Abstract
Murine osteoblasts express Toll-like receptor 5 (TLR5), and this expression is upregulated following exposure to bacteria or to the TLR5 agonist, flagellin. Importantly, flagellin activates transcriptional regulators and elicits proinflammatory cytokine production, suggesting TLR5 functionality. TLR5 may represent an important mechanism underlying the recognition of bacterial pathogens by osteoblasts during bone infections.
While the primary roles of osteoblasts are to synthesize the components of bone matrix and to regulate the activity of bone-resorbing osteoclasts, recent studies have revealed an additional function during bone diseases—namely, the initiation and maintenance of inflammatory immune responses. Previous studies by our laboratory have shown that bacterially exposed osteoblasts are a significant source of an array of soluble inflammatory mediators that include the cytokines interleukin 12 (IL-12) and IL-6 (3), growth factors such as granulocyte-macrophage colony-stimulating factor (1), and the chemokines inducible protein 10 and monocyte chemoattractant protein 1 (2, 6). Furthermore, the surprising ability of osteoblasts exposed to bacteria to activate T lymphocytes by presentation of antigen in the context of major histocompatibility complex class II molecules (18) and the ability to express the key costimulatory molecule CD40 (19) were recently described. Such a pattern of immune-molecule expression is one that can promote the recruitment of leukocytes to sites of bacterial infection and could serve to initiate and sustain inflammatory responses in bone tissue. To date, the mechanisms by which osteoblasts perceive and respond to bacteria are poorly understood.
The discovery of a family of pattern recognition receptors with a high degree of homology to the Toll family of proteins in Drosophila has shed light on the possible mechanisms by which host cells recognize a wide range of pathogens without the need for prior exposure (16, 22). To date, 10 homologues of these Toll-like receptors (TLR) have been identified in humans and mice, and bacterially derived ligands have been reported for TLR2, TLR4, TLR5, and TLR9 (5, 7, 9, 10, 13, 17, 21, 23). Importantly, several recent studies have provided evidence for the presence of a number of these TLR homologues on osteoblasts. Kikuchi and coworkers (12) reported the presence of message encoding TLR2 and TLR4 in murine osteoblasts. A study from our laboratory confirmed the functional expression of TLR4 on murine osteoblasts by demonstrating the ability of the specific TLR4 ligand, lipopolysaccharide (LPS), to initiate chemokine production by these cells (6). Such induction was abolished following the addition of blocking antibodies directed against TLR4 (6). Interestingly, while mRNA encoding TLR2 was found in murine osteoblasts, the TLR2-specific ligand, peptidoglycan, failed to elicit cellular activation in this study, which argued against a role for this receptor in bacterial recognition in this cell type (6). The presence of TLR9 on osteoblasts has recently been inferred by the ability of activating oligonucleotides to initiate production of proinflammatory molecules (24). Such a finding is supported by the detection of mRNA encoding TLR9 in this cell type by our laboratory (unpublished data). However, the involvement of TLR4 and TLR9 does not preclude a role for other TLR homologues in the recognition of bacteria by this cell type. Recently, antigens of various bacterial flagella have been shown to signal via TLR5 (8). To date, the functional expression of such receptors has not been investigated in osteoblasts.
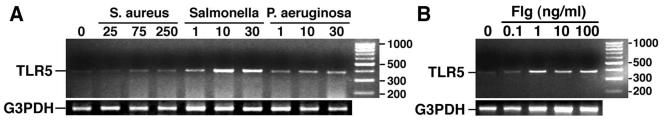
To begin to determine whether bacterial components may be recognized via TLR5 in these cells, we investigated the expression of mRNA encoding TLR5 in both resting cultures of murine osteoblasts and in cells after exposure to bacterial pathogens. Primary osteoblast cell cultures were prepared from BALB/c mouse neonate calvaria by sequential collagenase-protease digestion and cultured as previously described by our laboratory (1-3, 6, 18, 19). Cells were cultured in the absence of bacteria or in the presence of various numbers of Staphylococcus aureus strain UAMS-1 (ATCC 49230; American Type Culture Collection, Manassas, Va.), Salmonella enterica (serovar Dublin strain SL 1363), or Pseudomonas aeruginosa (ATCC 27853) bacteria for 45 min, followed by elimination of viable extracellular bacteria as previously described (1-3, 6, 18, 19). At 7 h postinfection, RNA was isolated and semiquantitative reverse transcription-PCR (RT-PCR) was performed for the presence of mRNA encoding TLR5 as previously described (1-4, 6, 14, 15, 18, 19). Positive- and negative-strand PCR primers used, respectively, were CCAGAACATCAGAGATCCTGA and CCAATGGCCTTAAGAGCATTG to amplify mRNA encoding murine TLR5 (365-bp fragment). PCR primers were designed, using Oligo 4.0 primer analysis software (National Biosciences, Plymouth, Mass.), from the published sequence of TLR5 (20), as previously described (1-4, 6, 14, 15, 18, 19). PCR amplification of the housekeeping gene, G3PDH, was performed on cDNA from each sample to ensure similar input of RNA and efficiencies of reverse transcription. The identity of the PCR-amplified fragments was verified by size comparison with DNA standards (Promega). As shown in Fig. 1A, resting osteoblasts constitutively express low levels of mRNA encoding TLR5. Exposure of osteoblasts to flagellated bacterial species, i.e., Salmonella and P. aeruginosa, elicited maximal 8.4- and 4.9-fold increases in TLR5 mRNA expression, respectively, as determined by densitometric analyses normalized to G3PDH expression. Interestingly, exposure of cells to nonflagellated Staphylococcus aureus elicited only a 2.5-fold maximal increase in TLR5 mRNA expression.
FIG. 1.
Expression of mRNA encoding Toll-like receptor 5 (TLR5) in resting osteoblasts and cells exposed to bacterial species and the specific TLR5 ligand, flagellin. (A) Cells were unexposed (0) or exposed to Staphylococcus aureus, Salmonella, or P. aeruginosa at the indicated number of bacteria to osteoblasts. (B) Cells were unexposed (0) or exposed to purified flagellin (0.1, 1, 10, and 100 ng/ml). At 7 h, RNA was isolated and RT-PCR was performed for the presence of TLR5 mRNA. The migration of DNA fragments of known sizes is indicated on the right. These studies were performed three times with similar results.
Furthermore, we have characterized the ability of the specific TLR5 ligand, flagellin, to elicit increases in the level of expression of mRNA encoding TLR5 in osteoblasts. Flagellin protein preparations were isolated from Salmonella enterica serovar Typhimurium serotype 12023 as previously described by our laboratory (4). This isolation procedure has been demonstrated to yield a single protein product with a molecular mass of approximately 48 kDa (4), a size that is within the previously described size range for flagellin from Salmonella serotypes (11). Endotoxin was removed from flagellin preparations using a Detoxi-Gel endotoxin-removing gel column (Pierce, Rockford, Ill.) according to the manufacturer's instructions. Residual endotoxin content was determined to be below detectable levels for the Pyrogent Limulus amoebocyte lysate assay (BioWhittaker, Inc., Walkersville, Md.) (<0.05 μg of LPS/μg of flagellin). Cells were unexposed or exposed to purified flagellin (0.1, 1, 10, and 100 ng/ml) for 7 h prior to RNA isolation and RT-PCR. As indicated by the representative experimental results shown in Fig. 1B, 1 ng of flagellin per ml induces a maximal 5.2-fold increase in TLR5 mRNA expression, as determined by densitometric analysis normalized to G3PDH expression, over that seen in resting osteoblasts. Importantly, concentrations of LPS of up to 100 ng/ml failed to elicit detectable elevations in mRNA encoding TLR5 (data not shown). Taken together, these data suggest that osteoblasts express mRNA encoding TLR5 and that this expression is elevated following bacterial challenge or exposure to the specific TLR5 ligand, flagellin.
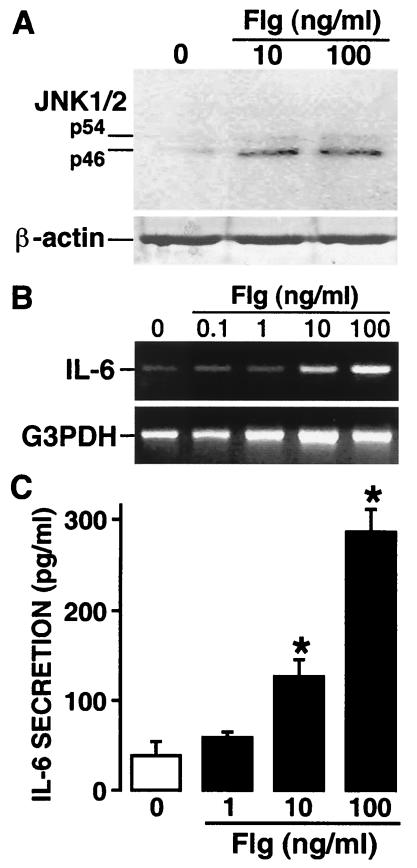
To begin to determine whether the expression of mRNA encoding TLR5 in osteoblasts translates into expression of functional receptor molecules on these cells, we investigated the ability of flagellin, a specific ligand for TLR5, to elicit cellular responses that result in the production of proinflammatory mediators by osteoblasts. Cells were unexposed or exposed to purified flagellin (10 and 100 ng/ml) for 90 min. Following treatment, protein samples were obtained from osteoblasts as previously described by our laboratory (4, 14), electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel, and transferred to Immobilon-P Transfer Membranes (Millipore, Bedford, Mass.). The level of activation of the important inflammatory transcription factor JNK1/2 was determined by Western blot analysis using an anti-Phospho-SAPK/JNK (THR183/Tyr185) antibody purchased from Cell Signaling Technology (Beverly, Mass.). As shown in Fig. 2A, flagellin treatment results in increases in the level of phosphorylated—and hence activated—JNK1/2 in cultured osteoblasts. Differences in expression could not be attributed to unequal protein loading due to the presence of similar levels of β-actin on reprobed membranes (Fig. 2A).
FIG. 2.
The TLR5 ligand flagellin elicits transcriptional factor activation and inflammatory cytokine production in murine osteoblasts. (A) Cells were unexposed or exposed to purified flagellin (10 and 100 ng/ml) for 90 min. Whole-cell lysates were analyzed by Western blotting for the presence of phosphorylated JNK1/2. (B) Cells were unexposed or exposed to purified flagellin (0.1, 1, 10, and 100 ng/ml) for 7 h prior to RNA isolation and RT-PCR for mRNA encoding IL-6. These studies were performed three times with similar results. (C) Cells were unexposed or exposed to purified flagellin (1, 10, and 100 ng/ml) for 24 h. Culture supernates were analyzed for the presence of IL-6 protein by specific capture ELISA. Results shown are the means ± standard errors of the means of replicate measurements in four separate preparations. *, significantly different from untreated cells (P < 0.05) when tested by Student's paired t test.
To determine whether this increase in transcription factor activation results in enhanced inflammatory mediator production, we investigated the ability of flagellin to induce expression of mRNA encoding the proinflammatory cytokine IL-6. Osteoblasts were unexposed or exposed to purified flagellin (0.1, 1, 10, and 100 ng/ml) for 7 h prior to RNA isolation and RT-PCR for mRNA encoding IL-6 as previously described (3). As indicated by the representative experimental results shown in Fig. 2B, 100 ng of flagellin per ml induces a maximal 3.5-fold increase in IL-6 mRNA expression, as determined by densitometric analysis normalized to G3PDH expression, over that seen in resting osteoblasts. To address whether the elevations in mRNA encoding IL-6 following flagellin treatment result in secretion of this cytokine, specific capture enzyme-linked immunosorbent assays were performed as described previously (3), using a capture antibody against IL-6 (clone MP5-20F3; BD PharMingen, San Diego, Calif.) and a biotinylated anti-mouse IL-6 detection antibody (clone MP5-32C11; BD PharMingen). Culture supernatants of untreated osteoblasts or cells exposed to purified flagellin (0.1, 1, 10, and 100 ng/ml) were taken after 24 h and assayed for the presence of IL-6. Figure 2C shows that flagellin elicits dose-dependent increases in IL-6 secretion that mirror the mRNA increases shown in Fig. 2B. Taken together, these data demonstrate the ability of a specific TLR5 ligand to activate transcription factors that lead to upregulation of mRNA levels and protein secretion of an important proinflammatory cytokine.
The ability of a specific TLR5 ligand to elicit transcription factor activation and subsequent IL-6 secretion in osteoblasts suggests the presence of functional TLR5 molecules on murine osteoblasts. In addition, the ability of flagellin to upregulate the level of expression of mRNA encoding this receptor further supports this notion. The recent commercial availability of polyclonal antibodies directed against TLR5 has enabled us to perform experiments to confirm the presence of this receptor in murine osteoblasts. Cells were either untreated or treated with flagellin (1, 10, and 100 ng/ml). After 12 h, protein samples were obtained from osteoblasts as previously described by our laboratory (4, 14) and were probed for the presence of TLR5 protein using an anti-TLR5 polyclonal antibody purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). As shown in Fig. 3, Western blot analysis revealed a single prominent band with a molecular mass of 92 kDa, which is in agreement with previous work that used this antibody to study TLR5 expression in other cell types (7). Resting osteoblasts expressed low levels of TLR5 constitutively (Fig. 3), consistent with the low-level expression of mRNA encoding this protein (Fig. 1). Importantly, flagellin, a specific ligand for TLR5, elicited an almost twofold maximal increase in the expression of TLR5 as determined by densitometric analysis of protein bands (Fig. 3). These increases conform to the pattern of induction in TLR5 mRNA expression seen following flagellin treatment (Fig. 1B).
FIG. 3.
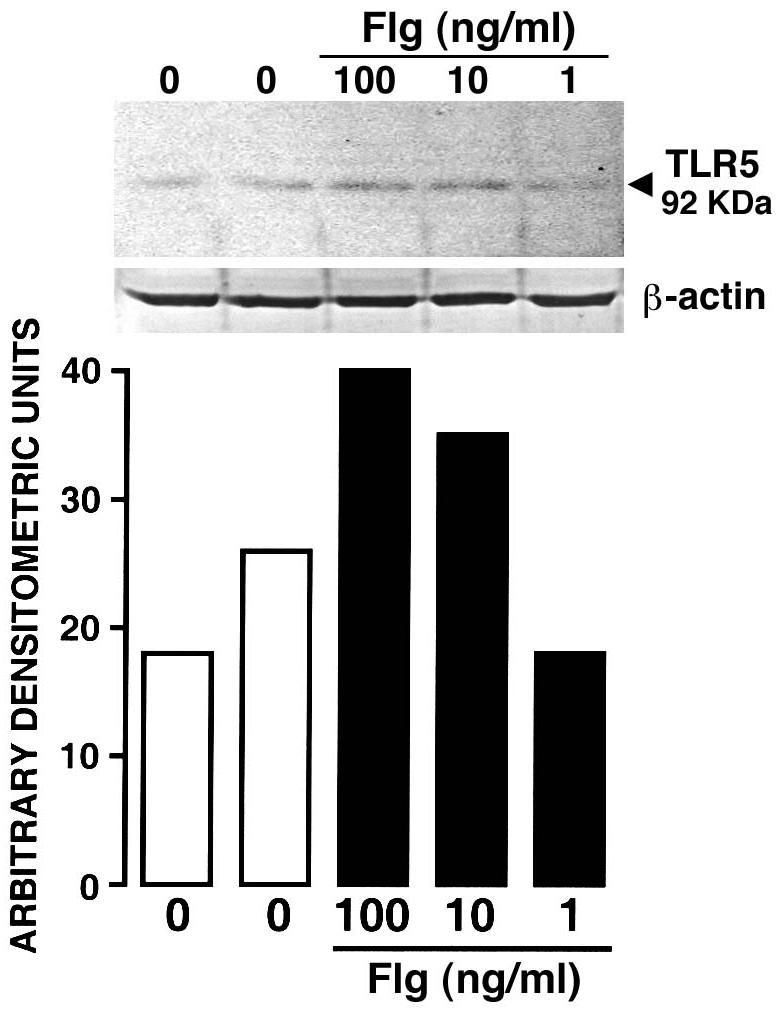
Expression of TLR5 protein in murine osteoblasts. Cells were unexposed or exposed to purified flagellin (1, 10, and 100 ng/ml) for 12 h. Whole-cell lysates were prepared and analyzed by Western blotting for the presence of TLR5. Below, densitometric analysis of this representative experiment is shown as arbitrary densitometric units corrected for background intensity for each lane. These studies were performed three times with similar results.
The present demonstration that osteoblasts recognize bacterial flagellins via TLR5 represents a potentially important means by which this bone cell type can respond to bacterial pathogens. While the reasons that both flagellated and nonflagellated bacterial species can upregulate TLR5 expression in osteoblasts are unclear, it may be that other bacterial products act via their specific TLR homologues to secondarily promote TLR5 expression. Such a phenomenon in central nervous system astrocytes was recently described in a report from our laboratory (4). As such, these data suggest that exposure of osteoblasts to bacteria or their components may serve to sensitize these cells to subsequent bacterial challenge. Taken together, these results indicate that the presence of TLR5 on osteoblasts may represent an important mechanism whereby these cells can perceive bacterial challenges and contribute to inflammatory immune responses during the progression of bone disease.
Acknowledgments
This work was supported by grants AR47585 and AR48842 to I.M. from the National Institutes of Health.
Editor: J. T. Barbieri
REFERENCES
- 1.Bost, K. L., J. L. Bento, J. K. Ellington, I. Marriott, and M. C. Hudson. 2000. Induction of colony-stimulating factor expression following Staphylococcus or Salmonella interaction with mouse or human osteoblasts. Infect. Immun. 68:5075-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bost, K. L., J. L. Bento, C. C. Petty, L. W. Schrum, M. C. Hudson, and I. Marriott. 2001. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. J. Interferon Cytokine Res. 21:297-304. [DOI] [PubMed] [Google Scholar]
- 3.Bost, K. L., W. K. Ramp, N. Nicholson, J. L. Bento, I. Marriott, and M. C. Hudson. 1999. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of IL-6 and IL-12 production. J. Infect. Dis. 180:1912-1920. [DOI] [PubMed] [Google Scholar]
- 4.Bowman, C. C., A. Rasley, S. L. Tranguch, and I. Marriott. 2003. Cultured astrocytes express Toll-like receptors for bacterial products. Glia 43:281-291. [DOI] [PubMed] [Google Scholar]
- 5.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor 4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 6.Gasper, N. A., C. C. Petty, L. W. Schrum, I. Marriott, and K. L. Bost. 2002. Bacteria-induced CXCL10 secretion by osteoblasts can be mediated, in part, through TLR4. Infect. Immun. 70:4075-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 11.Ibrahim, G. F., G. H. Fleet, M. J. Lyons, and R. A. Walker. 1985. Method for the isolation of highly purified Salmonella flagellins. J. Clin. Microbiol. 22:1040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi, T., T. Matsuguchi, N. Tsuboi, A. Mitani, S. Tanaka, M. Matsuoka, G. Yamamoto, T. Hishikawa, T. Noguchi, and Y. Yoshikai. 2001. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J. Immunol. 166:3574-3579. [DOI] [PubMed] [Google Scholar]
- 13.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marriott, I., and K. L. Bost. 2000. IL-4 and IFN-γ up-regulate substance P receptor expression in murine peritoneal macrophages. J. Immunol. 165:182-191. [DOI] [PubMed] [Google Scholar]
- 15.Marriott, I., K. L. Bost, and M. J. Mason. 1998. Differential kinetics for induction of interleukin-6 mRNA expression in murine peritoneal macrophages: evidence for calcium-dependent and independent-signalling pathways. J. Cell. Physiol. 177:232-240. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 17.Poltorak, A., I. Smirnova, X. He, M. Y. Liu, C. Van Huffel, O. McNally, D. Birdwell, E. Alejos, M. Silva, X. Du, P. Thompson, E. K. Chan, J. Ledesma, B. Roe, S. Clifton, S. N. Vogel, and B. Beutler. 1998. Genetic and physical mapping of the Lps locus: identification of the Toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 24:340-355. [DOI] [PubMed] [Google Scholar]
- 18.Schrum, L. W., K. L. Bost, M. C. Hudson, and I. Marriott. Bacterial infection induces expression of functional MHC class II molecules in murine and human osteoblasts. Bone, in press. [DOI] [PubMed]
- 19.Schrum, L. W., I. Marriott, E. K. Thomas, B. R. Butler, M. C. Hudson, and K. L. Bost. 2003. Functional CD40 expression induced following bacterial infection of mouse and human osteoblasts. Infect. Immun. 71:1209-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine Toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 21.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor 2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 24.Zou, W., A. Amcheslavsky, and Z. Bar-Shavit. 2003. CpG oligodeoxynucleotides modulate the osteoclastogenic activity of osteoblasts via Toll-like receptor 9. J. Biol. Chem. 278:16732-16740. [DOI] [PubMed] [Google Scholar]