg |
| 61 | Neg | Neg | Neg | Neg |
Antibody titers determined 10 days prior to challenge and 11 days postchallenge are expressed as the reciprocal of the highest dilution that gave a positive signal on immunoblots against B. bovis CM antigen.
Neg, titer of < 100, the lowest serum dilution tested.
CD4+-T-lymphocyte responses postchallenge.
To determine whether CD4+-T-lymphocyte responses were suppressed by the challenge infection, short-term cell lines from vaccinees were tested 6 weeks after challenge for RAP-1-specific responses, once the animals had recovered from clinical disease. All RAP-1 vaccinees and the remaining three RAP-1 NT vaccinees responded to B. bovis CM and all recombinant RAP-1 proteins (Table 7). The responses cannot be directly compared to those obtained prechallenge, since the SIs depend on background levels of proliferation, which can differ considerably from experiment to experiment. However, these results suggest that challenge infection did not impair the RAP-1-specific response. The response to RAP-1 CT by RAP-1 NT vaccinees indicates that these calves were primed to the CT region by the challenge infection. Additional assays performed two or more times 5 to 6 months following challenge of control calves demonstrated that in three animals that responded to B. bovis, RAP-1 was recognized by CD4+ T cells, confirming earlier studies (6, 26). This response was attributable to RAP-1 NT in two of the three calves and to RAP-1 CT in all three animals (Table 7), results that were confirmed by peptide proliferation assays (Table 8). These results differ from those of previous studies, in which the NT region was predominantly recognized (26). Short-term T-cell lines from calf 60 did not survive in culture beyond 1 week, and specific responses to B. bovis and RAP-1 antigens were not detected in either PBMC or cells cultured for 1 week (Table 8 and data not shown).
TABLE 7.
RAP-1-specific proliferation by CD4+ T lymphocytes after B. bovis challenge
| Group and calf no.a | Proliferation (SI) againstb:
|
|||
|---|---|---|---|---|
| B. bovis CM | RAP-1 | RAP-1 NT | RAP-1 CT | |
| RAP-1 (II) | ||||
| 45 (27/23) | 39.3 | 59.9 | 15.1 | 58.3 |
| 46 (16/14) | 74.0 | 82.4 | 33.8 | 75.8 |
| 51 (27/24) | 16.3 | 26.2 | 25.7 | 23.2 |
| 55 (8/24) | 6.5 | 16.0 | 14.9 | 4.2 |
| RAP-1 NT (III) | ||||
| 47 (27/14) | 135.9 | 10.8 | 4.5 | 13.0 |
| 52 (16/24) | 19.1 | 76.8 | 76.7 | 5.4 |
| 53 (23/11) | 205.0 | 124.7 | 107.8 | 95.8 |
| PBS(I) | ||||
| 49 (11/27) | 431.2 | 15.7 | 5.3 | 13.9 |
| 54 (27/7) | 114.7 | 159.8 | 0.8 | 209.0 |
| 60 (16/22) | 0.8 | 2.4 | 0.8 | 2.0 |
| 61 (11/28) | 86.2 | 133.5 | 29.4 | 7.2 |
DRB3 haplotypes for the indicated calves are in parentheses.
CD4+-T-cell lines were set up 6 weeks after challenge of immunized cattle and 5 months after challenge of control calves. T cells were cultured for 1 week with B. bovis CM (calf 60) or for 1 week with B. bovis CM and 1 week without antigen (other calves). Lymphocytes were tested against the indicated antigens, and SIs are reported for the optimal response to 1 or 10 μg of antigen /ml. SIs were calculated as the mean counts per minute to B. bovis CM divided by the mean counts per minute to URBC or as the mean counts per minute to recombinant RAP-1, RAP-1 NT, or RAP-1 CT divided by the mean counts per minute to MSP-5. Significant responses (SI ≥ 3.0) are indicated in boldface type. These responses were also significant when individual counts per minute were compared by the one-tailed Student t test (P < 0.05).
TABLE 8.
Responses of CD4+ T cell lines to NT and CT region peptides after challenge
| Peptide | Proliferation (SI) by T-cell lines from calvesa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RAP-1 vaccinees
|
RAP-1 NT vaccinees
|
Controls
|
||||||||
| 45 | 46 | 51 | 55 | 47 | 52 | 53 | 49 | 54 | 61 | |
| NT peptides | ||||||||||
| P0 | 1.4 | 53.3 | 35.7 | 19.4 | 5.7 | 33.3 | 95.6 | 4.1 | 0.8 | 1.1 |
| 60-0 | 1.4 | 1.3 | 2.2 | 2.8 | 1.7 | 1.8 | 1.3 | 0.7 | 2.2 | 1.0 |
| P1 | 1.4 | 2.5c | 4.6b | 10.9 | 1.8c | 1.1c | 2.7c | 1.0 | 2.1 | 1.0 |
| P2 | 2.1 | 27.6 | 2.3 | 15.0 | 2.1c | 6.0b | 3.5 | 1.0 | 6.4 | 1.5 |
| P3 | 4.4b | 0.4 | 7.6 | 11.7 | 1.2c | 7.9 | 79.8 | 29.5 | 1.2 | 11.0 |
| P4 | 3.5b | 2.1c | 7.9 | 1.9c | 1.0c | 2.0c | 86.0 | 5.1 | 2.6 | 0.6 |
| P5 | 1.6 | 18.3 | 2.9 | 1.1 | 1.2 | 25.0 | 75.3 | 1.1 | 2.4 | 0.4 |
| P6 | 1.8 | 1.4c | 2.0 | 1.7 | 1.0c | 2.2c | 2.2c | 2.4 | 8.2 | 0.4 |
| P7 | 64.2 | 3.6 | 8.0 | 4.0 | 1.5c | 14.2 | 119.9 | 3.2 | 1.9 | 1.6 |
| P8 | 2.1 | 0.9c | 1.0 | 1.8c | 1.1c | 1.3c | 0.9c | 2.3 | 1.3 | 0.5 |
| P9 | 2.7 | 29.9 | 2.6 | 6.0 | 1.6c | 2.6 | 4.3 | 0.8 | 2.1 | 1.0 |
| CT peptides | ||||||||||
| CT1 | 1.7 | 3.4 | 1.5 | 2.6 | 1.11 | 2.7 | 1.6 | 1.9 | 0.8 | 1.6 |
| CT2 | 1.9 | 1.0 | 1.6 | 1.4 | 1.4 | 0.6 | 1.0 | 3.0 | 1.1 | 0.8 |
| CT3 | 74.5 | 1.5 | 1.9 | 3.4b | 1.5 | 3.2b | 21.7b | 2.2 | 1.0 | 0.9 |
| CT4 | 21.3 | 1.3 | 2.6 | 2.0 | 1.9 | 10.0b | 1.2 | 1.9 | 46.4 | 2.0 |
| CT5 | 76.7 | 1.4 | 1.6 | 2.9 | 1.7 | 4.3b | 118.3b | 13.0 | 0.8 | 14.2 |
| CT6 | 72.6 | 1.2 | 1.8 | 2.7 | 0.8 | 0.9 | 110.8b | 4.3 | 43.8 | 8.5 |
| CT7 | 79.4 | 31.4 | 1.3 | 3.3b | 1.1 | 1.8 | 117.9b | 4.4 | 22.3 | 2.1 |
| CT8 | 2.1 | 28.1 | 2.3 | 3.3b | 1.2 | 2.8 | 14.7b | 1.9 | 12.9 | 0.9 |
| CT9 | 77.6 | 34.1 | 33.3 | 4.2b | 3.2b | 2.1 | 25.9b | 38.1 | 84.9 | 1.2 |
| CT10 | 28.8b | 131.6 | 28.9 | 6.8 | 26.2b | 2.0 | 1.0 | 1.4 | 2.0 | 1.0 |
| CT11 | 2.0 | 1.8 | 1.8 | 3.6b | 1.4 | 1.4 | 0.9 | 1.9 | 5.8 | 1.3 |
| CT12 | 1.8 | 1.1 | 3.1 | 2.6 | 1.2 | 1.1 | 1.6 | 1.6 | 0.9 | 1.4 |
| CT13 | 80.1 | 1.0 | 1.8 | 3.0 | 0.9 | 2.9 | 0.9 | 1.9 | 0.7 | 1.8 |
CD4+-T-cell lines were set up 6 weeks after challenge of immunized cattle and 5 months after challenge of control cattle. T cells were cultured for 1 week with B. bovis CM and 1 week without antigen. Lymphocytes were tested against the indicated peptides, and SIs are reported for the optimal response to 1 or 10 μg of peptide /ml. SIs were calculated as the mean counts per minute to peptide divided by the mean counts per minute to control the MSP2 peptide, P1. Significant responses (SI ≥ 3.0) are indicated in boldface type. These responses were also significant when individual counts per minute were compared by the one-tailed Student t test (P < 0.05).
Responses were negative before challenge.
Responses were positive before challenge.
Although B. bovis challenge did not appear to result in any obvious suppression of the RAP-1-specific response in the vaccinees, it was of interest to determine whether there were differences in the epitopes recognized after immunization and after challenge. In RAP-1 vaccinees, the development of new peptide-specific responses, as well as the loss of epitope-specific responses, occurred after challenge (Table 8). When the NT peptide-specific responses of all vaccinees were compared with those before challenge, four new peptide-specific responses (P1, P2, P3, and P4) by an individual that were not detected before challenge were detected, whereas NT peptides P1, P2, P3, P4, P6, P7, P8, and P9 did not recall responses in one or more vaccinees. In contrast, CT peptide-specific responses were recalled in all RAP-1 vaccinees, and individuals in this group also responded to six new CT peptides (Table 8). The majority of changes in the peptide repertoire that vaccinees responded to after versus before challenge occurred in the three RAP-1 NT calves. Importantly, with one exception (peptide CT11), those peptides that were first recognized by an individual after challenge were also identified by other individuals prior to challenge, and all peptides identified by control calves after challenge were also recognized by one or more vaccinees. These data indicate that the repertoires of epitope-specific responses induced by immunization and infection are similar.
DISCUSSION
We report four main findings. First, immunization of calves with full-length recombinant RAP-1 protein using RIBI adjuvant and IL-12 stimulated strong and significant IgG and CD4+-T-lymphocyte-proliferative and IFN-γ responses against both recombinant and native proteins. Second, immunization with RAP-1 or infection with B. bovis resulted in priming of CD4+ T cells specific for multiple epitopes in both the NT and CT regions of the RAP-1 protein, showing that in these individuals, no consistent bias in the response toward one region was apparent. In addition, the majority of peptide-specific responses induced by infection were also induced by immunization with RAP-1. However, in an earlier study of three B. bovis-infected cattle, the CT region epitope-specific responses were not as prevalent, possibly the result of expression of different class II haplotypes (26). The DRB3 haplotypes expressed by these cattle were 15/34, 18/24, and 18/23, which were not shared among the nonimmunized calves challenged in the present study (Table 7). Third, immune responses induced by immunization with full-length RAP-1 or RAP-1 NT were similar, demonstrating that our first hypothesis, that the NT region of RAP-1, used as a vaccine with IL-12 and RIBI adjuvant to induce a type 1 response, would prime calves for antibody and Th cell responses comparable to or greater than those induced by full-length RAP-1, was correct. However, removal of the repeat-rich CT region did not result in stronger responses to the NT region or in the recognition of additional Th cell epitopes in this region. Fourth, in spite of the immunization protocol, which resulted in priming for strong cell-mediated and humoral responses against RAP-1 or RAP-1 NT that were recalled upon challenge, there was no evidence of protective immunity. Thus, we reject our second hypothesis, that induction of a RAP-1-specific IgG response coupled with a Th cell response characterized by IFN-γ production would be sufficient to provide protective immunity to B. bovis challenge.
The failure of recombinant RAP-1 to provide any protection in this study raises a number of questions, as previous reports had indicated that some level of protective immunity was evoked by this antigen. In 1992, Wright et al. reported that a truncated RAP-1- glutathione S-transferase fusion protein reduced parasitemias in vaccinated animals following challenge, although details of the immunization and challenge were not provided (43). It was not specified if the recombinant protein was truncated at the N or C terminus, but the CT repeat region was reportedly not protective. Prior to this, immunization with a 70-kDa gel-excised protein of B. bovis merozoites containing protease activity, which was subsequently shown to react with the RAP-1-specific MAb T21B4, resulted in significantly reduced parasitemias and reduced mortality following homologous-strain challenge (11, 43). However, vaccination did not alter other clinical signs indicative of B. bovis disease, including fever and anemia.
Our study differed from the earlier one by Commins et al. (11) in several ways: (i) we used 6-month-old calves with intact spleens in this experiment in lieu of splenectomized 2- to 3-month-old calves as in the earlier study, (ii) we used recombinant antigens rather than native proteins, (iii) we used different adjuvants, (iv) we used different intervals between vaccination and challenge, and (v) we used different parasite strains for challenge. First, younger calves are more resistant to B. bovis (40), and splenectomized calves develop much higher parasitemias than calves with intact spleens, which may have allowed detectable differences in parasitemias in the study by Commins et al. (11). Furthermore, all calves in our study were treated by day 12, so that differences in mortality were not determined. Second, the native RAP-1 antigen used by Commins et al. (11) possibly contained additional proteins, since the material with protease activity used for inoculation contained a 70-kDa band plus two additional bands when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Thus, responses to a combination of proteins, or proteins other than RAP-1, might have resulted in the reduced parasitemia. In addition, native and recombinant RAP-1 proteins may have different structures. There is no evidence for glycosylation of RAP-1, but recombinant and native proteins could fold differently, resulting in stimulation of antibody responses with different conformation specificities. Third, Commins et al. (11) used one immunization with Freund's complete adjuvant and three with incomplete adjuvant, whereas we used RIBI adjuvant plus IL-12 for the first immunization and RIBI adjuvant alone for the remaining three immunizations. Both Freund's complete adjuvant and IL-12 induce Th1 responses (15, 39). Furthermore, RIBI adjuvant contains monophosphoryl lipid A, which is postulated to activate toll-like receptor 4, leading to the production of high-affinity IgG antibody and inflammatory cytokines, including IL-1β, IL-12, and IFN-γ (27). In our study, administration of RIBI adjuvant plus IL-12 did stimulate the high IgG titers and IFN-γ responses that are believed to be appropriate for mediating protection against B. bovis. Although IL-4 and IL-10 production was not measured, previous immunization of calves with a soluble A. marginale protein antigen and IL-12 in alum resulted in a dominant IFN-γ response with little IL-4 and IL-10 production (41, 46). Fourth, the intervals between vaccination and challenge were different, as Commins et al., (11) challenged the calves 4 months after immunization whereas we challenged at 5 months, and it is possible that the antibody titers at the time of challenge were different. Finally, the Texas strain used for our challenge experiment is highly virulent, resulting in severe disease and high mortality in mature cattle (17). However, the Australian S strain is also virulent, resulting in 100% mortality by days 12 to 15 in splenectomized calves (11).
We prioritized RAP-1 for testing in cattle because animals immune to challenge following infection and treatment typically display strong CD4+-T-cell responses to this protein (6, 8, 26). Furthermore, RAP-1 NT- and CT-specific T cells produce IFN-γ (6, 26). The three control calves in the present study that were challenged with B. bovis and mounted recall responses to B. bovis responded to RAP-1. Thus, during B. bovis infection, RAP-1 appears to prime CD4+ T cells that produce IFN-γ, a response required for protective immunity against most intracellular pathogens. However, immunization with this protein using adjuvants known to stimulate both antibody and Th1 responses was not sufficient to protect calves against severe clinical babesiosis.
The use of recombinant RAP-1 protein raises the possibility that T-cell epitope-specific responses recalled after challenge infection differ from those induced by immunization. In fact, for a given individual, not all NT peptide-specific T-cell responses were recalled after challenge, and several new peptide-specific responses were evident postchallenge that were not detected prior to challenge. A potential explanation for the lack of recall responses to certain peptides could be that in the short-term cell lines these T cells were too few in number to detect by proliferation. The appearance of new epitope-specific responses could be explained by priming against these epitopes during challenge or the T-cell responses against these epitopes could have been below the limits of detection prior to being recalled by challenge. However, in all but one case (the response of calf 55 to peptide CT11), a peptide recognized after but not before challenge by an individual was recognized by other vaccinees before challenge. Although the T-cell epitopes were not precisely mapped, these results suggest that the majority of epitopes recognized after immunization were the same as those recognized by the recall or primary response following infection. Thus, there is no evidence that immunization with RAP-1 or RAP-1 NT stimulated a repertoire of Th cell epitope specificities different from that induced by B. bovis infection.
Sera from RAP-1- and RAP-1 NT-immunized cattle had no inhibitory effect on the growth of B. bovis in vitro, in contrast to recent reports of antibody-mediated inhibition of sporozoite binding and merozoite growth (24, 45). The experiments demonstrating inhibition of sporozoite attachment by RAP-1-specific rabbit antiserum (24), and inhibition of merozoite growth by B. bovis MSA-1-specific bovine sera (19, 25), were performed by our group. Because the same culture conditions were used in the previous and present studies and the anti-MSA-1 serum (25) had the same merozoite-inhibitory effect in both studies, the inability of bovine RAP-1-specific immune sera to inhibit merozoite growth was not due to a technical problem. The epitope specificity of the merozoite-inhibitory MAb 1C1 was not determined (45), so we cannot evaluate the recognition of this epitope by the bovine antisera. However, the lack of protection against challenge may in part reflect the inability of the bovine RAP-1-specific sera to block merozoite growth in vitro.
In summary, immunization with recombinant RAP-1 protein or a fragment of this protein lacking the CT repeats, using adjuvants that induced strong IgG and Th1 responses in cattle, was not sufficient to provide protection against challenge with the virulent Texas strain of B. bovis. One possibility is that even though IgG responses were recalled by challenge, structural differences in recombinant and native proteins may result in antibody responses against recombinant RAP-1 incapable of neutralizing parasite infectivity. Alternatively, the level of antibody present at the time of challenge 5 months after immunization was too low to provide protection. Similarly, the amount of IFN-γ induced upon challenge may not have been sufficient for protection. It is also likely that the use of a single antigen is not sufficient for stimulating protective immunity against B. bovis and that a combination of proteins will prove more effective.
Development of effective recombinant vaccines against apicomplexan pathogens, such as Babesia, Plasmodium, and Theileria, has been frustratingly difficult. Progress in vaccine development will require improved understanding of the mechanism of immunity in natural hosts of these parasites and identification of immunogens that induce the protective mechanisms. While often disappointing, accurate reporting of studies in which failure to induce protection despite induction of the desired immune responses is fundamental to enhancing knowledge of the mechanisms and immunogens required to induce protective immunity and allow effective vaccine development.
Acknowledgments
We thank Kim Kegerreis, Shelley Whidbee, Deb Alperin, and Emma Karel for excellent technical help; Steve Hines and Will Goff for providing B. bovis parasites; Harris Lewin and Colleen Olmstead for performing BoLA DRB3 typing; Jeffrey Abbott for statistical analyses; and Genetics Institute for providing human IL-12.
This research was supported by National Institutes of Health grant R01-AI30136.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bennett, S., and E. M. Riley. 1992. The statistical analysis of data from immunoepidemiological studies. J. Immunol. Methods 146:229-239. [DOI] [PubMed] [Google Scholar]
- 2.Beyer, J. C., R. W. Stich, W. C. Brown, and W. P. Cheevers. 1998. Cloning and expression of caprine interferon-gamma. Gene 210:103-108. [DOI] [PubMed] [Google Scholar]
- 3.Brown, W. C., K. S. Logan, G. G Wagner, and C. L. Tetzlaff. 1991. Cell-mediated immune responses to Babesia bovis antigens in cattle following infection with tick-derived or cultured parasites. Infect. Immun. 59:2418-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, W. C., T. F. McElwain, I. Hötzel, B. J. Ruef, A. C. Rice-Ficht, R. W. Stich, C. E. Suarez, D. M. Estes, and G. H. Palmer. 1998. Immunodominant T-cell antigens and epitopes of Babesia bovis and Babesia bigemina. Ann. Trop. Med. Parasitol. 92:473-482. [PubMed] [Google Scholar]
- 5.Brown, W. C., T. F. McElwain, G. H. Palmer, S. E. Chantler, and D. M. Estes. 1999. Bovine CD4+ T-lymphocyte clones specific for rhoptry-associated protein 1 of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect. Immun. 67:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, W. C., T. F. McElwain, B. J. Ruef, C. E. Suarez, V. Shkap, C. G. Chitko-McKown, A. C. Rice-Ficht, and G. H. Palmer. 1996. Babesia bovis rhoptry-associated protein-1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains. Infect. Immun. 64:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 (MSP2) of the ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., and G. H. Palmer. 1999. Designing blood stage-vaccines against Babesia bovis and B. bigemina. Parasitol. Today 15:275-281. [DOI] [PubMed] [Google Scholar]
- 9.Brown, W. C., B. J. Ruef, J. Norimine, K. A. Kegerreis, C. E. Suarez, P. G. Conley, R. W. Stich, K. H. Carson, and A. C. Rice-Ficht. 2001. A novel 20-kilodalton protein conserved in Babesia bovis and B. bigemina stimulates memory CD4+ T lymphocyte responses in B. bovis-immune cattle. Mol. Biochem. Parasitol. 118:97-109. [DOI] [PubMed] [Google Scholar]
- 10.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commins, M. A., B. V. Goodger, and I. G. Wright. 1985. Proteinases in the lysate of bovine erythrocytes infected with Babesia bovis: initial vaccination studies. Int. J. Parasitol. 15:491-495. [DOI] [PubMed] [Google Scholar]
- 12.Dalrymple, B. P. 1993. Molecular variation and diversity in candidate vaccines from Babesia. Acta Trop. 53:227-238. [DOI] [PubMed] [Google Scholar]
- 13.Dalrymple, B. P., R. E. Casu, J. M. Peters, C. M. Dimmock, K. R. Gale, R. Boese, and I. G. Wright. 1993. Characterisation of a family of multi-copy genes encoding rhoptry protein homologues in Babesia bovis, Babesia ovis and Babesia canis. Mol. Biochem. Parasitol. 57:181-192. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple, B. P., J. M. Peters, R. Bose, and I. G. Wright. 1996. A polymerase chain reaction method for the identification of genes encoding members of the Bv60/p58 family of rhoptry protein homologues in the genus Babesia. Exp. Parasitol. 84:96-100. [DOI] [PubMed] [Google Scholar]
- 15.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of Th1 and Th2 immunity in neonatal mice. Science 271:1728-1730. [DOI] [PubMed] [Google Scholar]
- 16.Goff, W. L., W. C. Davis, G. H. Palmer, T. F. McElwain, W. C. Johnson, J. F. Bailey, and T. C. McGuire. 1988. Identification of Babesia bovis merozoite surface antigens by using bovine sera and monoclonal antibodies. Infect. Immun. 56:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff, W. L., W. C. Johnson, S. M. Parish, G. M. Barrington, W. Tuo, and R. A. Valdez. 2001. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-γ, and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 23:463-471. [DOI] [PubMed] [Google Scholar]
- 18.Hines, S. A., T. F. McElwain, G. M. Buening, and G. H. Palmer. 1989. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol. Biochem. Parasitol. 37:1-9. [DOI] [PubMed] [Google Scholar]
- 19.Hines, S. A., G. H. Palmer, D. P. Jasmer, T. C. McGuire, and T. F. McElwain. 1992. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 55:85-94. [DOI] [PubMed] [Google Scholar]
- 20.Homer, M. J., I. Aguilar-Delfin; S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 22.McElwain, T. F., L. E. Perryman, W. C. Davis, and T. C. McGuire. 1987. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J. Immunol. 138:2298-2304. [PubMed] [Google Scholar]
- 23.McElwain, T. F., L. E. Perryman, A. J. Musoke, and T. C. McGuire. 1991. Molecular characterization and immunogenicity of neutralization-sensitive Babesia bigemina merozoite surface proteins. Mol. Biochem. Parasitol. 47:213-222. [DOI] [PubMed] [Google Scholar]
- 24.Mosqueda, J., T. F. McElwain, and G. H. Palmer. 2002. Babesia bovis merozoite surface antigen 2 proteins are expressed on the merozoite and sporozoite surface, and specific antibodies inhibit attachment and invasion of erythrocytes. Infect. Immun. 70:6448-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosqueda, J., T. F. McElwain, D. Stiller, and G. H. Palmer. 2002. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect. Immun. 70:1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norimine, J., C. E. Suarez, T. F. McElwain, M. Florin-Christensen, and W. C. Brown. 2002. Immunodominant epitopes in Babesia bovis rhoptry-associated protein 1 that elicit memory CD4+-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain. Infect. Immun. 70:2039-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldrige, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10:S32-S37. [DOI] [PubMed] [Google Scholar]
- 28.Ramasamy, R. 1998. Molecular basis for evasion of host immunity and pathogenesis in malaria. Biochim. Biophys. Acta 1406:10-27. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, S. D., G. H. Palmer, T. F. McElwain, T. C. McGuire, B. J. Ruef, C. G. Chitko-McKown, and W. C. Brown. 1996. CD4+ T helper lymphocyte responses against Babesia bigemina rhoptry-associated protein-1. Infect. Immun. 64:2079-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schetters, T. P. M., and S. Montenegro-James. 1995. Vaccines against babesiosis using soluble parasite antigens. Parasitol. Today 11:456-462. [DOI] [PubMed] [Google Scholar]
- 31.Schofield, L. 1991. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol. Today 7:99-105. [DOI] [PubMed] [Google Scholar]
- 32.Shoda, L. K. M., J. Florin-Christensen, M. Florin-Christensen, G. H. Palmer, and W. C. Brown. 2000. Babesia bovis-stimulated macrophages express interleukin-1β, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect. Immun. 68:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skuce, P. J., T. R. Mallon, and S. B. Taylor. 1996. Molecular cloning of a putative rhoptry protein homologue from Babesia divergens. Mol. Biochem. Parasitol. 77:99-102. [DOI] [PubMed] [Google Scholar]
- 34.Suarez, C. E., T. F. McElwain, E. B. Stephens, V. S. Misha, and G. H. Palmer. 1991. Sequence conservation among merozoite apical complex proteins of Babesia bovis, Babesia bigemina and other apicomplexa. Mol. Biochem. Parasitol. 49:329-332. [DOI] [PubMed] [Google Scholar]
- 35.Suarez, C. E., G. H. Palmer, S. A. Hines, and T. F. McElwain. 1993. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect. Immun. 61:3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suarez, C. E., G. H. Palmer, I. Hötzel, and T. F. McElwain. 1998. Structure, sequence, and transcriptional analysis of the Babesia bovis rap-1 multigene locus. Mol. Biochem. Parasitol. 93:215-224. [DOI] [PubMed] [Google Scholar]
- 37.Suarez, C. E., G. H. Palmer, D. P. Jasmer, S. A. Hines, L. E. Perryman, and T. F. McElwain. 1991. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol. Biochem. Parasitol. 46:45-52. [DOI] [PubMed] [Google Scholar]
- 38.Suarez, C. E., S. M. Thompson, T. F. McElwain, S. A. Hines, and G. H. Palmer. 1994. Conservation of oligopeptide motifs in rhoptry protein from different genera of erythroparasitic protozoa. Exp. Parasitol. 78:246-251. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 12:251-276. [DOI] [PubMed] [Google Scholar]
- 40.Trueman, K. F., and G. W. Blight. 1978. The effect of age on resistance of cattle to Babesia bovis. Aust. Vet. J. 54:301-305. [DOI] [PubMed] [Google Scholar]
- 41.Tuo, W., G. H. Palmer, T. C. McGuire, D. Zhu, and W. C. Brown. 2000. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect. Immun. 68:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Eijk, M. J. T., J. A. Stewart-Haynes, and H. A. Lewin. 1992. Extensive polymorphism of the BoLA-DRB3 gene distinguished by PCR-RFLP. Anim. Genet. 23:483-496. [DOI] [PubMed] [Google Scholar]
- 43.Wright, I. G., R. Casu, M. A. Commins, B. P. Dalrymple, K. R. Gale, B. V. Goodger, P. W. Riddles, D. J. Waltisbuhl, I. Abetz, D. A. Berrie, Y. Bowles, C. Dimmock, T. Hayes, H. Kalnins, G. Leatch, R. McCrae, P. E. Montague, I. T. Nisbet, F. Parrodi, J. M. Peters, P. C. Scheiwe, W. Smith, K. Rode-Bramanis, and M. A. White. 1992. The development of a recombinant Babesia vaccine. Vet. Parasitol. 44:3-13. [DOI] [PubMed] [Google Scholar]
- 44.Wright, I. G., B. V. Goodger, and I. A. Clark. 1988. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol. Today 4:214-218. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama, N., B. Suthisak, H. Hirata, T. Matsuo, N. Inoue, C. Sugimoto, and I. Igarashi. 2002. Cellular localization of Babesia bovis merozoite rhoptry-associated protein-1 and its erythrocyte-binding activity. Infect. Immun. 70:5822-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Y., G. H. Palmer, J. R. Abbott, C. J. Howard, J. C. Hope, and W. C. Brown. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307-3318. [DOI] [PubMed] [Google Scholar]