Abstract
We have demonstrated that lipopolysaccharide (LPS) obtained from Burkholderia cepacia, an important opportunistic pathogen, has unique characteristics in both structure and activity. One of the structural characteristics is that the B. cepacia LPS has 4-amino-4-deoxy-l-arabinose (Ara4N) in its inner core region. Polymyxin B (PmxB) is known to act as an LPS antagonist, but LPS with Ara4N is suggested to be PmxB resistant by decreasing the binding capability of PmxB. Interaction of B. cepacia LPS with PmxB was investigated and compared with that of a reference LPS of Salmonella enterica serovar Abortusequi, referred to hereafter as the reference LPS. B. cepacia LPS suffered no suppressive effect of PmxB in its activity to stimulate murine peritoneal macrophages for induction of tumor necrosis factor alpha (TNF-α) and IL-6 even when the activity of the reference LPS was completely suppressed. A characteristic of B. cepacia LPS is that it has selectively weak interleukin-1β (IL-1β)-inducing activity while activity to induce TNF-α and IL-6 has been shown to be as strong as that of the reference LPS. Remarkably, PmxB augmented the IL-1β-inducing activity of B. cepacia LPS to the level of that of the reference LPS and, in contrast, completely suppressed the strong activity of the reference LPS. Using PmxB-immobilized beads, the adsorbances of these LPSs to the beads were compared, and it was found that B. cepacia LPS bound to PmxB with a high affinity similar to that of the reference LPS. These results indicate an unusual interaction of B. cepacia LPS with PmxB whereby B. cepacia LPS not only allows the binding of PmxB with high affinity, even though it contains Ara4N, but also suffers no suppressive effect of PmxB on its activity. Moreover, a remarkable increase in its IL-1β-inducing activity was also observed.
In recent years, Burkholderia cepacia has been recognized as an important opportunistic pathogen in hospitalized patients and other compromised individuals. Especially in patients with cystic fibrosis, some strains of B. cepacia are highly transmissible from person to person (9, 15), and the infection can lead to the development of serious pneumonia and sepsis with a high mortality rate, i.e., “cepacia syndrome” (10). This organism is also intrinsically resistant to a wide range of antibiotics, such as β-lactams, aminoglycosides, and polymyxins, which gives rise to therapeutic problems (14, 24). B. cepacia is an aerobic glucose-nonfermentative gram-negative bacillus and a type species in the new genus Burkholderia (35), separated from the genus Pseudomonas. Lipopolysaccharide (LPS) of this organism has been suggested as a candidate for the virulence factor, since LPS is a major component of the outer membrane in gram-negative bacteria and LPSs obtained from representative gram-negative bacteria, such as Escherichia coli and Salmonella, have been shown to play central roles in the induction of septic shock and endotoxin shock (1, 19). We have isolated and purified an LPS of B. cepacia, and the characteristic features of the LPS in both chemical structure (12) and biological activity (30) have been demonstrated.
Typical LPSs have one to three molecules of 3-deoxy-d-manno-octulosonic acid (KDO) in the inner core region attached to the lipid A moiety, and the lipid A-KDO-KDO structure is well conserved among a wide range of bacterial species. In the inner core region of the LPS of B. cepacia, a unique component, d-glycero-d-talo-2-octulosonic acid (KO), has been found in the structure of lipid A-KDO-KO, and it has been shown that the KO is partially replaced at the C-8 position with 4-amino-4-deoxy-l-arabinose (Ara4N) (12). In addition, the substitution of Ara4N in phosphate groups in the lipid A moiety of LPS obtained from a deep-rough mutant of B. cepacia has recently been reported (11). LPSs containing Ara4N have been obtained from polymyxin-resistant strains and mutants of enterobacterial species, such as E. coli, Salmonella enterica, and Proteus mirabilis, and it has been indicated that substitutions of Ara4N occur in the ester-linked phosphate group in the lipid A region and/or in the KDO in the inner core region (4, 22, 25, 33). Polymyxin B (PmxB) is an amphiphilic cyclic polycationic peptide antibiotic that exhibits antimicrobial activity against gram-negative bacteria by augmentation of permeability in the bacterial outer membrane and causes injury to the bacterial cytoplasmic membrane. It has been well established that PmxB has high affinity for negatively charged bacterial cell surface components, including LPS, and that its binding to LPS plays an essential role in its bactericidal activity (32). PmxB is also known as a potent LPS antagonist which suppresses various biological activities of LPS, such as lethal toxicity and induction of cytokines, by forming PmxB-LPS complexes with electrostatic and hydrophobic interactions (7, 26, 31). The presence of Ara4N with a positive charge that reduces the electronegativity of LPS is considered to be responsible for the decreased binding of PmxB, causing LPS to be resistant to the antagonistic action of PmxB. The effect of PmxB on the activity of the LPS of B. cepacia containing Ara4N has not been investigated prior to this study.
In the present study, purified LPS of B. cepacia was incubated with or without PmxB, and the activity of the LPS and LPS-PmxB in stimulating mouse peritoneal macrophages for induction of cytokines was investigated. Surprisingly, it was found that the weak activity of the B. cepacia LPS to induce interleukin 1β (IL-1β) was remarkably augmented by PmxB to a level comparable to that of a reference LPS of Salmonella and that the B. cepacia LPS bound to PmxB with high affinity similar to that of the reference LPS without Ara4N.
MATERIALS AND METHODS
Mice and cell culture.
LPS-responsive C3H/HeN mice purchased from Clea Japan, Inc. (Tokyo, Japan) and LPS-hyporesponsive C3H/HeJ mice from Charles River Japan Inc. (Kanagawa, Japan) were used at the age of 8 to 10 weeks. All animal experiments in the present study were conducted according to the guidelines of the Laboratory Animal Center, Jichi Medical School. For preparation of murine peritoneal macrophages, peritoneal exudate cells taken from mice that had received 2 ml of thioglycolate broth (Difco Laboratories, Detroit, Mich.) intraperitoneally 4 days in advance were used. RPMI 1640 medium (Flow Laboratories, Inc., Rockville, Md.) supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.2% NaHCO3, and 5% heat-inactivated fetal calf serum (culture medium) was used for cell culture. The peritoneal exudate cells were washed and suspended in the culture medium (106/ml), and a 0.5-ml aliquot was dispensed into each well of a 48-well culture plate (Sumitomo Bakelite Co. Ltd., Tokyo, Japan). After 3 h of culture, nonadherent cells were washed off, and the remaining adherent cells were used as peritoneal macrophages. All the cells were cultured in a humidified chamber at 37°C with 5% CO2.
LPS preparations and their treatment with PmxB.
LPS was prepared from B. cepacia GIFU 645, a type strain of B. cepacia and the same strain as ATCC 25416 or NCTC 10743, which belongs to genomovar I. the B. cepacia LPS used in the present study was a highly purified preparation, the same preparation as the BcLPS-3 in a preceding paper (30), which scarcely activates the cells of C3H/HeJ mice. LPS of Salmonella enterica serovar Abortusequi, referred to hereafter as the reference LPS, isolated and purified as described elsewhere (8), a kind gift from C. Galanos (Max-Planck-Institut für Immunbiologie, Freiburg, Germany), was used as the reference LPS. These LPS preparations, dissolved in pyrogen-free distilled water (PFDW), were incubated at room temperature for 30 min in the presence or absence of PmxB (Sigma Chemical Co., St. Louis, Mo.) and then diluted appropriately with the culture medium for use as stimulants of macrophage cultures.
Measurement of cytokines.
The activity of tumor necrosis factor alpha (TNF-α) in the macrophage culture supernatant at 4 h was determined by a cytotoxic assay against actinomycin D-treated L929 cells basically according to methods described elsewhere (27). Viable cells in the overnight culture were stained with crystal violet, and absorbance of blue color extracted with 30% acetic acid was measured at 540 nm by a Biomek 1000 spectrophotometer (Beckman Instruments, Palo Alto, Calif.). The activity of TNF-α (in units per milliliter) was calculated from the dilution factors of test samples necessary for 50% cell lysis, with correction by an internal standard of a recombinant human TNF-α in each assay.
IL-6 activity in the macrophage culture supernatant at 48 h was determined by a proliferation assay of IL-6-dependent mouse hybridoma MH60.BSF2 cells (17), a kind gift from N. Nishimoto of Osaka University (Osaka, Japan). The cells were cultured for 3 days, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma Chemical Co.) was added for the last 4 h of culture for formation of formazan blue crystals by viable cells (20). The supernatant was removed, and the precipitated formazan crystals were dissolved with isopropanol solution containing 5% formic acid to measure the absorbance at 540 nm. IL-6 activity (in units per milliliter) was calculated from the dilution factors of test samples required to induce 50% cell growth, with correction by an internal standard of a recombinant human IL-6 in each assay.
The amount of IL-1β in the macrophage culture supernatant at 48 h was determined by a specific sandwich enzyme-linked immunosorbent assay (Endogen, Woburn, Mass.) basically according to the manufacturer's instructions using matched antibody pairs as described elsewhere (30). Quantification of IL-1β (in picograms per milliliter) was performed based on a standard IL-1β curve in each assay.
Detection of pro-IL-1β.
Peritoneal macrophages stimulated with LPS for 20 to 24 h were lysed in lysis buffer (1% Triton X-100, 20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 1 mM Na3VO4, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 0.15 U of aprotin per ml) by three freeze-thaw cycles. Insoluble materials in the lysate were removed by centrifugation, and a 30-μg aliquot of protein was electrophoresed on a sodium dodecyl sulfate-10% polyacrylamide gel according to the method of Laemmli (13). Proteins separated on the gel were transferred to a polyvinylidene difluoride membrane (Millipore Co., Bedford, Mass.). Pro-IL-1β protein was analyzed using anti-mouse IL-1β monoclonal antibody (Endogen) and ECL Western blotting reagent (Amersham Life Science, Little Chalfont, United Kingdom) according to the manufacturers' instructions.
Adsorption of LPS by PmxB-immobilized agarose beads.
Appropriate amounts of PmxB-immobilized beads (Detoxi-gel; Pierce Inc., Rockford, Ill.), or agarose beads without PmxB as the negative control, were washed aseptically with PFDW and suspended in PFDW. The suspension of the beads was mixed with an equal volume of LPS solution (2 μg/ml of PFDW) and incubated with shaking for 30 min at room temperature. The agarose beads were removed by centrifugation, and the recovered solution, with its initial LPS concentration regarded as 1 μg/ml, was diluted appropriately with the culture medium to test for cytokine-inducing activity.
RESULTS
Effect of PmxB on activity of B. cepacia LPS to stimulate murine peritoneal macrophages for production of TNF-α and IL-6.
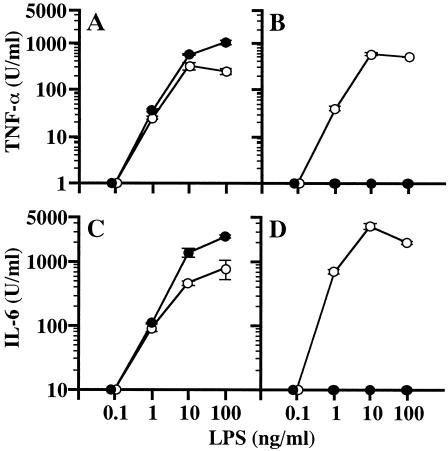
B. cepacia LPS was incubated with PmxB at room temperature for 30 min at various ratios up to 1:500 (LPS-PmxB, by weight), and the mixture (B. cepacia LPS-PmxB) diluted appropriately with the culture medium was added to the macrophage culture for stimulation. No suppressive effect of PmxB on the activity of B. cepacia LPS for induction of either TNF-α or IL-6 was observed even at the highest ratio (1:500) examined, while a suppressive effect on the activity of the reference LPS was clearly observed (Fig. 1). Neither of the two cytokines was induced by the reference LPS-PmxB (1:500) up to the highest LPS dose examined (100 ng/ml). At a lower ratio (1:50) of the reference LPS-PmxB, activity to induce TNF-α was suppressed completely, although the activity of IL-6 induction was not completely suppressed (data not shown). These results indicate that B. cepacia LPS escapes in some way from the action of PmxB to inactivate the endotoxic activity of typical LPSs, such as the reference LPS.
FIG. 1.
Effect of PmxB on activity of B. cepacia LPS to stimulate murine peritoneal macrophages for production of TNF-α and IL-6. B. cepacia LPS (A and C) or reference S. enterica serovar Abortusequi LPS (B and D) was incubated with PmxB (•) in PFDW at a ratio of 1:500 (LPS-PmxB, by weight) or without PmxB (○) at room temperature for 30 min. The specimens were added to the culture of peritoneal macrophages of C3H/HeN mice by diluting them appropriately with the culture medium to the final LPS concentrations indicated. The cells were cultured, and the culture supernatants were obtained at 4 and 48 h for determination of TNF-α (A and B) and IL-6 (C and D), respectively. The data are the means ± standard errors of the mean of triplicate samples. A representative result obtained from three independent experiments is shown.
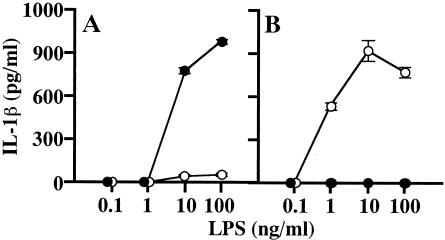
Effect of PmxB on IL-1β-inducing activity of B. cepacia LPS.
The cytokine-inducing activity of B. cepacia LPS has a unique characteristic, i.e., it shows weak activity in the induction of IL-1β relative to the strong activity in its induction of other cytokines, such as TNF-α and IL-6, as reported previously (30). The IL-1β-inducing activity of B. cepacia LPS-PmxB prepared as described above was next compared with the activities of B. cepacia LPS, the reference LPS, and the reference LPS-PmxB to investigate the effect of PmxB on these activities. As shown in Fig. 2, the weak activity of B. cepacia LPS was augmented remarkably by PmxB, and a large amount of IL-1β was produced by the macrophages in response to B. cepacia LPS-PmxB at LPS doses of 10 and 100 ng/ml. The amount of IL-1β was comparable to that produced in response to the reference LPS at the corresponding doses. In contrast, the strong activity of the reference LPS was completely suppressed by PmxB, and no IL-1β production was detected even at the highest LPS dose (100 ng/ml) of the reference LPS-PmxB, as seen in the activity for induction of the other cytokines, such as TNF-α and IL-6. These results suggest that B. cepacia LPS interacts with PmxB positively to open its masked potential, such as IL-1β-inducing activity, but not negatively to escape from the action of PmxB.
FIG. 2.
Effect of PmxB on IL-1β-inducing activity of B. cepacia LPS. Experimental procedures were basically the same as those in the legend to Fig. 1. B. cepacia LPS (A) or the reference LPS (B) incubated with (•) or without (○) PmxB was added to the macrophage culture. The culture supernatant obtained at 48 h was assayed for determination of IL-1β by enzyme-linked immunosorbent assay. The data are the means ± standard errors of the mean of triplicate samples. A representative result obtained from three independent experiments is shown.
In addition to the peritoneal macrophages from C3H/HeN mice, those from C3H/HeJ mice, which have a functional defect in Toll-like receptor 4 (TLR4) and are hyporesponsive to LPS, were also used in these experiments. Like the reference LPS and the reference LPS-PmxB, neither B. cepacia LPS nor B. cepacia LPS-PmxB stimulated the HeJ macrophages for the induction of IL-1β (data not shown).
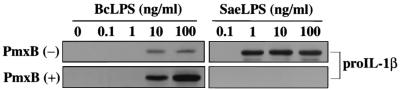
Augmentation by PmxB of B. cepacia LPS-induced pro-IL-1β production in murine peritoneal macrophages.
Mature IL-1β is released from cells after the processing of pro-IL-1β by the action of caspase-1. Production of pro-IL-1β in the macrophages stimulated with B. cepacia LPS-PmxB was analyzed by immunoblotting and compared with the production by those stimulated with B. cepacia LPS, the reference LPS, and the reference LPS-PmxB. As shown in Fig. 3, the weak production of pro-IL-1β observed in response to B. cepacia LPS at doses of 10 and 100 ng/ml was remarkably augmented in response to B. cepacia LPS-PmxB at the same LPS doses. On the other hand, the strong production of pro-IL-1β observed in response to the reference LPS at doses of 1, 10, and 100 ng/ml was completely eliminated in response to the reference LPS-PmxB. These patterns of pro-IL-1β production correlated well with those of mature IL-1β production shown in Fig. 2 and indicate that the altered IL-1β production induced by PmxB is caused by a change in some upstream step(s) of pro-IL-1β production rather than in the activation step of caspase-1 for the processing of pro-IL-1β.
FIG. 3.
Production of pro-IL-1β in murine peritoneal macrophages upon stimulation with B. cepacia LPS (BcLPS) and effect of PmxB on the production. B. cepacia LPS or reference S. enterica serovar Abortusequi LPS (SaeLPS) incubated with (+) or without (−) PmxB as indicated in Fig. 1 was diluted appropriately, and the murine peritoneal macrophages were stimulated. The macrophages, cultured for 20 to 24 h, were lysed in the lysis buffer by three freeze-thaw cycles. The cell lysate containing 30 μg of total proteins was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the separated proteins were transferred to a polyvinylidene difluoride membrane, and pro-IL-1β was determined using anti-mouse IL-1β antibody and ECL Western blotting reagent.
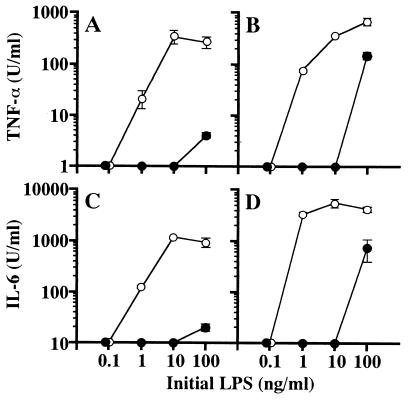
Adsorption of B. cepacia LPS on PmxB-immobilized agarose beads.
To investigate the binding of B. cepacia LPS to PmxB, B. cepacia LPS was incubated with PmxB-immobilized agarose beads and recovery of the B. cepacia LPS was determined by its activity to induce TNF-α and IL-6. As a negative PmxB control, agarose beads without PmxB were used for incubation with the LPS. As shown in Fig. 4A and C, at initial LPS concentrations over 1 ng/ml, clear activity to induce both TNF-α and IL-6 was detected in the recovered specimen of B. cepacia LPS after incubation with the negative control PmxB agarose beads. This means that nearly 100% of B. cepacia LPS was recovered without nonspecific binding to the agarose beads, since the original B. cepacia LPS exhibited the activity with a similar dose response (Fig. 1A and C). In the specimen of B. cepacia LPS recovered after incubation with PmxB-agarose, a concentration that was >100 times higher than that of the initial LPS was required to detect the activity (Fig. 4A and C). This means that >99% of the initial B. cepacia LPS was adsorbed on the PmxB-agarose. At the same time, the reference LPS was examined in the same manner. As shown in Fig. 4B and D, the reference LPS was similarly adsorbed on the PmxB-agarose, and ∼99% of the LPS was estimated to be adsorbed. These results indicate that B. cepacia LPS binds to PmxB with a high affinity similar to that of the reference LPS.
FIG. 4.
Adsorption of B. cepacia LPS by PmxB-immobilized agarose beads. B. cepacia LPS (A and C) or the reference LPS (B and D) at 2 μg/ml in PFDW was incubated at room temperature for 30 min with an equal-volume suspension of PmxB-immobilized agarose beads (•) or control agarose beads without PmxB (○). The solution recovered after removal of the agarose beads by centrifugation, with its initial LPS concentration assumed to be 1 μg/ml, was diluted appropriately with the culture medium and added to the macrophage culture to the initial LPS concentrations indicated. The culture supernatants obtained at 4 and 48 h were assayed for determination of TNF-α (A and B) and IL-6 (C and D), respectively. The data are the means ± standard errors of the mean of triplicate samples. A representative result obtained from three independent experiments is shown.
DISCUSSION
In previous studies, we demonstrated that the activity of B. cepacia LPS to stimulate murine macrophages for the production of IL-1β is very weak relative to its strong activities for the production of other cytokines, such as TNF-α and IL-6 (30). It has also been shown that the LPS contains Ara4N in its structure (12). PmxB is a well-characterized pharmacological LPS antagonist, and it completely suppressed the cytokine-inducing activity of the reference LPS (Fig. 1 and 2). LPS modified with Ara4N, like B. cepacia LPS, is, however, indicated to be PmxB resistant, since positively charged Ara4N makes LPS less anionic and prevents it from binding to PmxB. Activity of B. cepacia LPS to induce either TNF-α or IL-6 was not suppressed by PmxB (Fig. 1), as expected, but to our surprise, the weak activity of the LPS for induction of IL-1β was remarkably augmented by PmxB (Fig. 2) to a level comparable to the strong activity of the reference LPS without PmxB. This result indicated the possibility that B. cepacia LPS interacts positively with PmxB but does not escape from the binding of PmxB. It was shown in a previous report (18) that LPS isolated from PmxB-resistant Pseudomonas cepacia (the former name of B. cepacia) bound to PmxB with high affinity, although the intact cells of the organism did not. There was no indication in the report of the presence or absence of Ara4N in the LPS. The presence of Ara4N in LPS of P. cepacia was reported by another group (6), and the LPS was assumed to have low affinity for PmxB without binding experiments. The B. cepacia LPS used in the present study certainly has Ara4N and was revealed to bind PmxB with high affinity (Fig. 4). Binding of PmxB to Ara4N-containing LPS has been directly indicated for the first time in the present study. In addition to electrostatic interaction, hydrophobic interaction between acyl groups in LPS and a fatty acid residue in PmxB is also considered to contribute to the binding of PmxB to LPS (7). Such hydrophobic interaction, rather than electrostatic interaction, is assumed to play the main role in the binding of the B. cepacia LPS to PmxB. Binding of PmxB to LPS with stronger electrostatic interaction may induce a stronger suppressive effect on LPS activities, but binding with stronger hydrophobic interaction may not correlate with the suppressive effect and may induce different effects, as observed in augmentation of IL-1β induction by B. cepacia LPS. Further investigations are necessary to elucidate the underlying mechanisms.
In LPS-responsive cells like macrophages, the important role of TLR4 as an LPS signal transducer has recently been elucidated by the finding that the mutation of the Tlr4 gene in C3H/HeJ mice is the cause of the defective LPS signaling (23). We have shown in a preceding paper (30) that highly purified B. cepacia LPS was not active for HeJ mice in activities such as lethal toxicity, mitogenic activity to spleen cells, and TNF-α- and IL-6-inducing activity to macrophages. These activities of B. cepacia LPS were exhibited in a manner similar to that of the reference LPS, but the IL-1β-inducing activity of B. cepacia LPS was weak, unlike the strong activity of the the reference LPS. On the other hand, the activity of B. cepacia LPS-PmxB was strong, unlike the undetectable activity of the reference LPS-PmxB, as shown in Fig. 2. We thought that such unusual activity might possibly be exhibited via a TLR4-independent signaling pathway and investigated the activity using HeJ macrophages, but neither B. cepacia LPS nor B. cepacia LPS-PmxB showed the ability to stimulate HeJ macrophages to induce IL-1β. This result indicates that the strong signal of B. cepacia LPS-PmxB to induce IL-1β is also transduced via TLR4. It is now widely accepted that expression of CD14 (34) and MD-2 (29), in addition to TLR4, is necessary to form an LPS receptor complex that induces optimal response to LPS (5). The action mechanisms by which the receptor complex recognizes the LPS molecule and transduces the signals from outside the cells to inside have not been elucidated. B. cepacia LPS-PmxB may be recognized by a receptor complex similarly to the reference LPS, but B. cepacia LPS itself may act in a somewhat different way to transduce a weak signal for IL-1β, and the reference LPS-PmxB may be ignored.
In the process of LPS-induced IL-1β production, IL-1β is synthesized as an inactive precursor protein (pro-IL-1β) that is cleaved by a protease, caspase-1, to generate a mature secretory protein, IL-1β (16). Caspase-1 is constitutively expressed as its precursor form, which completely lacks cleavage activity (3), and is activated in response to LPS stimulation that contributes to LPS-induced IL-1β production (28). In other words, both synthesis of pro-IL-1β and activation of caspase-1 are required for the production of IL-1β upon LPS stimulation. In the case of IL-1β induction by B. cepacia LPS and B. cepacia LPS-PmxB, good correlation between the production of IL-1β and pro-IL-1β was observed (Fig. 2 and 3), indicating that the unusual behavior of B. cepacia LPS for the induction of IL-1β is caused at least by abnormal signaling for the synthesis of pro-IL-1β, although alteration in caspase-1 activation was not clarified. Stimulation of macrophages with B. cepacia LPS and B. cepacia LPS-PmxB to analyze the differences in activation of signaling factors between these stimulants is a promising method for obtaining valuable information for elucidation of the specific signaling steps leading to the synthesis of pro-IL-1β.
It is very interesting that B. cepacia LPS, in spite of containing Ara4N, binds to PmxB with as high an affinity as the reference LPS does and that B. cepacia LPS suffers no suppressive effect on its bioactivities by the binding of PmxB. It was indicated recently that substitution of Ara4N in the LPS of B. cepacia always occurs at the outer sugar, mostly in the KO residue, but the substitution in LPSs of various serotypes of the genus Proteus occurs at the inner KDO residue (11). Such a difference in the Ara4N substitution patterns of LPSs has a possibility to induce interaction of B. cepacia LPS with PmxB different from that of enterobacterial LPSs containing Ara4N, like those of Proteus. An antagonistic effect of PmxB on endotoxic activities of such Ara4N-containing LPSs in relation to interaction between PmxB and the LPSs has not been investigated in detail, but such investigations, like the present study, are necessary to obtain useful information for treatment of infections by bacteria containing such LPSs. Recently, PmxB-immobilized fiber was developed for clinical application in hemoperfusion to eliminate blood endotoxin in septic shock patients (2, 21). Based on the information obtained in the present study, PmxB-immobilized fiber is expected to be effective for elimination of B. cepacia LPS, but soluble PmxB is not expected to be effective in suppressing endotoxic activity. Information about the binding capacities of various types of LPSs to PmxB, including those containing Ara4N, is also important. The present study provides new insights into the interaction between LPS and PmxB.
Acknowledgments
This work was supported in part by grant 13670280 (to M.M.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alexander, C., and E. T. Rietschel. 2001. Bacterial lipopolysaccharide and innate immunity. J. Endotoxin Res. 7:167-202. [PubMed] [Google Scholar]
- 2.Asanuma, Y., T. Furuya, J. Tanaka, T. Sato, S. Shibata, and K. Koyama. 1999. The application of immobilized polymyxin B fiber in the treatment of septic shock associated with severe acute pancreatitis: report of two cases. Surgery Today 29:1177-1182. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, J. M., T. T. Yamin, L. A. Egger, J. Chin, M. J. Kostura, and D. K. Miller. 1994. IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J. Immunol. 153:2592-2599. [PubMed] [Google Scholar]
- 4.Boll, M., J. Radziejewska-Lebrecht, C. Warth, D. Krajewska-Pietrasik, and H. Mayer. 1994. 4-Amino-4-deoxy-l-arabinose in LPS of enterobacterial R-mutants and its possible role for their polymyxin reactivity. Immunol. Med. Microbiol. 8:329-342. [DOI] [PubMed] [Google Scholar]
- 5.Correia, J. D. S., K. Soldau, U. Christen, P. S. Tobias, and R. J. Ulevitch. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. J. Biol. Chem. 276:21129-21135. [DOI] [PubMed] [Google Scholar]
- 6.Cox, A. D., and S. G. Wilkinson. 1991. Ionizing groups in lipopolysaccharides of Pseudomonas cepacia in relation to antibiotic resistance. Mol. Microbiol. 5:641-646. [DOI] [PubMed] [Google Scholar]
- 7.Coyne, C. P., and B. W. Fenwick. 1993. Inhibition of lipopolysaccharide-induced macrophage tumor necrosis factor-α synthesis by polymyxin B sulfate. Am. Vet. Res. 54:305-314. [PubMed] [Google Scholar]
- 8.Galanos, C., O. Lüderitz, and O. Westphal. 1979. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-pyrexal). Zentbl. Bakteriol. Hyg. Abt. 1 Orig. A 243:226-244. [PubMed] [Google Scholar]
- 9.Govan, J. R. W., P. H. Brown, J. Maddison, C. J. Doherty, J. W. Nelson, M. Dodd, A. P. Greening, and A. K. Webb. 1993. Evidence of transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet 342:15-19. [DOI] [PubMed] [Google Scholar]
- 10.Govan, J. R. W., and J. W. Nelson. 1992. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 48:912-930. [DOI] [PubMed] [Google Scholar]
- 11.Gronow, S., C. Noah, A. Blumentha, B. Lindner, and H. Brade. 2003. Construction of a deep-rough mutant of Burkholderia cepacia ATCC 25416 and characterization of its chemical and biological properties. J. Biol. Chem. 278:1647-1655. [DOI] [PubMed] [Google Scholar]
- 12.Issiki, Y., K. Kawahara, and U. Zähringer. 1998. Isolation and characterisation of disodium (4-amino-4-deoxy-β-l-arabinopyranosyl)-(1→8)-(d-glycero-α-d-talo-octo-2-ulopyranosylonate)-(2→4)-(methyl-3-deoxy-d-manno-octo-2-ulopyranoside) onate from the lipopolysaccharide of Burkholderia cepacia. Carbohydr. Res. 313:21-27. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Lipuma, J. J. 1998. Burkholderia cepacia. Management issues and new insights. Clin. Chest Med. 19:473-486. [DOI] [PubMed] [Google Scholar]
- 15.Lipuma, J. J., S. E. Dasen, D. W. Neilson, R. C. Stern, and T. L. Stull. 1990. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet 336:527-532. [DOI] [PubMed] [Google Scholar]
- 16.Lonneman, G., S. Endres, J. W. V. D. Meer, K. M. Koch, and C. A. Dinarello. 1989. Differences in the synthesis and kinetics of release of interleukin 1. Eur. J. Immunol. 19:1531-1536. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda, T., T. Hirano, and T. Kishimoto. 1987. Establishment of an interleukin-6 (IL-6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL-6 monoclonal antibodies. Eur. J. Immunol. 18:951-956. [DOI] [PubMed] [Google Scholar]
- 18.Moore, R. A., and R. E. T. Hancock. 1986. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob. Agents Chemother. 30:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison, D. C., and J. L. Ryan. 1987. Endotoxins and disease mechanisms. Annu. Rev. Med. 38:417-432. [DOI] [PubMed] [Google Scholar]
- 20.Mosman, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, T., I. Ebihara, H. Shoji, C. Ushiyama, S. Suzuki, and H. Koide. 1999. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm. Res. 48:171-175. [DOI] [PubMed] [Google Scholar]
- 22.Nummila, K., I. Kilpelainen, U. Zähringer, M. Vaara, and I. M. Helander. 1995. Lipopolysaccharide of polymyxin B-resistant mutants of Escherichia coli are extensively substituted by 2-aminoethyl pyrophosphate and contain aminoarabinose in lipid A. Mol. Microbiol. 16:271-278. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 24.Prince, A. 1986. Antibiotic resistance of Pseudomonas species. J. Pediatr. 108:830-834. [DOI] [PubMed] [Google Scholar]
- 25.Radziejewska-Lebrecht, J., U. R. Bhat, M. Brade, and H. Mayer. 1988. Structural studies on the core and lipid A region of a 4-amino-arabinose-lacking Rc-type mutant of Proteus mirabilis. Eur. J. Biochem. 183:573-581. [DOI] [PubMed] [Google Scholar]
- 26.Rifkind, D. 1967. Prevention by polymyxin B of endotoxin lethality in mice. J. Bacteriol. 93:1463-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruff, M. R., and G. E. Gilfford. 1980. Purification and physiological characterization of rabbit tumor necrosis factor. J. Immunol. 125:1671-1677. [PubMed] [Google Scholar]
- 28.Schumann, R. R., C. Belka, D. Reuter, N. Lamping, C. J. Kirschning, J. R. Weber, and D. Pfeil. 1998. Lipopolysaccharide activates caspase-1 (interleukin-1-converting enzyme) in cultured moncytic and endothelial cells. Blood 91:577-584. [PubMed] [Google Scholar]
- 29.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimomura, H., M. Matsuura, S. Saito, Y. Hirai, Y. Issiki, and K. Kawahara. 2001. Lipopolysaccharide of Burkholderia cepacia and its unique character to stimulate murine macrophages with relative lack of interleukin-1β-inducing ability. Infect. Immun. 69:3663-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes, D. C., J. L. Shenep, M. Fishmen, W. K. Hildner, G. K. Bysani, and K. Rufus. 1989. Polymyxin B prevents lipopolysaccharide-induced release of tumor necrosis factor-α from alveolar macrophages. J. Infect. Dis. 160: 52-57. [DOI] [PubMed] [Google Scholar]
- 32.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Rev. Microbiol. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaara, M., T. Vaara, M. Jensen, I. Helander, M. Numinen, E. T. Rietschel, and P. H. Mäkelä. 1981. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutant of Salmonella typhimurium. J. Bacteriol. 139:664-667. [DOI] [PubMed] [Google Scholar]
- 34.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 35.Yabuuchi, E., Y. Kosako, H. Oyaizu, I. Yano, H. Hotta, Y. Hashimoto, T. Ezaki, and M. Arakawa. 1992. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species of Burkholderia cepacia (Palleroni and Holmes) comb. nov. Microbiol. Immunol. 36:1251-1275. [DOI] [PubMed] [Google Scholar]