Abstract
Johne's disease (paratuberculosis) of cattle is widespread and causes significant economic losses for producers due to decreased production and poor health of affected animals. The chronic nature of the disease and the lack of a reproducible model of infection hinder research efforts. In the present study, instillation of Mycobacterium avium subsp. paratuberculosis into the tonsillar crypts of neonatal calves resulted in peripheral colonization as detected by antemortem culture of feces and postmortem (320 days postchallenge) culture of intestinal tissues. Antigen-specific blastogenic, gamma interferon (IFN-γ), and nitric oxide responses by blood mononuclear cells from infected calves exceeded prechallenge responses beginning 194 days postchallenge. Upon in vitro stimulation with paratuberculosis antigens, CD4+ cells from infected calves proliferated, produced IFN-γ, and increased expression of CD26 and CD45RO (indicative of an activated memory phenotype). Utilizing a lipoarabinomannan-based enzyme-linked immunosorbent assay, specific serum immunoglobulin was detected as early as 134 days postchallenge and generally increased after this time point. Two antigens of ∼50 and ∼60 kDa were particularly immunodominant early in infection, as shown by immunoblot with serum collected within 2 weeks postchallenge. Findings indicate that the intratonsillar inoculation route will prove useful as an experimental model for paratuberculosis infection. Additionally, this study confirms that mycobacteria-specific antibody is detectable early in the course of experimental Johne's disease, even preceding the development of specific cell-mediated responses.
Mycobacterium avium subsp. paratuberculosis infection of cattle is widespread, with estimates of 20 to 40% of U.S. dairy herds affected and costs of $220 million per year to the dairy industry (7, 57). Considering the poor sensitivity and specificity of present paratuberculosis diagnostic tests (reviewed in reference 25), negative effects of this disease may be even greater than present estimates indicate. These diagnostic deficiencies result from, among other things, the absence of a consistent experimental infection model for diagnostic test development and by confounding responses to other closely related mycobacteria. Furthermore, the discovery that M. avium subsp. paratuberculosis colonizes rabbits, elk, deer, foxes, bighorn sheep, and other mammals that may serve as reservoir hosts adds another significant obstacle for implementation of an effective eradication campaign (5, 6, 24, 58). Developed countries with wildlife reservoirs of Mycobacterium bovis (i.e., bovine tuberculosis) have been unable to eradicate tuberculosis from their domestic herds and are exploring control measures, such as vaccination, as alternatives to traditional test and slaughter campaigns (3, 13). A consistent calf model of experimental M. avium subsp. paratuberculosis infection would facilitate development and validation of new diagnostic tests and evaluation of candidate vaccines.
Oral inoculation of goats with multiple doses of M. avium subsp. paratuberculosis results in consistent infection (46). Cellular and humoral immune responses follow similar kinetics in experimentally infected goats. In contrast, it is often stated that cellular and humoral immune responses of M. avium subsp. paratuberculosis-infected cattle are divergent, with gamma interferon (IFN-γ) responses detected early and antibody responses detected late in infection (reviewed in references 10 and 43). This dogma, however, has recently been questioned (29). Detection of specific antibody responses is highly dependent on the isotypes and antigens used in the assay. Additionally, many conclusions on the immune response to paratuberculosis infection come from evaluation of responses in naturally infected cattle. The dose of M. avium subsp. paratuberculosis, exposure to other mycobacterial species, housing conditions, number of lactations, exposure to other pathogens (e.g., bovine leukemia virus), administration of antibiotics and other therapies, and age of the naturally exposed animal, however, are often unknown. Experimental inoculation of calves housed in a controlled environment should minimize these variable influences.
Intratonsillar inoculation of cattle with M. bovis results in consistent infection with associated lesions and host responses similar to those of natural infection (37). Mycobacteria that enter through oral and/or nasal routes encounter tonsillar tissue (9, 31, 36, 38-40). Although rarely detected in tonsillar tissue of naturally infected animals, M. avium subsp. paratuberculosis has been isolated from the tonsils of calves for up to 6 months after oral inoculation (39, 40). It is hypothesized that M. avium subsp. paratuberculosis disseminates via the reticuloendothelial system, with M cells acting as portals of entry into the lymphatic system (38, 34). For pathogens infecting macrophages, tonsillar tissue offers one of the first opportunities for intracellular invasion and interaction with specific host cell defenses. The primary objective of the present study was to determine if instillation of M. avium subsp. paratuberculosis into the tonsillar crypts of young calves results in a detectable host response and/or colonization of the bacterium. A secondary objective was to determine and compare the kinetics of host cellular and humoral responses.
MATERIALS AND METHODS
Animals, bacterial culture, antigens, and challenge and necropsy procedures.
Three castrated male Holstein calves were challenged with M. avium subsp. paratuberculosis by instillation of 0.2 ml of challenge inoculum into each of the two tonsillar crypts weekly from 2 to 5 weeks of age. Challenge inoculum consisted of ∼1.6 × 107 CFU of mid-log-phase M. avium subsp. paratuberculosis (i.e., four weekly doses of ∼4 × 106 CFU/2 tonsillar crypts) grown in Middlebrook's 7H9 medium (Becton Dickinson, Cockeysville, Md.) supplemented with 2 mg of mycobactin J (Allied Monitor Inc., Fayette, Mo.)/liter and 10% oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Mo.). Bacilli were harvested from the culture media by centrifugation at 10,000 × g, were washed with cold phosphate-buffered saline solution (PBS; 0.15 M, pH 7.2), and were diluted to the appropriate cell density for use as a challenge inoculum in PBS. Enumeration of bacilli was by serial dilution plating on Herrold's egg yolk slants containing mycobactin J (2 mg/liter). Whole-cell sonicates (WCS) of M. avium subsp. paratuberculosis strains K10 and 19698 were prepared for use as antigens in immunoassays. M. avium subsp. paratuberculosis organisms were cultured in 500 ml of Middlebrook's 7H9 medium at 37°C to an optical density at 540 nm (OD540) reading of 0.2 to 0.4. Mycobacteria were pelleted (10,000 × g for 20 min) and washed twice with cold PBS. The pellet was resuspended in PBS and was sonicated on ice with a probe sonicator. Sonication consisted of three cycles of 10-min bursts at 18 W on ice with 10-min chilling periods between sonications. Debris was removed by centrifugation (12,000 × g for 5 min), and supernatants were harvested and stored at −20°C. Protein concentration was determined by using the Bio-Rad protein assay (Richmond, Calif.).
Animals were euthanized 320 days after inoculation with intravenous sodium pentobarbital. A thorough postmortem examination was done, and the following tissues were collected for microscopic examination and bacteriologic isolation of M. avium subsp. paratuberculosis: palatine tonsil; medial retropharyngeal; mandibular and parotid lymph nodes; mid-duodenum and associated lymph node; one section each of proximal, middle, and distal jejunum and associated lymph nodes; proximal, middle, and distal ileum and associated lymph nodes; ileocecal valve and ileocecal lymph node; cecum; spiral colon and associated lymph node; transverse and descending colon; and hepatic and ileac lymph nodes. Tissue specimens were fixed by immersion in neutral buffered 10% formalin. Tissues were routinely processed, embedded in paraffin, cut at 4 to 6 μm, and stained with hematoxylin and eosin and acridine orange/aurimine O. Adjacent sections were cut from blocks containing tissues with lesions suggestive of paratuberculosis and were stained by the Ziehl-Neelsen technique to visualize acid-fast bacteria.
Culture of M. avium subsp. paratuberculosis and PCR analyses.
Culture of M. avium subsp. paratuberculosis was performed on fecal samples by a double centrifugation, double decontamination procedure (45). Tissues were homogenized in 0.75% hexadecylpyradinium chloride solution (Sigma) with a stomacher for 1 min and were incubated overnight to decontaminate cultures. Each fecal and tissue sample was inoculated onto four culture tubes of Herrold's egg yolk medium containing mycobactin J (2 mg/liter) and antibiotics (naladixic acid [50 μg/ml], vancomycin [50 μg/ml], and amphotericin B [50 μg/ml]). Colonies were enumerated after 12 weeks of incubation at 37°C. Confirmation of colonies on agar slants was performed by PCR analysis. Briefly, agar slants were flooded with 1 ml of sterile 1 mM Tris-0.05 mM EDTA buffer (pH 7.6), and slants were scraped. The solution was then decanted into a sterile microcentrifuge tube, and the tubes were placed within a boiling water bath for 10 min to release the DNA from the bacteria. After cooling to room temperature (RT), tubes were briefly centrifuged at 16,000 × g to pellet the bacterial debris. Bacterial lysate (5 μl) was added to 45 μl of PCR mix containing GeneAmp 10× PCR buffer II (Perkin-Elmer, Foster City, Calif.), 3.0 mM MgCl2, 0.25 mM deoxynucleoside triphosphates, 0.6 mM primers, and 2 U of AmpliTaq gold. Primer sequences for the M. avium subsp. paratuberculosis-specific genetic element, IS900, were used in the reaction mixture as follows: 5′-CCGCTAATTGAGAGATGCGATTGG-3′, forward primer, and 5′-AATCAACTCCAGCAGCGCGGCCTCG-3′, reverse primer, to yield a 229-bp product as previously described (52). Controls consisting of reaction mixture alone and positive controls containing 50 ng of genomic DNA from M. avium subsp. paratuberculosis were run according to the following protocol: 1 cycle at 94°C, 10 min; 50 cycles at 94°C, 1 min, 60°C, 30 s, and 72°C, 1 min; followed by a final extension cycle at 72°C, 10 min. PCR amplicons and a 50- to 1,000-bp marker (BioWhittaker Molecular Applications, Rockland, Maine) were then electrophoresed in a 4% NuSieve 3:1 Plus agarose gel (FMC Bioproducts, Rockland, Maine) in 1× Tris-Borate-EDTA (1 M Tris-HCl, 0.9 M boric acid, 0.01 M EDTA; GIBCO BRL) buffer at 65 V for 1 h. Gels were then stained with ethidium bromide, visualized, and photographed on a Bio-Rad Gel Doc 1000 Imager System (Hercules, Calif.).
Mononuclear cell culture and blastogenesis.
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat fractions of peripheral blood collected in 2× acid citrate dextrose (8). Wells of 96-well round-bottom microtiter plates (Falcon; Becton-Dickinson, Lincoln Park, N.J.) were seeded with 2 × 105 mononuclear cells in a total volume of 200 μl per well. Medium was RPMI 1640 supplemented with 25 mM HEPES buffer, 100 U of penicillin/ml, 0.1 mg of streptomycin/ml, 50 μM 2-mercaptoethanol (Sigma), and 10% (vol/vol) fetal bovine serum (FBS). Wells contained medium plus 5 μg of M. avium-purified protein derivative (PPDa; CSL Limited)/ml, 10 μg of M. avium subsp. paratuberculosis strain 19698 WCS/ml, 10 μg of M. avium subsp. paratuberculosis strain K10 WCS/ml, 1 μg of pokeweed mitogen (PWM)/ml, or medium alone (no stimulation). Responses to PWM were performed to confirm a general responsiveness of the cell population to polyclonal stimulation (i.e., a positive control). Leukocyte cultures were incubated for 5 days at 37°C in 5% CO2 in air. After 5 days, 0.5 μCi of [methyl-3H]thymidine (specific activity, 6.7 Ci mmol−1; Amersham Life Science, Arlington Heights, Ill.) in 50 μl of medium was added to each well, and cells incubated for an additional 20 h. Well contents were harvested onto fiber filters with a 96-well plate harvester (EG & G Wallace, Gaithersburg, Md.), and the incorporated radioactivity (counts per minute [cpm]) were measured by liquid scintillation counting. Treatments were run in triplicate. Data are presented as stimulation indices (i.e., mean cpm for stimulated samples/mean cpm for samples receiving medium alone).
PKH67 proliferation assay.
Staining of PBMC with PKH67 was performed according to manufacturer instructions (Sigma) and as described previously (55). Briefly, 2 × 107 PBMC were centrifuged (10 min, 400 × g), supernatants were aspirated, and cells were resuspended in 1 ml of diluent provided in the PKH67 kit. Cells in diluent were added to 1 ml of PKH67 green fluorescent dye (2 μM) and were incubated for 5 min followed by a 1-min incubation with 2 ml of FBS to adsorb the excess dye and to stop further dye uptake by cells. Cells were then washed (twice) with RPMI 1640, and wells of 96-well round-bottom microtiter plates (Falcon; Becton-Dickinson) were seeded with 2 × 105 PKH67-stained mononuclear cells in a total volume of 200 μl per well (six replicates for each treatment). Cells were stimulated as described in the cell culture procedure, incubated for 7 days at 37°C in 5% CO2 in air, and harvested according to treatment for flow cytometric analysis.
Flow cytometry.
Mononuclear cells (∼2 × 106/ml) in 100 μl of balanced salt solution with 1% FBS and 0.1% sodium azide (fluorescence-activated cell sorter [FACS] buffer) were stained with 100 μl of primary antibody to leukocyte surface antigens ([CACT138A, immunoglobulin G1 {IgG1}, anti-bovine CD4], [BAQ111A, IgM, anti-bovine CD8α], [CACT61A IgM, anti-bovine γδ T-cell receptor {TCR}, specific for the delta chain], [BAQ4A, IgG1, anti-bovine WC1, a subset of bovine γδ T cells], [PIG45A, IgG2b, anti-bovine IgM], [CACT114A, IgG2b, anti-bovine CD26], [GC44A, IgG3, anti-bovine CD45RO]). All primary antibodies were obtained from Veterinary Medical Research and Development, Pullman, Wash. After a 15-min incubation, cells were centrifuged (400 × g, 2 min) and resuspended in 100 μl of appropriate secondary antibody (fluorescein isothiocyanate [FITC; 1 μg/well]-conjugated goat anti-mouse IgG3 [Caltag, Burlingame, Calif.], phycoerythrin [PE; 1 μg/well]-conjugated goat anti-mouse IgM, IgG2a, or IgG2b [Southern Biotechnology Associates, Birmingham, Ala.], peridinin chlorophyll protein [12 μl/well]-conjugated rat anti-mouse IgG1 [Becton Dickinson], or TRICOLOR-conjugated goat anti-mouse IgG1 [Caltag]). Cells were then incubated an additional 15 min, centrifuged (400 × g, 2 min), resuspended in 200 μl of FACS buffer, centrifuged again (400 × g, 2 min), and resuspended in 200 μl of FACS buffer. Cells were then analyzed by using a Becton Dickinson FACScan flow cytometer (10,000 events, live gate, 3-color analysis, 488-nm-wavelength laser). Modfit Proliferation Wizard (Verity Software House Inc., Topsham, Maine) and CellQuest software (Becton Dickinson) were used for cell proliferation and phenotype analyses, respectively. Proliferation profiles were determined as the number of cells proliferating in antigen-stimulated wells minus the number of cells proliferating in nonstimulated wells for both gated (i.e., CD4+, CD8+, γδ TCR+, and IgM+ cells) or ungated (total PBMC) populations and are presented as the mean (± standard error of the mean [SEM]) number of cells that had proliferated/10,000 PBMC. Appropriate isotype control antibodies were used for both the nonstimulated as well as antigen-stimulated wells as a control for nonspecific binding of antibodies to activated cells.
IFN-γ: ELISA and intracellular staining protocols.
Supernatants of PBMC cultures (2 × 105 cells/well, 4 replicates/treatment) stimulated with antigen, PWM, or medium alone (as described above for the cell culture procedure) were harvested after a 48- and 72-h incubation at 37°C in a 5% CO2 humidified chamber. Analysis of IFN-γ concentrations was performed by a commercial enzyme-linked immunosorbent assay (ELISA)-based kit (Bovigam; CSL Limited). For intracellular staining, PBMC were cultured as described above with the addition of 10 μg of brefeldin A (Sigma)/ml, 1 μg of ionomycin (Sigma)/ml, and 50 ng of phorbol 12-myristate 13-acetate (PMA; Sigma)/ml for the terminal 5 h of incubation. After stimulation, PBMC were surface labeled with 10 μg of primary antibody/ml to leukocyte surface antigens IL-A11, IgG2a, anti-bovine CD4 [VMRD]; GB21A, IgG2b, anti-bovine γδ TCR [VMRD]; CC63, IgG2a, anti-bovine CD8 [Serotec, Kidlington, Oxford, United Kingdom] or appropriate isotype control antibodies) for 60 min at 4°C. Cells were washed (FACS buffer), stained with biotinylated anti-mouse IgG2a/2b (0.1 μg/106 cells; Becton Dickinson), washed again (FACS buffer), and incubated with streptavidin cy-chrome (0.06 μg/106 cells; Becton Dickinson). Cells were fixed in 1% paraformaldehyde, permeabilized (PBS plus 1% normal swine serum, 0.1% NaN3, and 0.1% [wt/vol] saponin), and incubated with 10 μg of mouse anti-bovine IFN-γ (MCA 1783, IgG1; Serotec)/ml or isotype control antibody. Cells were washed (FACS buffer), stained with rat anti-mouse IgG1-PE (0.06 μg/ml; Southern Biotechnology Associates) for detection of intracellular IFN-γ, and analyzed by flow cytometry.
Nitric oxide assay.
Nitrite is the stable oxidation product of NO, and the amount of nitrite within culture supernatants is indicative of the amount of NO produced by cells in culture. Nitrite was measured by using the Griess reaction (41) performed in 96-well microtiter plates (Immunolon 2; Dynatech Laboratories, Inc., Chantilly, Va.). Culture conditions were as described above for the cell culture procedure (2 × 105 cells/well, 4 replicates/treatment), and supernatants were harvested after 48- and 72-h incubations at 37°C in a 5% CO2 humidified chamber. Culture supernatant (100 μl) was mixed with 100 μl of Griess reagent (0.5% sulfanilamide; Sigma) in 2.5% phosphoric acid (Mallinckrodt Chemicals, Inc., Paris, Ky.) and 0.05% N-(1-naphthyl) ethylenediamine dihydrochloride (Sigma). The mixture was incubated at 21°C for 10 min. Absorbances of test and standard samples at 550 nm were measured by using an automated ELISA plate reader (Molecular Devices, Menlo Park, Calif.). All dilutions were made by using culture medium (RPMI 1640 medium with 2 mM l-glutamine and 10% [vol/vol] FBS). Absorbances of standards, controls, and test samples were converted to nanograms per milliliter of nitrite by comparison with absorbances of sodium nitrite (Fisher Chemicals, Fair Lawn, N.J.) standards within a linear curve fit. In prior studies (54), NG-monomethyl-l-arginine (1.15 mM; equimolar to the amount of l-arginine in the culture medium; Calbiochem, La Jolla, Calif.), a competitive inhibitor of the enzyme nitric oxide synthase (NOS), was added to parallel cultures to verify that the nitrite produced was due to the activity of NOS.
ELISA for antibody to M. avium subsp. paratuberculosis.
Antigen used for the assay was prepared from strain 19698 of M. avium subsp. paratuberculosis. Cells were washed and heat-inactivated (100°C for 20 min) and then were processed by a method designed to extract and concentrate lipoarabinomannan (LAM) from the cell wall (26). This procedure involved cell disruption with a French press, protein digestion with proteinase K, and purification by centrifugation and ultrafiltration. Carbohydrate concentration of the final stock antigen was 4.652 mg/ml, as measured by a phenol-sulfuric acid technique (16), and protein concentration was 1.415 μg/ml (Bio-Rad Protein Assay). Stock antigen was diluted 1:4,000 in PBS, pH 7.5, and 100 μl was added to each well of 96-well plates (3911, Falcon; U-bottom, polyvinyl chloride; Becton Dickinson and Co., Franklin Lakes, N.J.). The plates were incubated for 3 days at 4°C and then were sealed and stored at −70°C until use. Prior to addition of sera for the test, plates were washed (EL 404 Microplate Autowasher; Bio-Tek Instruments, Winooski, Vt.) three times in wash buffer (PBS with 1 mM EDTA and 0.05% Tween 20), followed by a 30-min incubation at RT in blocking buffer (Milk Diluent/Blocking Solution Concentrate; Kirkegaard & Perry Laboratories Inc., Gaithersburg, Md.) diluted 1:20 in PBS with 1 mM EDTA and 0.05% Tween 20. Serum samples to be tested were diluted 1:100 in blocking buffer containing 4 μg of Mycobacterium phlei (Allied Monitor, Fayette, Mo.) protoplasmic extract/ml. This extract was prepared by incubating washed cells overnight at 4°C in Tris buffer, pH 8.5, with 0.1% Triton X-100, followed by centrifugation and ultrafiltration of the supernatant for purification. Negative and positive control sera, diluted 1:100 and 1:400, respectively, were included in each plate for determination of test sera sample/positive (S/P) ratios. Each serum was tested in duplicate by using 100 μl per well. Incubation was carried out at 4°C overnight, after which the plate was washed nine times in wash buffer.
Antigen-antibody reactions were detected with an indirect ELISA that used the following conditions for all steps: 100 μl reagent per well, reagent dilutions prepared in blocking buffer, nine washes between each reagent, and incubations at RT. The reaction steps were the following: biotinylated secondary antibody (biotinylated anti-goat IgG heavy and light chains [rabbit origin] [Vector Laboratories, Burlingame, Calif.]) diluted 1:700, incubation for 1 h; avidin (NeutrAvidin Biotin-Binding Protein, Pierce, Rockford, Ill.) diluted 1:2,000, incubation for 30 min; biotinylated alkaline phosphatase (standard, reagent B; Vectastain ABC-Alkaline Phosphatase kit; Vector Laboratories) diluted 1:50, incubation for 30 min; enzyme substrate (BluePhos Microwell Phosphatase Substrate System; Kirkegaard & Perry Laboratories Inc.), incubation (in the dark) until absorbance of the positive control reached 0.400. The S/P ratios of test samples were calculated from absorbance values by using the formula: (sample − negative control)/(positive sample − negative control).
Serological responses were also evaluated by a commercially available kit (IDEXX Laboratories, Westbrook, Maine) according to manufacturer's instructions.
Immunoblot assay.
Antibody responses of calves were evaluated over time by immunoblot analysis. Electrophoresis and immunoblot assays were performed by using previously described procedures (2) with the following modifications. Comparisons of the reactivity of serial serum samples from each of the calves against M. avium subsp. paratuberculosis K10 WCS antigen were conducted by using a slot-blotting device (Bio-Rad). Antigen (K10 WCS) was electrophoresed through preparative 12% (wt/vol) polyacrylamide gels and was transferred to nitrocellulose filters. These filters were placed in a blocking solution consisting of PBS plus 0.1% Tween 20 (PBST) and 2% (wt/vol) bovine serum albumin (PBST-BSA). After blocking, the filters were placed into the slot blot device, and individual sera, diluted 1:200 in PBST-BSA, were added to independent slots. After a 2-h incubation with gentle rocking, blots were washed three times with PBST and were incubated with horseradish peroxidase-conjugated anti-goat IgG heavy and light chains (rabbit origin; Vector Laboratories) diluted 1:20,000 in PBST-BSA for 1.5 h. Blots were again washed three times with PBST and were developed for chemiluminescence in SuperSignal detection reagent (Pierce Chemical Co).
Statistics.
Data were analyzed by either repeated measures, one-way analysis of variance followed by Tukey-Kramer multiple comparisons test, or Student's t test with a commercially available statistics program (InStat 2.00; GraphPAD Software, San Diego, Calif.).
RESULTS
Bacterial culture and clinical status.
Two-week-old calves received four weekly doses (∼4 × 106 CFU/dose) of M. avium subsp. paratuberculosis by direct instillation of the inoculum into the tonsillar crypts. Prior to necropsy, fecal culture and IS900 PCR confirmed M. avium subsp. paratuberculosis colonization in each of the three M. avium subsp. paratuberculosis-inoculated calves. Fecal shedding was intermittent and light (1 to 4 colonies/slant, with an average of 4 replicate slants). M. avium subsp. paratuberculosis was first detected at 146, 167, or 271 days postchallenge for individual calves, respectively. Clinical signs of M. avium subsp. paratuberculosis infection were not observed for any of the calves over the course of the study. Infected calves were euthanized 320 days postchallenge, and macroscopic lesions were not detected. However, M. avium subsp. paratuberculosis was isolated from tonsil, duodenum, ileum, and jejunum as well as ileal, jejunal, and spiral colon lymph nodes. While M. avium subsp. paratuberculosis was isolated from each calf, M. avium subsp. paratuberculosis was not detected in all tissues from each calf. As reported previously for animals with early M. avium subsp. paratuberculosis infection (42), neither histologic lesions nor acid-fast bacteria were detected. Collectively, these data demonstrate that calves did not enter the clinical stage of infection as defined by Cocito et al. (12).
Recall lymphocyte proliferative responses.
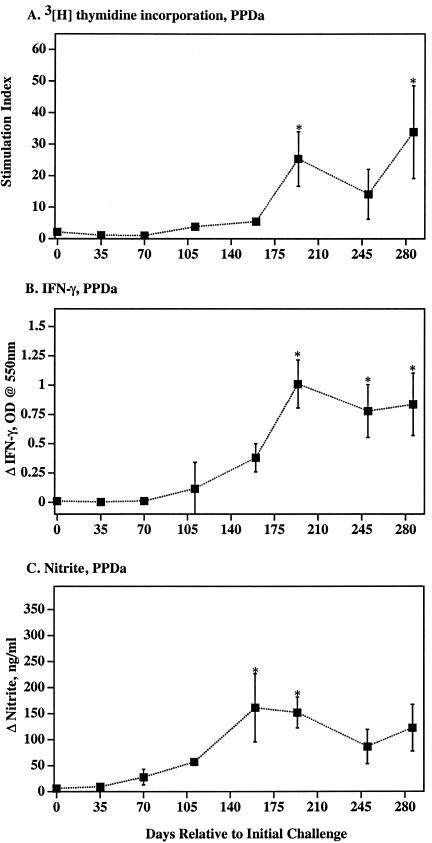
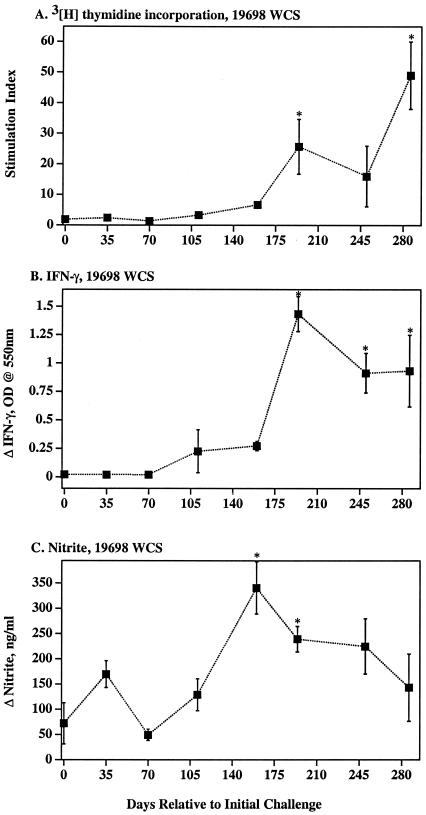
Blood mononuclear cells from naturally infected cattle with subclinical paratuberculosis often exhibit mycobacterium-specific T-cell in vitro proliferative responses (53). At 194 and 286 days postchallenge, antigen-specific DNA synthesis by PBMC from M. avium subsp. paratuberculosis-infected calves exceeded (P < 0.05) prechallenge responses (Fig. 1 and 2). Antigen-specific lymphocyte proliferation was confirmed by a flow cytometric method with significant (P < 0.05) responses by CD4+ and γδ TCR+ cell subsets (Table 1). Percentages and actual numbers of CD4+ cells, but not CD8+ or γδ TCR+ cells, increased in PBMC cultures from infected calves upon mycobacterial antigen stimulation (data not shown). Additionally, stimulation of PBMC cultures from infected animals resulted in increased percentages of CD4+ cells expressing CD26 and CD45RO (Table 2), indicative of activation of memory T cells.
FIG. 1.
Cellular immune response kinetics to M. avium PPDa. Mean (± SEM) DNA synthesis (A), IFN-γ (B), and NO (C) responses by PBMC from M. avium subsp. paratuberculosis-infected calves (closed squares) (n = 3). DNA synthesis was measured by [3H]thymidine uptake, IFN-γ was measured by ELISA, and nitrite (as an indication of NO synthesis) was measured by Griess reaction. *, Differs (P < 0.05) from response at day 0 (i.e., prechallenge response).
FIG. 2.
Cellular immune response kinetics to M. avium subsp. paratuberculosis strain 19698 WCS. Mean (± SEM) DNA synthesis (A), IFN-γ (B), and NO (C) responses by PBMC from M. avium subsp. paratuberculosis-infected calves (closed squares) (n = 3) are depicted. DNA synthesis was measured by [3H]thymidine uptake, IFN-γ was measured by ELISA, and nitrite (as an indication of NO synthesis) was measured by Griess reaction. *, Differs (P < 0.05) from response at day 0 (i.e., prechallenge response.
TABLE 1.
Lymphocyte subset proliferation to strain 19698 WCSa
| Group | Proliferation by subset:
|
||||
|---|---|---|---|---|---|
| Ungatedb | CD4+ | CD8+ | γδ TCR+ | IgM+ | |
| Infected (n = 3) | 3,937 (422)** | 2,013 (524)* | 129 (120) | 1,215 (325)* | 1,007 (382) |
| Noninfected (n = 3)c | 59 (59) | 105 (97) | 0 (0) | 0 (0) | 496 (250) |
Data represent the mean (± SEM) number of cells that proliferated to 10 μg of strain 19698 WCS/ml minus the response to no stimulation per 10,000 PBMC, 286 days postchallenge. Similar responses by infected animals to strain K10 WCS at 286 days postchallenge and to strain 19698 WCS at 211 days postchallenge were also detected (data not shown). *, P < 0.05; **, P < 0.01, differs from responses of noninfected animals, same subset (i.e., vertical comparisons).
Ungated refers to response of the total live PBMC population.
Noninfected animals were of the same age, breed, sex, and herd origin as infected animals, but they were housed outdoors at a local dairy facility (infected animals were housed indoors at the National Animal Disease Center).
TABLE 2.
Percent expression of CD45RO and CD26 on CD4+ cells from M. avium subsp. paratuberculosis- infected cattle upon antigen stimulation
| Subseta | % Expression
|
|||
|---|---|---|---|---|
| Freshly isolated | Medium | PPDa | WCS | |
| CD45RO− | 42.3 (2.85) | 52.3 (3.18) | 4.0 (0)*** | 3.0 (0.58)*** |
| CD45RO+ | 56.7 (2.33) | 45.3 (2.60) | 95.3 (0.67)*** | 97.0 (1.00)*** |
| CD26− | 70.3 (1.86) | 89.7 (2.73) | 19.5 (6.5)** | 30.3 (11.85)* |
| CD26+ | 29.3 (2.19) | 7.3 (3.38) | 80.5 (6.50)** | 69.3 (12.17)* |
At 286 days postchallenge, PBMC were cultured with either medium alone, 4 μg of PPDa/ml, or 20 μg of strain K10 WCS/ml for 7 days, harvested, and analyzed by flow cytometry. Data represent CD26 or CD45RO expression [mean percent positive or negative (± SEM)] by CD4+ cells. *, P < 0.05; **, P < 0.01; ***, P < 0.001; differs from response to media alone and expression by freshly isolated PBMC, same subset (i.e., horizontal comparisons).
Recall IFN-γ and NO responses.
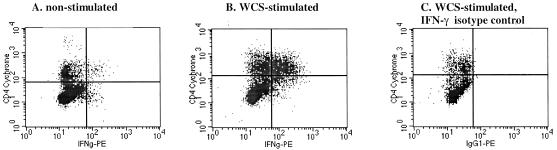
Both IFN-γ and NO are essential for proficient killing of highly virulent mycobacteria (14, 18, 19, 21, 22, 32, 35). The role for IFN-γ and particularly NO in the killing of less virulent mycobacteria, including M. avium, is less clear (1, 17, 56, 59). As with DNA synthesis, antigen-specific IFN-γ responses by PBMC from infected calves exceeded (P < 0.05) prechallenge responses beginning 194 days postchallenge (Fig. 1 and 2). Antigen-specific NO exceeded (P < 0.05) prechallenge responses at 160 and 194 days postchallenge (Fig. 1 and 2). DNA synthesis, IFN-γ, and NO responses to strain K10 WCS stimulation (data not shown) were similar to responses to strain 19698 WCS and PPDa. In general, DNA synthesis, IFN-γ, and NO responses followed similar kinetics and magnitude of response (Fig. 1 and 2). As determined for calves receiving M. avium subsp. paratuberculosis by an oral route (4), CD4+ cells were the predominant subset of T cells producing IFN-γ (7 days poststimulation) in response to soluble mycobacterial antigens (Table 3 and Fig. 3).
TABLE 3.
Intracellular staining for IFN-γ production by lymphocyte subsets in response to mycobacterial antigen stimulation
| Treatmenta | IFN-γ production by subset:
|
|||
|---|---|---|---|---|
| Ungatedb | CD4+ | CD8+ | γδ TCR+ | |
| No stimulation | 1.07 (0.49) | 0.51 (0.22) | 0.34 (0.22) | 0.11 (0.11) |
| WCS stimulation | 12.16 (4.57)* | 9.65 (3.27)** | 0.92 (0.44) | 0.63 (0.16) |
| PPDa stimulation | 7.10 (2.65) | 3.92 (1.10) | 0.97 (0.71) | 0.68 (0.32) |
At 313 days postchallenge, PBMC were cultured with either medium alone, 5 μg of PPDa/ml, or 10 μg of strain 19698 WCS (WCS/ml) for 7 days (ionomycin, PMA, and brefeldin A added for the terminal 5 h), harvested, and analyzed by flow cytometry for phenotype and intracellular IFN-γ by flow cytometry. Data are presented as mean (±SEM, n = 3) percent of cells staining positive for IFN-γ.
Ungated refers to the response of the total live PBMC population. Differs (*, P = 0.07; **, P < 0.05) from nonstimulated samples, same subset (i.e., vertical comparisons within group). Responses were not detected for samples obtained from noninfected animals of the same sex, age, breed, and herd of origin as infected animals (data not shown; n = 3).
FIG. 3.
IFN-γ response by CD4+ cells to M. avium subsp. paratuberculosis strain 19698 WCS. At 313 days postchallenge, PBMC were cultured with either medium alone (A) or with 10 μg strain 19698 WCS/ml (B) for 7 days (ionomycin, PMA, and brefeldin A were added for the terminal 5 h), harvested, and analyzed by flow cytometry for CD4 expression and intracellular IFN-γ. CD4+ and IFN-γ+ are in the upper right quadrants. Panel C was stained with the isotype control antibody for IFN-γ.
Serum Ig response.
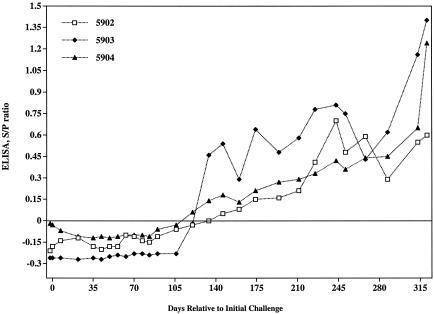
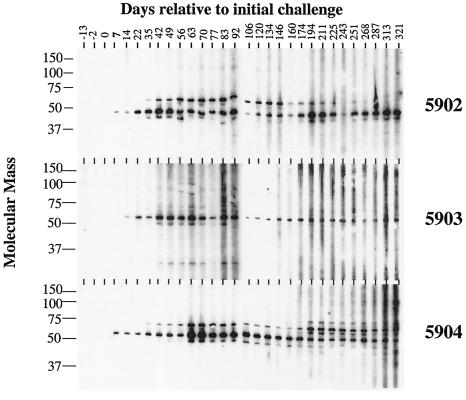
In contrast to previous dogma, it has recently been determined that humoral responses to M. avium subsp. paratuberculosis are detectable early after infection (29). By using an ELISA-based assay to detect antibody to LAM antigen, Mycobacterium-specific antibody was detected as early as 134 days postchallenge (Fig. 4), and this response followed kinetics similar to those of the cellular response (Fig. 1 and 2). A commercially available ELISA kit (IDEXX Laboratories) did not detect serum antibody responses in these calves at any time point during the study. Analysis of serial serum samples from each calf by immunoblot showed reactivity to a ∼50-kDa protein in K10 WCS protein lysates beginning 7 to 14 days postchallenge and continuing throughout the study (Fig. 5). A ∼60-kDa protein was also detected by day 35 in animals 5902 and 5904. Reactivity to the 60-kDa antigen decreased over time in animal 5902 and was variable in intensity in animal 5904. Immunoblot analysis of serum samples from four M. bovis-infected cattle (housed in a manner similar to that of M. avium subsp. paratuberculosis-infected calves, intratonsillarly inoculated, and ∼1 year of age) did not react to the 50- and 60-kDa antigens (data not shown), demonstrating the specificity of these two antigens.
FIG. 4.
Response kinetics of serum antibody specific for LAM-enriched antigen. Serum from M. avium subsp. paratuberculosis-infected animals was collected at the indicated time points relative to the initial challenge and was analyzed for reactivity to LAM by ELISA. The LAM antigen used for the assay was prepared from strain 19698 of M. avium subsp. paratuberculosis as described previously (26). This procedure involved cell disruption with a French press, protein digestion with proteinase K, and purification by centrifugation and ultrafiltration. The S/P ratios of test samples were calculated from absorbance values by using the formula (sample − negative control)/(positive sample − negative control).
FIG. 5.
Preparative immunoblots of M. avium subsp. paratuberculosis strain K10 WCS antigen probed with sera from experimentally infected cattle. Note that serum samples from the initial three bleed dates prior to challenge show no reactivity. Animal numbers are indicated in the right margin, days relative to initial challenge of sampling are indicated along the top margin, and protein size markers (in kilodaltons) are indicated in the left margin.
DISCUSSION
The primary objective of this study was to determine if inoculation of M. avium subsp. paratuberculosis into the tonsillar crypts of neonatal calves would result in infection and/or a detectable immune response, as previously demonstrated with M. bovis (37). Colonization of gastrointestinal tissues and fecal shedding of M. avium subsp. paratuberculosis were indicative of infection. Both humoral and cellular immune responses were also detected. Specific CD4+ cell proliferative and IFN-γ responses occurred concurrently with humoral responses to a LAM-enriched antigen preparation. Both cellular responses and LAM-specific antibody were indicative of infection and not simply sensitization without colonization, as they were not detected until 4 to 5 months after the final M. avium subsp. paratuberculosis inoculation. Despite detection of a specific immune response and intestinal colonization, intestinal lesions were not detected in inoculated calves. Natural progression of cattle to clinical disease and associated lesion formation generally occur after a prolonged subclinical period of at least 3 to 5 years. More prolonged studies are under way to determine if this route of inoculation will induce clinical disease.
Sera from inoculated calves reacted with at least two M. avium subsp. paratuberculosis antigens by immunoblot analysis within 2 weeks after the initial intratonsillar challenge, and this antibody response persisted to the termination of the experiment. While the identity of these antigens is unknown, efforts to identify them from a M. avium subsp. paratuberculosis genomic expression library are being pursued. In contrast to prevailing dogma (10, 43), present findings (in conjunction with another recent report by Koets et al. [29]) indicate that tests for M. avium subsp. paratuberculosis-specific antibody may provide an early diagnosis of M. avium subsp. paratuberculosis-infected cattle. A serologic assay for detection of recently infected calves would greatly enhance paratuberculosis eradication efforts and likely prove more practical than cell-based assays.
Present findings confirm the predominant contribution of CD4+ cells to the peripheral IFN-γ (4) and proliferative response during the early, subclinical course of infection. Responding CD4+ cells also upregulated CD26 and CD45RO expression, indicating conversion to an effector-memory phenotype. Progression of cattle from subclinical to clinical Johne's diseases is associated with a decreased ability of mononuclear cells to produce IFN-γ, both specifically and nonspecifically, at the site of infection and in the blood (30, 44, 46, 51 and reviewed in references 10 and 43). Unlike tuberculous mycobacteria (20), M. avium subsp. paratuberculosis is relatively resistant to the killing actions of IFN-γ and NO (56, 59, 60). Additionally, the ability of M. avium subsp. paratuberculosis to induce IL-10 secretion, both in vitro and at the site of infection, likely suppresses macrophage activation and subsequent intracellular killing ability (15, 27, 56). Compartmentalization and loss of specific effector cells, both host and pathogen mediated, also limit containment of M. avium subsp. paratuberculosis (11, 28). Additional studies are under way to further characterize the sustainability of this response as experimentally infected cattle progress from subclinical to clinical disease.
LAM-based ELISA assays have demonstrated utility for diagnosis of mycobacterial diseases, including M. avium subsp. paratuberculosis infection (23, 26, 29, 33, 48-50). It is speculated that selective binding of the lipid portion of LAM to the test plate leaves the more immunogenic carbohydrate portion of the molecule free to react with antibody in the sample. In the present study, sensitivity of the LAM-based assay was maximized through use of a very low M. phlei concentration for preabsorption of sera to remove cross-reactive antibodies against antigenically similar bacteria. Failure of the commercial ELISA to detect an antibody response may have been due to preabsorption with a higher M. phlei concentration, or the commercial test may have been designed to detect only antibodies against protoplasmic protein antigens expressed later in the course of the disease. Utilization of comparative genomics to uncover unique M. avium subsp. paratuberculosis genes (especially in comparison to other M. avium species) will be necessary to develop antigens specifically recognized by samples from M. avium subsp. paratuberculosis-infected animals. Experimental models of infection, as described in this study, should prove useful for the evaluation of such unique antigens.
Two compelling research issues concerning M. avium subsp. paratuberculosis are (i) the mechanisms responsible for progression of cattle from subclinical to clinical disease and (ii) the potential for early diagnosis. An experimental model(s) of infection, as described in this report, will be necessary to fully address these two issues. Present findings indicate that mycobacteria-specific antibody is detectable early in the course of disease and concurrently with a robust CD4+, IFN-γ response. Further studies are under way to evaluate and compare responses of cattle experimentally infected with M. avium subsp. paratuberculosis, M. avium subsp. avium, or M. bovis to develop tools for specific mycobacterial disease diagnosis and to evaluate specific immunopathogenesis.
Acknowledgments
We thank Dennis Orcutt, Janis Hansen, Trudy Bosworth, Shelly Zimmerman, Theresa Rahner, and Rebecca Lyon for technical support and Nate Horman, John Kent, and Larry Wright for animal care.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Appelberg, R., I. S. Leal, T. F. Pais, J. Pedrosa, and M. Florida. 2000. Differences in resistance of C57BL/6 and C57BL/10 mice to infection by Mycobacterium avium are independent of gamma interferon. Infect. Immun. 68:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannantine, J., and J. R. Stabel. 2000. HspX is present within Mycobacterium paratuberculosis-infected macrophages and is recognized by sera from some infected cattle. Vet. Microbiol. 76:343-358. [DOI] [PubMed] [Google Scholar]
- 3.Barrow, P. A., and J. Gallagher. 1981. Aspects of the epidemiology of bovine tuberculosis in badgers and cattle. I. The prevalence of infection in two wild animal populations in south-west England. J. Hyg. 86:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassey, E. O., and M. T. Collins. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 65:4869-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard, P. M., K. Stevenson, A. Pirie, K. Rudge, D. Buxton, S. M. Rhind, M. C. Sinclair, L. A. Wildblood, D. G. Jones, and J. M. Sharp. 2001. Experimental paratuberculosis in calves following inoculation with a rabbit isolate of Mycobacterium avium subsp. paratuberculosis. J. Clin. Microbiol. 39:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard P. M., M. J. Daniels, D. Henderson, A. Pirie, K. Rudge, D. Buxton, S. Rhind, A. Greig, M. R. Hutchings, I. McKendrick, K. Stevenson, and J. M. Sharp. 2001. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedictus, G., A. A. Dijkhuizen, and J. Stelwagen. 1987. Economic losses due to paratuberculosis in dairy cattle. Vet. Rec. 121:142-146. [DOI] [PubMed] [Google Scholar]
- 8.Burton, J. L., and M. E. Kehrli. 1996. Effects of dexamethasone on bovine circulating T lymphocyte populations. J. Leukoc. Biol. 59:90-99. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy, J. P., D. G. Bryson, and S. D. Neill. 1999. Tonsillar lesions in cattle naturally infected with Mycobacterium bovis. Vet. Rec. 144:139-142. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini, R. J. 1996. Immunology: resistance to paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:313-341. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini, R. J., and W. C. Davis. 1992. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb. Pathog. 13:447-463. [DOI] [PubMed] [Google Scholar]
- 12.Cocito, C., P. Gilot, M. Coene, M. de Kesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman, J. D. 1988. Distribution, prevalence and epidemiology of bovine tuberculosis in brushtail possums, Trichosurus vulpecula. Austr. Wildl. Res. 15:651-663. [Google Scholar]
- 14.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 78:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens, P. M., C. J. Colvin, K. Wiersma, A. Abouzied, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric methods for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 17.Ehlers, S., S. Kutsch, J. Benini, A. Cooper, C. Hahn, J. Gerdes, I. Orme, C. Martin, and E. T. Rietschel. 1999. NOS2-derived nitric oxide regulates the size, quantity and quality of granuloma formation in Mycobacterium avium-infected mice without affecting bacterial loads. Immunology 98:313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flesch, I. E., and S. H. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flesch, I., and S. H. Kaufmann. 1987. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:4408-4413. [PubMed] [Google Scholar]
- 20.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 21.Flynn, J. L., C. A. Scanga, K. E. Tanaka, and J. Chan. 1998. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 160:1796-1803. [PubMed] [Google Scholar]
- 22.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaborick, C., M. D. Salman, R. P. Ellis, and J. Triantis. 1996. Evaluation of a five-antigen ELISA for diagnosis of tuberculosis in cattle and Cervidae. J. Am. Vet. Med. Assoc. 209:962-966. [PubMed] [Google Scholar]
- 24.Greig, A., K. Stevenson, D. Henderson, V. Perez, V. Hughes, I. Pavlik, M. E. Hines, I. McKendrick, and J. M. Sharp. 1999. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J. Clin. Microbiol. 37:1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jark, U., I. Ringena, B. Franz, G. F. Gerlach, M. Beyerbach, and B. Franz. 1997. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet. Microbiol. 51:189-198. [DOI] [PubMed] [Google Scholar]
- 27.Khalifeh, M. 2003. Ph.D. thesis. Iowa State University, Ames.
- 28.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Muller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of γδ T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koets, A. P., V. P. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, H., J. R. Stabel, and M. E. Kehrli, Jr. 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82:73-85. [DOI] [PubMed] [Google Scholar]
- 31.Lugton, I. W. 1999. Mucosa-associated lymphoid tissues as sites for uptake, carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunol. Cell Biol. 77:364-372. [DOI] [PubMed] [Google Scholar]
- 32.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, R. A., S. Dissanayake, and T. M. Buchanan. 1983. Development of an enzyme-linked immunosorbent assay using arabinomannan from Mycobacterium smegmatis: a potentially useful screening test for the diagnosis of incubating leprosy. Am. J. Trop. Med. Hyg. 32:555-564. [DOI] [PubMed] [Google Scholar]
- 34.Momatani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 35.Ottenhof, T. H., D. Kumararatne, and J. L Casanova. 1998. Novel human immunodeficiency reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 36.Palmer, M. V., D. L. Whipple, K. L. Butler, S. D. Fitzgerald, C. S. Brunning-Fann, and S. M. Schmitt. 2002. Tonsillar lesions in white-tailed deer (Odocoileus virginianus) naturally infected with Mycobacterium bovis. Vet. Rec. 151:149-150. [DOI] [PubMed] [Google Scholar]
- 37.Palmer, M. V., D. L. Whipple, J. C. Rhyan, C. A. Bolin, and D. A. Saari. 1999. Granuloma development in cattle after intratonsilar inoculation with Mycobacterium bovis. Am. J. Vet. Res. 60:310-315. [PubMed] [Google Scholar]
- 38.Payne, J. M., and J. B. Derbyshire. 1963. Portals of entry for bacterial infection in calve and piglets with particular reference to the tonsil. J. Pathol. Bacteriol. 85:171-178. [DOI] [PubMed] [Google Scholar]
- 39.Payne, J. M., and J. D. Rankin. 1961. The pathogenesis of experimental Johne's disease in calves. Res. Vet. Sci. 2:167-174. [Google Scholar]
- 40.Payne, J. M., and J. D. Rankin. 1961. A comparison of the pathogenesis of experimental Johne's disease in calves and cows. Res. Vet. Sci. 2:175-179. [Google Scholar]
- 41.Rajaraman, V., B. J. Nonnecke, S. T. Franklin, D. C. Hammell, and R. L. Horst. 1998. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy Sci. 81:3278-3285. [DOI] [PubMed] [Google Scholar]
- 42.Rideout, B., S. T. Brown, W. C. Davis, J. M. Gay, R. A. Giannella, M. E. Hines II, W. D. Hueston, and L. J. Hutchinson. 2003. Diagnosis and control of Johne's disease. The National Academies Press, Washington, D.C. [PubMed]
- 43.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 44.Stabel, J. R. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61:754-760. [DOI] [PubMed] [Google Scholar]
- 45.Stabel, J. R. 1997. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other models. J. Vet. Diagn. Investig. 9:375-380. [DOI] [PubMed] [Google Scholar]
- 46.Stabel, J. R. 1996. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 47.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. S. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection an immunological and microbiological study. Vet. Immunol. Immunopathol. 80:271-287. [DOI] [PubMed] [Google Scholar]
- 48.Sugden, E. A., K. Stilwell, and A. Michaelides. 1997. A comparison of lipoarabinomannan with other antigens used in the absorbed enzyme immunoassays for the serological detection of cattle infected with Mycobacterium paratuberculosis. J. Vet. Diagn. Investig. 9:413-417. [DOI] [PubMed] [Google Scholar]
- 49.Sugden, E. A., A. H. Corner, B. S. Samagh, B. W. Brooks, C. Turcotte, K. H. Nielsen, R. B. Stewart, and J. R. Duncan. 1989. Serodiagnosis of ovine paratuberculosis, using lipoarabinomannan in an enzyme-linked immunosorbent assay. Am. J. Vet. Res. 6:850-854. [PubMed] [Google Scholar]
- 50.Sugden, E. A., B. S. Samagh, D. R. Bundle, and J. R. Duncan. 1987. Lipoarabinomannan and lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect. Immun. 55:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney, R. W., D. E. Jones, P. Habecker, and P. Scott. 1998. Interferon-γ and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 59:842-847. [PubMed] [Google Scholar]
- 52.Vary, P. H., P. R. Andersen, E. Green, J. Hermon-Taylor, and J. J. McFadden. 1990. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J. Clin. Microbiol. 28:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters, W. R., J. R. Stabel, R. E. Sacco, J. A. Harp, B. A. Pesch, and M. J. Wannemuehler. 1999. Antigen-specific B cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect. Immun. 67:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters, W. R., B. J. Nonnecke, T. E. Rahner, M. V. Palmer, D. L. Whipple, and R. L. Horst. 2001. Modulation of Mycobacterium bovis specific responses of bovine peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin. Diagn. Lab. Immunol. 8:1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waters, W. R., M. V. Palmer, B. A. Pesch, S. C. Olsen, M. J. Wannemuehler, and D. L. Whipple. 2003. Expression of L-selectin (CD62L), CD44, and CD25 on activated bovine T cells. Infect. Immun. 71:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wells, S. J., S. L. Ott, and A. H. Seitzinger. 1998. Key health issues for dairy cattle-new and old. J. Dairy Sci. 81:3029-3035. [DOI] [PubMed] [Google Scholar]
- 58.Williams, E. S., S. P. Snyder, and K. L. Martin. 1983. Experimental infection of some North American wild ruminants and domestic sheep with Mycobacterium paratuberculosis: clinical and bacteriological findings. J. Wildl. Dis. 19:185-191. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, B., M. T. Collins, and C. J. Czuprynski. 1997. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect. Immun. 65:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurbrick, B. G., and C. J. Czuprynski. 1987. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect. Immun. 55:1588-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]