Abstract
Antibodies are believed to play a role in the protection against Candida albicans infections by a number of mechanisms, including the inhibition of adhesion or germ tube formation, opsonization, neutralization of virulence-related enzymes, and direct candidacidal activity. Although some of these biological activities have been demonstrated individually in monoclonal antibodies (MAbs), it is not clear if all these anti-C. albicans activities can be displayed by a single antibody. In this report, we characterized a monoclonal antibody raised against the main target of salivary secretory immunoglobulin A in the cell wall of C. albicans, which exerts three anti-C. albicans activities: (i) inhibition of adherence to HEp-2 cells, (ii) inhibition of germination, and (iii) direct candidacidal activity. MAb C7 reacted with a proteinic epitope from a mannoprotein with a molecular mass of >200 kDa predominantly expressed on the C. albicans germ tube cell wall surface as well as with a number of antigens from Candida lusitaniae, Cryptococcus neoformans, Aspergillus fumigatus, and Scedosporium prolificans. MAb C7 caused a 31.1% inhibition in the adhesion of C. albicans to HEp-2 monolayers and a 55.3% inhibition in the adhesion of C. albicans to buccal epithelial cells, produced a 38.5% decrease in the filamentation of C. albicans, and exhibited a potent fungicidal effect against C. albicans, C. lusitaniae, Cryptococcus neoformans, A. fumigatus, and S. prolificans, showing reductions in fungal growth ranging from 34.2 to 88.7%. The fungicidal activity showed by MAb C7 seems to be related to that reported by antibodies mimicking the activity of a killer toxin produced by the yeast Pichia anomala, since one of these MAbs also reacted with the C. albicans mannoprotein with a molecular mass of >200 kDa. Results presented in this study support the concept of a family of microbicidal antibodies that could be useful in the treatment of a wide range of microbial infections when used alone or in combination with current antimicrobial agents.
Members of genus Candida, and in particular Candida albicans, are opportunistic pathogens that are frequently isolated from the mucosal surfaces of normal individuals. In patients with predisposing conditions, including immunodeficiencies, pregnancy, diabetes mellitus, or those necessitating the use of broad-spectrum antibiotics, C. albicans may produce mucosal infections such as oral and vaginal candidiasis (34). Among the variety of mechanisms that are believed to play a role in the protection against C. albicans at the mucosal surfaces, secretory immunoglobulin A (sIgA) is thought to play a key role by inhibiting Candida adherence to host cells (12, 13, 40). However, it has been reported that antibodies can exert anti-Candida activities, such as inhibition of germination and direct candidacidal activity, which may also contribute to the ability to control Candida multiplication at the mucosal surfaces. Casanova et al. (6) described the inhibition of germ tube formation by Fab fragments from a monoclonal antibody (MAb) directed against an antigen specifically expressed on the germ tube surface, and San Millán et al. (35) described two monoclonal antibodies directed against two antigens expressed on the cell wall surface that decreased the filamentation of C. albicans. A monoclonal anti-idiotypic antibody mimicking the activity of a killer toxin from the yeast Pichia anomala has been shown to be candidacidal in vitro (31) and to confer significant immunoprotection against mucosal candidiasis (18). This antibody proved to be representative of the protective mucosal and systemic humoral immune response elicited in mice by intravaginal and parenteral idiotypic vaccination with a yeast killer toxin-neutralizing MAb (27, 28). Interestingly, candidacidal anti-killer toxin cell wall receptor sIgA antibodies have been identified in the vaginal fluid of women affected by recurrent vulvovaginal candidiasis, suggesting that these antibodies may be naturally present in humans as part of the humoral defensive repertoire (30).
In previous studies, we have demonstrated that salivary sIgA reacts with a group of stress mannoproteins located on the cell wall surface—mannoproteins whose expression is modulated by a number of factors, including the temperature of growth, composition of growth medium, yeast-mycelium transition, and pH (3, 29, 31, 39). We have also demonstrated that salivary sIgA inhibits the adhesion of C. albicans to plastic and composite restorative dental materials and that the inhibitory effect can be mimicked by MAbs directed against cell wall antigens of C. albicans (24, 36). In this report, we have characterized a monoclonal antibody raised against the main target of salivary sIgA in the cell wall of C. albicans in an attempt to assess whether the monoclonal antibody shows—in addition to the ability to inhibit adhesion of C. albicans to host surfaces—other biological properties such as fungicidal activity, which may be implicated in the protection against mucosal candidiasis.
MATERIALS AND METHODS
Fungal strains and culture conditions.
The strains used in this study were obtained from the National Collection of Pathogenic Fungi (NCPF; Bristol, United Kingdom), the American Type Culture Collection (Manassas, Va.) or the Colección Española de Cultivos Tipo (Valencia, Spain) and included C. albicans serotype A NCPF 3153, Candida lusitaniae ATCC 200951, Cryptococcus neoformans ATCC 90113, Aspergillus fumigatus CECT 2071, and Scedosporium prolificans NCPF 2799. The strains were maintained at 4°C on slants containing 20 g of glucose, 10 g of yeast extract, and 20 g of agar per liter. C. albicans yeast cells and germ tubes were obtained in medium 199 (Sigma Chemical Co., St. Louis, Mo.) as previously described (33). Briefly, 48-h-old blastospores grown in Sabouraud Dextrose Agar plates were transferred to Erlenmeyer flasks containing medium 199 at 5 × 107 blastospores/ml, and they were incubated at 25°C for 18 h in a rotatory shaker set at 200 rpm. After incubation, blastospores were harvested by centrifugation at 1,000 × g for 10 min and inoculated in new medium at 25°C for 24 h at 200 rpm to obtain blastospores or at 37°C for 4 h at 200 rpm to obtain germ tubes. A. fumigatus and S. prolificans conidia, as well as Cryptococcus neoformans and C. lusitaniae blastospores, were grown in medium 199 at 25°C for 18 h at 200 rpm. The fungal cells were washed in phosphate-buffered saline (PBS) and adjusted to the appropriate concentrations by hemocytometer counting.
In one experiment, the expression of the C7 epitope in Lee's medium (17), horse serum (Difco, Detroit, Mich.) and Sabouraud broth (40 g of glucose and 10 g of peptone per liter) at both 25 and 37°C for 0.5, 1, 1.5, 2, 3, 4, 6, and 24 h was also assessed.
Antibodies.
The experimental protocols were approved by the Institutional Review Board of the School of Medicine and Odontology at the University of Basque Country, and all the subjects gave informed consent prior to participation. Unstimulated whole saliva from healthy C. albicans carriers and uninfected individuals was collected. In every case, the saliva was centrifuged at 400 × g for 10 min and the supernatant was stored at −80°C until used. The saliva samples were studied by indirect immunofluorescence assay (IFA) against different C. albicans oral isolates, and the most reactive saliva samples were used.
Monoclonal antibodies were produced by following standard methods with splenocytes from BALB/c mice immunized by intraperitoneal injections of a C. albicans high-molecular-weight stress mannoprotein recognized by salivary sIgA (32, 33). The animal studies were performed according to the guidelines of the Department of Agriculture of the Basque Government. For comparative purposes, a candidacidal anti-idiotypic antibody (i.e., MAb K10) representing the internal image of a P. anomala killer toxin characterized by a large spectrum of microbicidal activity against microorganisms presenting specific cell wall receptors was also studied. MAbs were purified from ascites fluid by affinity chromatography on an ImmunoPure IgM purification kit (Pierce, Rockford, Ill.) essentially as described by the manufacturer. Purified antibodies were dialyzed against PBS and filter sterilized before use.
In one experiment, MAb C7 was adsorbed with C. albicans germ tubes by incubating the purified MAb with an equal volume of a 1 × 1010 formalin-killed germ tubes/ml suspension in saline for 2 h at room temperature. After incubation, the suspension was centrifuged and the supernatant was recovered. The adsorption was repeated, and the absence of MAb C7 in the supernatant was checked by Western blotting.
Immunofluorescence.
IFA was carried out as described previously (1). Briefly, the fungal species studied were grown on Sabouraud agar plates for 48 h at 24 or 37°C, resuspended in PBS at a cell density of 106 cells/ml, and placed on Teflon-coated immunofluorescence slides. The slides were incubated with the MAbs or saliva samples diluted 1:5 in PBS supplemented with Evans blue (0.05% [wt/vol]) and Tween 20 (0.05% [vol/vol] PBS-Tween-Evans blue) and washed, and the reacting antibodies were revealed by incubation with fluorescein isothiocyanate-conjugated goat anti-mouse IgM or goat anti-human IgA (Sigma) diluted 1:150 in PBS-Tween-Evans blue) for 30 min at 37°C. Slides were mounted with carbonate-glycerol mounting fluid and examined with an epifluorescence microscope.
Antigenic extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting.
Cell walls of the different fungi studied were extracted in the presence of dithiothreitol (DTT) as described by Smail and Jones (37). In some experiments, removal of carbohydrate groups from cell wall antigens was accomplished either by oxidation with sodium meta-periodate or with a GlycoFree deglycosylation kit (Glyko, Inc., Bicester, United Kingdom). For sodium meta-periodate oxidation, DTT antigenic extracts transferred to Immobilon membranes (Millipore Ibérica, Madrid, Spain) were treated with 0.05 M sodium meta-periodate in 0.05 M acetate buffer (pH 4.5) for 18 h at 4°C and, after washing with the buffer, reactive groups were blocked with 1% glycine in acetate buffer. In the GlycoFree deglycosylation kit, N- and O-linked glycosyl moieties were removed from the eluted >200-kDa antigen with anhydrous trifluoromethanesulfonic acid according to the manufacturer's instructions.
SDS-PAGE was performed by the method of Laemmli (16) in a minigel system (Bio-Rad Laboratories, Richmond, Calif.). The total amount of protein loaded per lane was 15 to 20 μg for each extract. Electrophoresis was carried out in 10% (wt/vol) acrylamide gels at 200 V for 45 min. Standard molecular weight markers were obtained from Bio-Rad or Invitrogen (Invitrogen S. A., Barcelona, Spain). Subsequently, the gels were either stained with Coomassie blue or were electrophoretically transferred to an Immobilon membrane (Millipore Ibérica) by using a fast blot system (Biometra, Göttingen, Germany). After the transfer, the membranes were washed in Tris-buffered saline, dried, and incubated with a 1:10 dilution of the saliva or with a 1:20 dilution of the MAbs. After washing, they were incubated with peroxidase-labeled affinity-purified goat anti-human IgA or goat anti-mouse IgM (Sigma). Immunoreactive bands were visualized after staining for 30 min with a substrate solution (0.05% [wt/vol] 4-chloro-1-naphtol [Sigma] and 0.015% [vol/vol] H2O2 in Tris-buffered saline).
In some experiments, proteins were eluted after electrophoresis by using the Bio-Rad Mini Whole-Gel Eluter. Briefly, polyacrylamide gels were overlaid on the Mini Whole-Gel Eluter, and the individual bands were eluted into separate fractions in 50 mM Tris-25 mM Boric acid (pH 8.7) buffer at 100 mA for 20 min. After collection by vacuum harvesting, individual fractions were analyzed by SDS-PAGE and Western blotting with MAb C7. Fractions containing components with molecular masses of >200 kDa reacting with MAb C7 were pooled and stored frozen until used.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was used for the screening of the hybridomas and for identifying the isotype of each MAb and was carried out by a modification of a previously described method (32). Briefly, each well of plates (Costar, Cambridge, Mass.) was coated with 100 μl of either the C. albicans germ tube or blastoconidium extract obtained with DTT and incubated overnight at 4°C. Plates were blocked by adding 200 μl of PBS containing 1% (wt/vol) bovine serum albumin (fraction V; Sigma) for 1 h at 37°C. The extracts were then incubated with the supernatants containing the MAbs (100 μl per well) for 1 h at 37°C. Plates were washed and incubated with peroxidase-conjugated goat anti-mouse Total Immunoglobulin (Sigma) diluted 1:2,000 in PBS-bovine serum albumin containing 0.05% (wt/vol) Tween 20 for 1 h at 37°C. After washing, 100 μl of a solution containing 0.05% (wt/vol) ortho-phenylenediamine dihydrochloride (Sigma) and 0.015% (wt/vol) hydrogen peroxide in phosphate-citrate buffer 0.15 M (pH 5.0) were added to each well, and the plates were incubated in the dark at room temperature for 30 min. The reaction was stopped with 50 μl of 1 N H2SO4, and optical densities were read with an ELISA plate reader (Bio-Tek, Winooski, Vt.) at 450 nm. To characterize the isotype of the selected MAbs, peroxidase-conjugated goat anti-mouse IgM, IgG, or IgA (Sigma) were used in the second incubation instead of the anti-mouse Total Immunoglobulin conjugate.
Adhesion.
HEp-2 cells (a larynx carcinoma cell line from the European Collection of Cell Cultures, Salisbury, United Kingdom) were inoculated at 2 × 105 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum and incubated for 48 h at 37°C and then washed with PBS. The HEp-2 cells' monolayers were then incubated at 37°C for 120 min with 2.5 × 106 C. albicans cells/ml grown to stationary phase in medium 199 (pH 6.7) at 25°C for 18 h. The total number of C. albicans cells was quantified by phase-contrast microscopy in four different fields (0.64 mm2 each). Following a saline wash to remove unbound cells, adhering C. albicans cells were quantified in the same fields. HEp-2 adherence data were expressed as a percentage of the number of adhering cells (Percent adherence = [adhered cells/total] × 100).
Adhesion of C. albicans to human buccal epithelial cells (BECs) was studied by a modification of methods described previously (11, 40). Briefly, BECs from four healthy adults were collected each morning at the same time of the day by gently rubbing the right and left buccal mucosa using sterile wood spatulas and suspended in 10 ml of PBS. The pooled BEC suspension was washed four times in PBS by centrifugation at 1,000 × g for 10 min. Finally, the BECs were resuspended to a concentration of 1 × 105 cells/ml in PBS by hemocytometer counting. C. albicans NCPF 3153 was grown in medium 199 (pH 6.7) for 18 h at 21°C to reach the exponential phase, washed in PBS by centrifugation at 1,000 × g for 5 min, and resuspended to a final concentration of 1 × 107 cells/ml in PBS.
In the adherence assay, 500 μl of the BEC suspension was incubated with 50 μl of the yeast suspension for 2 h at 37°C with gentle agitation in the presence of 20 μg of MAb C7 or MAb C7 adsorbed with C. albicans germ tubes. The cultures were then washed twice with PBS by centrifugation at 1,000 × g for 10 min. Air-dried smears of the sedimented epithelial cells were made on glass slides, fixed in methanol, and Gram stained. The number of adherent yeast cells was quantified by light microscopy at ×400 magnification. Fifty BECs were observed for adherent yeast cells.
Inhibition of germ tube formation.
Quantification of germination by C. albicans cells was performed by a modification of the method described by Cenci et al. (7). Briefly, cells in the stationary phase were inoculated in Sabouraud broth at a final concentration of 5 × 104 cells/ml in the presence of 12.5 μg/ml of the MAb C7 and incubated with gentle agitation at 37°C. After 90 min of incubation, both the total number of cells and the number of yeast cells bearing germ tubes were counted. Germination was quantified with a phase-contrast microscope in four different fields (0.64 mm2 each). Results were expressed as mean values derived from at least four independent assays. An irrelevant IgM (Sigma) was used as control. The percentage of germ tubes per field was calculated by the following equation: Percent filamentation = (number of yeast cells bearing germ tubes/total number of cells) × 100.
Candidacidal activity.
The candidacidal activity of MAbs was performed as described by Magliani et al. (18). Briefly, 150 viable fungal cells suspended in 10 μl of PBS were added to 100 μl of the purified MAb at a concentration of 100 μg/ml and incubated for 18 h at 37°C. An irrelevant IgM (Sigma) was used as control. After incubation with the respective MAbs, the fungal cells were spread on Sabouraud dextrose agar and incubated at 30°C. The number of CFU was recorded after 48 h of incubation. All values quoted represent mean figures derived from four independent assays performed in triplicate. A similar protocol was followed to assess the growth inhibition in C. lusitaniae, Cryptococcus neoformans, A. fumigatus, and S. prolificans.
In one experiment, the candidacidal activity of MAb C7 was studied by the 3-(4,5-dimethythiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction assay. Briefly, 100 μl of a suspension of 5 × 105 cells/ml in PBS was incubated with 10 μg of MAb C7 for 18 h at 37°C in 96-well microtiter plates (Greiner Bio-One GmbH, Frickenhausen, Germany). After centrifugation at 600 × g for 8 min, the supernatants were discarded and 100 μl of MTT (Sigma) at a concentration of 0.5 mg/ml in RPMI 1640 medium (Sigma) was added to each well. The plates were incubated for 4 h at 37°C, and supernatants were discarded after centrifugation at 600 × g for 8 min. One hundred microliters of dimethyl sulfoxide (Sigma) was added to each well, and the formation of the dark formazan product developed by the cells with active mitochondria was read in an ELISA plate reader (Bio-Tek) at a wavelength of 550 nm. An irrelevant IgM (Sigma) was used as control. All values quoted represent mean figures derived from four independent assays performed in triplicate.
Statistics.
The analysis of variance test was used to assess the significance of differences between means in the growth inhibition assays. Values of P < 0.05 were considered significant.
RESULTS
Preliminary characterization of the MAbs.
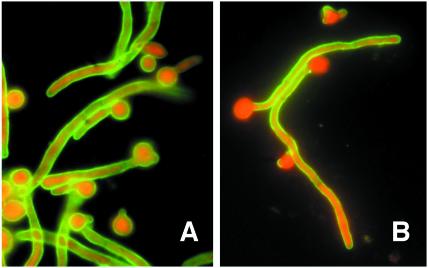
The fusion yielded eight IgM-secreting hybridomas that reacted with cell wall antigens from C. albicans by ELISA. Different types of reactivity were observed when the MAbs were tested by IFA against both blastoconidia and germ tubes. MAbs C6, 28G11, 30D1, 12D12, and 28F7 stained the surface of both germ tubes and blastoconidia (Table 1), although the reactivity of MAb C6 was higher than that of the other MAbs (Fig. 1A). MAbs C7 and 29D1 preferentially stained the germ tube surface (Fig. 1B), and a very low reactivity was observed when both blastoconidia and germ tubes were incubated with MAb 30C1 (Table 1). The very low reactivity of MAb C7 with the blastoconidium cell wall surface was confirmed with cells grown in Lee's medium, horse serum, and Sabouraud broth at both 25 and 37°C for 0.5, 1, 1.5, 2, 3, 4, 6, and 24 h (data not shown).
TABLE 1.
Reactivity of MAbs against blastoconidium and germ tube antigens by IFA and Western blotting (WB)
| MAb | IFA reactivitya
|
WB reactivityb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLc | GTc | >200d | 100 | 90 | 65 | 60 | 55 | 51 | 48 | 44 | 42 | 40 | 35 | 28 | |
| C6 | + | + | + | ± | − | − | + | + | + | + | + | + | + | + | ± |
| C7 | ± | + | + | ± | − | − | + | + | − | + | − | − | − | − | − |
| 28G11 | + | + | + | − | ± | + | − | + | − | + | − | − | − | − | − |
| 30C1 | ± | ± | + | − | − | − | − | ± | − | + | − | − | − | − | − |
| 30D1 | + | + | + | − | − | − | − | ± | − | + | − | − | ± | − | − |
| 12D12 | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 28F7 | + | + | + | + | + | ± | − | − | − | − | − | − | − | − | − |
| 29D1 | ± | + | + | − | − | − | − | − | − | − | − | − | ± | − | − |
±, faint fluorescence; +, fluorescence.
−, no band; +, strong band; ±, faint band.
BL, blastoconidia; GT, germ tubes.
Apparent molecular mass in kilodaltons.
FIG. 1.
Immunofluorescence photographs of C. albicans blastoconidia and germ tubes oxidized with sodium meta-periodate and stained with MAbs C6 (A) and C7 (B). Magnification, ×1,000.
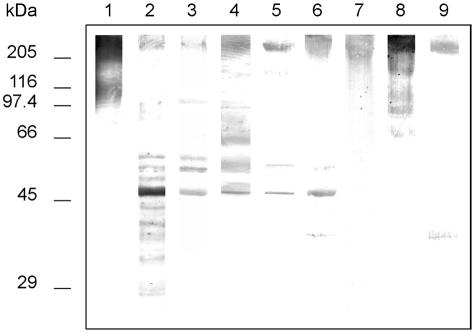
Although all MAbs reacted with components of high molecular mass (>200 kDa) recognized by salivary sIgA in cell wall extracts from germ tubes (Fig. 2), differences were observed in the reactivity of the MAbs with medium to low-weight antigenic components (Table 1 and Fig. 2). A component with a molecular mass of 48 kDa was stained by five out of the eight MAbs.
FIG. 2.
Western blots of 10% slab gels loaded with DTT extracts from C. albicans germ tubes stained with salivary secretory IgA (lane 1) and MAbs C6 (lane 2), C7 (lane 3), 28G11 (lane 4), 30C1 (lane 5), 30D1 (lane 6), 12D12 (lane 7), 28F7 (lane 8), and 29D1 (lane 9). Extracts in lanes 2 to 9 were oxidized with sodium meta-periodate. Molecular masses (in kDa) of standard proteins are listed to the left of the gel.
Characterization of MAb C7.
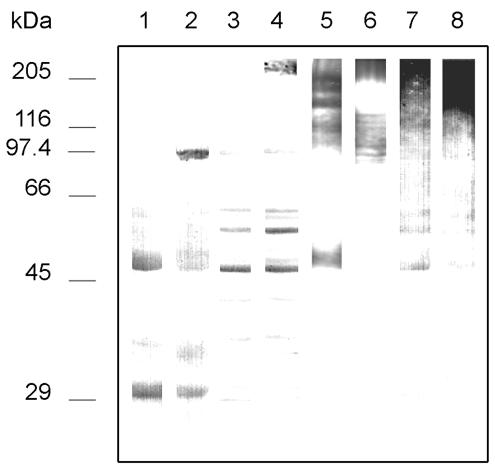
After a careful analysis of the reactivities of the MAbs by IFA and Western blotting, MAb C7 was selected for further characterization since it stained the germ tube surface and reacted with a group of components in DTT extracts from the germ tube including antigens with molecular masses of >200 and 100 kDa. Since MAb C7 showed a very low reactivity with blastoconidia by IFA, it was of interest to assess its reactivity with extracts from the blastoconidium cell wall. In those extracts, MAb C7 reacted with discrete bands of a wide range of molecular masses and especially with a component of 48 kDa (Fig. 3). Periodate oxidation of both germ tubes and blastoconidia extracts resulted in a complete loss of concanavalin A reactivity (data not shown) and in the loss of reactivity of an antigen of 30 kDa observed in both extracts but produced an increase in the reactivity of MAb C7 with antigens of 48, 55, and 60 kDa present in both extracts and with the component of >200 kDa present in germ tube extracts (Fig. 3, lane 4). Since MAb C7 showed a higher reactivity against components present in germ tube extracts than in blastoconidium extracts, it was of interest to assess whether the differences in reactivity were due to the blastoconidium-germ tube transition or to heat shock. Interestingly, the reactivity of MAb C7 with extracts from C. albicans cells grown at 25 and 37°C in a medium in which only blastoconidia were produced was higher in the extract from blastoconidia grown at 37°C than in that grown at 25°C (Fig. 3, lanes 7 and 8).
FIG. 3.
Western blots of 10% slab gels loaded with DTT extracts from C. albicans germ tubes grown in medium 199 at 37°C (lanes 2, 4, and 6), blastoconidia grown in medium 199 at 25°C (lanes 1, 3, and 5) or blastoconidia grown on Sabouraud agar at 25 (lane 7) and 37°C (lane 8) stained with MAb C7 (lanes 1 to 4 and 7 to 8) and concanavalin A (lanes 5 and 6). Extracts in lanes 3, 4, 7, and 8 were oxidized with sodium meta-periodate. Molecular masses (in kDa) of standard proteins are listed to the left of the gel.
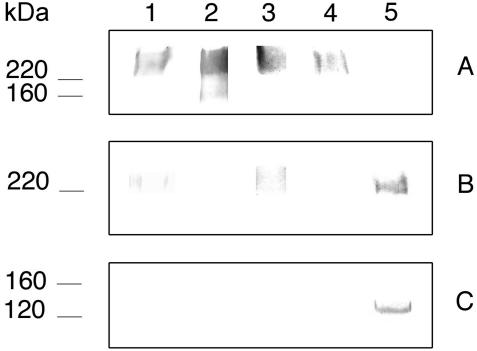
In an attempt to assess whether the component of >200 kDa recognized by MAb C7 was related to some of the medium- to low-molecular-mass components present in the extracts, the antigen of >200 kDa was purified by electroelution. The eluted component reacted strongly with concanavalin A and with antibodies against (1-6)-β-glucan, a weak reactivity was observed with salivary sIgA and MAb K10, and it showed no reactivity with MAb C7 and with antibodies against (1-3)-β-glucan (Fig. 4A). As expected, periodate oxidation of the component resulted in a reduction or complete loss of the reactivity with salivary sIgA, concanavalin A, MAb K10, and anti-(1-6)-β-glucan antibodies but enhanced the reactivity of MAb C7 (Fig. 4B). After deglycosylation of the component of >200 kDa prior to SDS-PAGE, all the antibodies but MAb C7 failed to react with the treated antigen. MAb C7 reacted strongly with a band of 130 kDa (Fig. 4C).
FIG. 4.
(A) Western blots of 10% slab gels loaded with an antigen of >200 kDa purified by electroelution from extracts of C. albicans germ tubes stained with salivary secretory IgA (lane 1), concanavalin A (lane 2), a polyclonal antibody against (1-6)-β-glucan (lane 3), and MAbs K10 (lane 4) and C7 (lane 5). (B and C) Reactivity of the antigen of >200 kDa after removal of carbohydrate groups by oxidation with sodium meta-periodate or with a deglycosylation kit, respectively. Molecular masses (in kDa) of standard proteins are listed to the left of the gel.
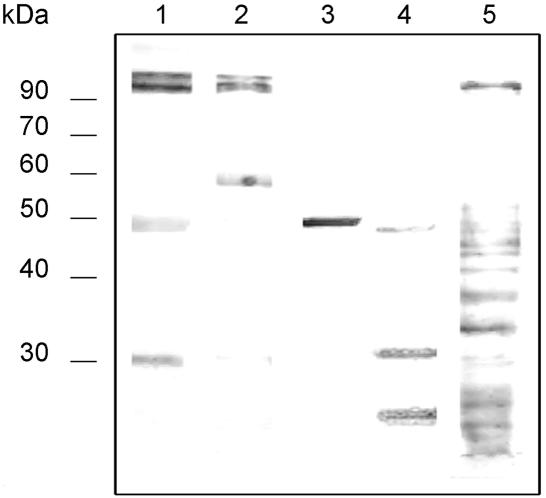
In addition to its reactivity with C. albicans components, MAb C7 also reacted with a wide range of antigens present in other fungi (Fig. 5). Main components stained by MAb C7 included an antigen of 48 kDa present in extracts from Cryptococcus neoformans and antigens of approximately 90 kDa in C. lusitaniae and S. prolificans. An antigen of 30 kDa was stained in the A. fumigatus extract. Periodate oxidation maintained the reactivity of MAb C7 with all extracts (data not shown).
FIG. 5.
Western blots of 10% slab gels loaded with dithiothreitol extracts from C. albicans (lane 1), C. lusitaniae (lane 2), Cryptococcus neoformans (lane 3), A. fumigatus (lane 4), and S. prolificans (lane 5) stained with MAb C7. Molecular masses (in kDa) of standard proteins are listed to the left of the gel.
Biological activities of MAb C7.
When compared to the control without MAb, MAb C7 caused a 31.1% inhibition in the adhesion of C. albicans to HEp-2 monolayers (56.3% ± 0.10% versus 81.7% ± 0.05% in the absence of C7; P < 0.0001) and a 55.3% inhibition in the adhesion of C. albicans to BECs (73.6% ± 8.0% versus 167.0% ± 27.8% in the absence of C7; P < 0.01). MAb C7 produced a 38.5% decrease in the filamentation of C. albicans (40.4% ± 0.07% versus 65.6% ± 0.04% in the absence of C7; P < 0.01).
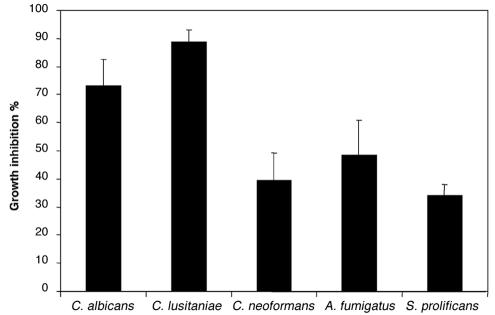
In addition to the inhibition of adhesion, MAb C7 exhibited a potent candidacidal effect since its presence produced a 73.4% reduction in the number of CFU compared to the control with an irrelevant MAb (39.4 ± 10.9 CFU versus 135.9 ± 32.2 CFU, respectively; Fig. 6). By comparison, MAb K10 produced a 92% reduction in the number of CFU. The effect of the dose of MAb C7 on the candidacidal activity was assessed in one experiment. Incubation of C. albicans cells with 2.5 μg (25 μg/ml) of MAb C7 caused an 80.7% inhibition of the fungal growth. Increasing the doses of MAb C7 produced an increase in the candidacidal activity (50 μg/ml, 81.1%; 100 μg/ml, 91.5%). No reduction in fungal growth was observed when MAb C7 was adsorbed with C. albicans germ tubes or replaced by PBS in the experiments. The fungicidal activity of MAb C7 was also studied by a MTT reduction assay, in which a 19.0% ± 0.01% inhibition of the fungal growth was observed when compared to the control with an irrelevant MAb. Since MAb C7 also reacted with antigens expressed in a variety of fungi, the fungicidal activity of MAb C7 was also studied in a number of fungi, including C. lusitaniae, Cryptococcus neoformans, A. fumigatus, and S. prolificans, which showed a marked reduction in fungal growth ranging from 34.2 to 88.7% (Fig. 6).
FIG. 6.
Fungicidal activity of MAb C7 against C. albicans, C. lusitaniae, Cryptococcus neoformans, A. fumigatus, and S. prolificans measured by the reduction in the number of CFU compared to the control with an irrelevant MAb. Values are means of triplicate determinations ± standard error of the mean.
DISCUSSION
To control infections caused by C. albicans, the host must generate a wide range of defensive responses directed to neutralize the virulence factors displayed by the fungus. Although the role of antibody immunity in protection against candidiasis has been controversial, recent evidence demonstrates that antibodies with defined specificities show different degrees of protection against systemic and mucosal candidiasis (10, 14, 23). The exact mechanisms by which these antibodies protect against Candida infection are unknown but are likely to include the inhibition of adhesion or germ tube formation, opsonization, neutralization of virulence-related enzymes, and direct candidacidal activity (5). Although these biological activities have been demonstrated individually in MAbs, it is not clear if all these anti-C. albicans activities may be displayed by a single antibody. In fact, complex mixtures of antibodies with different specificities such as those found in salivary sIgA have been shown to decrease adhesion of C. albicans to host surfaces but not to inhibit germination (36). However, results presented in this study show that an antibody that reacts with cell wall antigens of C. albicans recognized by salivary sIgA exerts at least three anti-C. albicans activities—namely, inhibition of adhesion of C. albicans to HEp-2 cells and BECs, inhibition of germination of C. albicans, and direct candidacidal activity.
Inhibition of adhesion of C. albicans to host surfaces is one of the best-documented activities mediated by antibodies, and different degrees of inhibition have been described with saliva, polyclonal antisera, and MAbs (2, 25, 38). The inhibition of adhesion is usually mediated by blocking the adhesins present on the fungal cell wall (36), but inhibition of germination may be another important mechanism since filamentation plays a key role in the adhesion process (15). Both mechanisms are likely to play a role in the inhibition mediated by MAb C7 since it binds the surface of C. albicans, specially in the filamentous form, and decreases the germination of C. albicans. However, the modest but significant ability of MAb C7 to inhibit both the adhesion of C. albicans to HEp-2 cells and BECs and the germination in C. albicans is likely to be related to the limited exposure of the epitope recognized by MAb C7 since, as will be discussed later, the proteinic epitope recognized by MAb C7 seems to be hidden by the sugar residues of the antigenic glycoprotein.
In contrast to the discrete inhibition of germination and adhesion to the HEp-2 cell line and BECs, MAb C7 exhibited a potent fungicidal effect on C. albicans. The candidacidal effect was initially demonstrated by the reduction in the number of CFU in the presence of MAb C7, but it was confirmed by a different assay based on the reduction of MTT. Interestingly, the fungicidal effect of MAb C7 was also demonstrated on a group of clinically relevant fungi, including Cryptococcus neoformans, A. fumigatus, and the amphotericin B-resistant S. prolificans and C. lusitaniae. The fungicidal activity showed by MAb C7 is reminiscent of that reported by antibodies mimicking the activity of a killer toxin produced by the yeast P. anomala, since they are characterized by a wide spectrum of antimicrobial activity that ranges from C. albicans, A. fumigatus, and Pneumocystis carinii to multidrug-resistant Mycobacterium tuberculosis and gram-positive cocci (7-9, 19). In an attempt to study the relationship between MAb C7 and the yeast killer toxin-like antibodies, we included one of these MAbs, designated K10, in some of the experiments. As expected, MAb K10 produced a marked (92%) reduction of the growth of C. albicans in comparison with the appropriate controls. Results presented in this study confirm and expand the observation concerning the antifungal activity of MAbs directed against selected antigens from the C. albicans cell wall and add a new agent to the armamentarium of potential antimycotics for future use in the therapy of fungal infections resistant to the conventional antifungals.
Investigations performed at the molecular level also confirmed the relationship between MAbs C7 and K10. Both MAbs react with the high-molecular-weight glycoprotein recognized by salivary sIgA. The glycoprotein contains mannan and (1-6)-β-glucan and is present in the cell wall of C. albicans but is preferentially located at the germ tube surface. Interestingly, MAb K10 has been reported to react with the cell wall surface of both germ tubes and young blastoconidia (26). However, both MAbs react with different epitopes in the same glycoprotein since MAb C7 reacts with a proteinic epitope, while MAb K10 reacts with a β-glucan moiety that is sensitive to both periodate oxidation and deglycosylation. In fact, results presented in this paper suggest that the proteinic epitope reactive with MAb C7 must be partially hidden by sugar residues, since the reactivity of MAb C7 with some of the antigens of the extracts from C. albicans—and in particular with the high-molecular-weight component—increases after periodic acid oxidation. This situation seems to happen at the cell wall surface also, since although the MAb does not label the cell wall surface of C. albicans germ tubes when tested by IFA, there was a strong labeling after periodic acid oxidation. Deglycosylation of the component of >200 kDa yielded a band of 130 kDa that contained the epitope recognized by MAb C7. This antigen does not seem to be related to antigens of 100, 60, 55, and 48 kDa that were stained by MAb C7 in cell wall extracts and suggests that MAb C7 reacts with an epitope distributed in a group of proteins from the cell wall of C. albicans, C. lusitaniae, Cryptococcus neoformans, A. fumigatus, and S. prolificans, thus explaining the reactivity and antifungal activity of MAb C7 with those fungi.
The increase in reactivity of MAb C7 with extracts from blastoconidia grown at 37°C in comparison with those grown at 25°C suggests that MAb C7 reacts with a heat shock mannoprotein. This reactivity was expected, since the mice from whom the splenocytes were obtained were immunized with one of the cell wall stress mannoproteins that have been found to be major targets for salivary sIgA (29). Heat shock proteins are highly conserved and have been shown to be among the dominant antigens recognized in immune responses to a broad spectrum of pathogens (41), including C. albicans and other fungi (22). Attempts to induce protective immunity in mice against systemic candidiasis have been successful with antibodies against Hsp 90 (20) but failed with antibodies against Hsp 60 and Hsp 70 (4, 21).
In conclusion, the results presented in this study suggest that the C. albicans mannoprotein of >200 kDa is the target of two different kinds of candidacidal antibodies represented by MAbs C7 and K10. Since the activity of these MAbs is not restricted to C. albicans, the existence of these MAbs supports the concept of a family of microbicidal antibodies. MAb K10 has been shown to protect mice from invasive pulmonary aspergillosis (7). If a similar activity is described for MAb C7, these antibodies could be useful in the treatment of a wide range of microbial infections when used alone or in combination with current antimicrobial agents.
Acknowledgments
The (1-3)-β- and (1-6)-β-glucan antisera were a gift from Hans Kapteyn.
This investigation was supported by grants 9/UPV 0093.327-13550/2001 from the Universidad del País Vasco, PM99-0033 and PB98-0248 from the Dirección General de Enseñanza Superior e Investigación Científica from the Spanish Ministerio de Educación y Cultura, and by AIDS grant no. 50D.26 of Istituto Superiore di Sanità, Italy.
Editor: T. R. Kozel
REFERENCES
- 1.Barturen, B., J. Bikandi, R. San Millán, M. D. Moragues, P. Regúlez, G. Quindós, and J. Pontón. 1995. Variability in expression of antigens responsible for serotype specificity in Candida albicans. Microbiology 141:1535-1543. [DOI] [PubMed] [Google Scholar]
- 2.Bendel, C. M., M. K. Hostetter, and M. McClellan. 1993. Distinct mechanisms of epithelial adhesion for Candida albicans and Candida tropicalis: identification of the participating ligands and development of inhibitory peptides. J. Clin. Investig. 92:1840-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikandi, J., M. D. Moragues, G. Quindós, L. Polonelli, and J. Pontón. 2000. Influence of the environmental pH on the reactivity of Candida albicans with salivary secretory IgA. J. Dent. Res. 79:1439-1442. [DOI] [PubMed] [Google Scholar]
- 4.Bromuro, C., R. La Valle, S. Sandini, F. Urbani, C. M. Ausiello, L. Morelli, C. Fè d'Ostiani, L. Romani, and A. Cassone. 1998. A 70-kDa recombinant heat shock protein of C. albicans is highly immunogenic and enhances systemic murine candidiasis. Infect. Immun. 66:2154-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A. 1995. Antibody immunity and invasive fungal infections. Infect. Immun. 63:4211-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova, M., J. P. Martínez, and W. L. Chaffin. 1990. Fab fragments from a monoclonal antibody against a germ tube mannoprotein block the yeast-to-mycelium transition in Candida albicans. Infect. Immun. 58:3810-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci, E., A. Mencacci, A. Spreca, C. Montagnoli, A. Bacci, K. Perruccio, A. Velardi, W. Magliani, S. Conti, L. Polonelli, and L. Romani. 2002. Protection of killer antiidiotypic antibodies against early invasive aspergillosis in a murine model of allogeneic T-cell-depleted bone marrow transplantation. Infect. Immun. 70:2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti, S., F. Fanti, W. Magliani, M. Gerloni, D. Bertolotti, A. Salati, A. Cassone, and L. Polonelli. 1998. Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J. Infect. Dis. 177:807-811. [DOI] [PubMed] [Google Scholar]
- 9.Conti, S., W. Magliani, S. Arseni, E. Dieci, R. Frazzi, A. Salati, P. E. Varaldo, and L. Polonelli. 2000. In vitro activity of monoclonal and recombinant yeast killer toxin-like antibodies against antibiotic-resistant gram-positive cocci. Mol. Med. 6:613-619. [PMC free article] [PubMed] [Google Scholar]
- 10.De Bernardis, F., M. Boccanera, D. Adriani, E. Spreghini, G. Santoni, and A. Cassone. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellepola, A. N. B., G. J. Panagoda, and L. P. Samaranayake. 1999. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol. Immunol. 14:358-363. [DOI] [PubMed] [Google Scholar]
- 12.Epstein, J. B., L. H. Kimura, T. W. Menard, E. L. Truelove, and N. N. Pearsall. 1982. Effects of specific antibodies on the interaction between the fungus Candida albicans and human oral mucosa. Arch. Oral Biol. 27:469-474. [DOI] [PubMed] [Google Scholar]
- 13.Fidel, P. L., Jr. 1999. Host defense against oropharyngeal and vaginal candidiasis: Site-specific differences. Rev. Iberoam. Micol. 16:8-15. [PubMed] [Google Scholar]
- 14.Han, Y., R. P. Morrison, and J. E. Cutler. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, L. H., and N. N. Pearsall. 1980. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect. Immun. 28:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lee, K. L., M. R. Buckley, and C. Campbell. 1975. An amino acid liquid synthetic medium for development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148-153. [DOI] [PubMed] [Google Scholar]
- 18.Magliani, W., S. Conti, F. De Bernardis, M. Gerloni, D. Bertolotti, P. Mozzoni, A. Cassone, and L. Polonelli. 1997. Therapeutic potential of antiidiotypic single chain antibodies with yeast killer toxin activity. Nat. Biotechnol. 15:155-158. [DOI] [PubMed] [Google Scholar]
- 19.Magliani, W., S. Conti, M. Gerloni, D. Bertolotti, and L. Polonelli. 1997. Yeast killer systems. Clin. Microbiol. Rev. 10:369-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews, R. C., J. P. Burnie, D. Howat, T. Rowland, and F. Walton. 1991. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology 74:20-24. [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews, R. C., J. P. Burnie, A. Fox, A. Baskerville, C. Wells, S. Strachan, and I. Clark. 1998. The diagnostic and therapeutic potential of a monoclonal antibody to the 60 kilodalton nuclear antigen of Candida albicans. Serodiagn. Immunother. 3:75-86. [Google Scholar]
- 22.Matthews, R. C., B. Maresca, J. P. Burnie, A. Cardona, L. Carratu, S. Conti, G. S. Deepe, A. M. Florez, S. Franceschelli, E. Garcia, L. S. Gargano, G. S. Kobayashi, J. G. McEwen, B. L. Ortiz, A. M. Oviedo, L. Polonelli, J. Ponton, A. Restrepo, and A. Storlazzi. 1998. Stress proteins in fungal diseases. Med. Mycol. 36(Suppl. 1): 45-51. [PubMed] [Google Scholar]
- 23.Matthews, R., and J. Burnie. 2001. Antifungal antibodies: a new approach to the treatment of systemic candidiasis. Curr. Opin. Investig. Drugs 2:472-476. [PubMed] [Google Scholar]
- 24.Maza, J. L., N. Elguezabal, C. Prado, J. Ellacuría, I. Soler, and J. Pontón. 2002. Candida albicans adherence to resin composite restorative dental material: influence of whole human saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94:589-592. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa, Y., T. Kuribayashi, K. Kagaya, M. Suzuki, T. Nakase, and Y. Fukazawa. 1992. Role of specific determinants in mannan of Candida albicans serotype-A in adherence to human buccal epithelial cells. Infect. Immun. 60:2493-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polonelli, L., F. Fanti, S. Conti, L. Campani, M. Gerloni, M. Castagnola, G. Morace, and C. Chezzi. 1990. Detection by immunofluorescent anti-idiotypic antibodies of yeast killer toxin cell wall receptors of Candida albicans. J. Immunol. Methods 132:205-209. [DOI] [PubMed] [Google Scholar]
- 27.Polonelli, L., R. Lorenzini, F. De Bernardis, M. Gerloni, S. Conti, G. Morace, W. Magliani, and C. Chezzi. 1993. Idiotypic vaccination: immunoprotection mediated by anti-idiotypic antibodies with antibiotic activity. Scand. J. Immunol. 37:105-110. [DOI] [PubMed] [Google Scholar]
- 28.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, M. Gerloni, G. Morace, W. Magliani, C. Chezzi, and A. Cassone. 1994. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory, yeast killer toxin-like anti-idiotypic antibodies. J. Immunol. 152:3175-3182. [PubMed] [Google Scholar]
- 29.Polonelli, L., M. Gerloni, S. Conti, P. Fisicaro, C. Cantelli, P. Portincasa, F. Almondo, P. L. Barea, F. L. Hernando, and J. Pontón. 1994. Heat-shock mannoproteins as targets of secretory IgA in Candida albicans. J. Infect. Dis. 169:1401-1405. [DOI] [PubMed] [Google Scholar]
- 30.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, W. Magliani, M. Gerloni, C. Cantelli, and A. Cassone. 1996. Human natural yeast killer toxin-like candidacidal antibodies. J. Immunol. 156:1880-1885. [PubMed] [Google Scholar]
- 31.Polonelli, L., N. Seguy, S. Conti, M. Gerloni, D. Bertolotti, C. Cantelli, W. Magliani, and J. C. Cailliez. 1997. Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin. Diagn. Lab. Immunol. 4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontón, J., A. Marot-Leblond, P. Ezkurra, B. Barturen, R. Robert, and J. M. Senet. 1993. Characterization of Candida albicans cell wall antigens with monoclonal antibodies. Infect. Immun. 61:4842-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pontón, J., J. Bikandi, M. D. Moragues, M. C. Arilla, R. Elósegui, G. Quindós, P. Fisicaro, S. Conti, and L. Polonelli. 1996. Reactivity of Candida albicans germ tubes with salivary secretory IgA. J. Dent. Res. 75:1979-1985. [DOI] [PubMed] [Google Scholar]
- 34.Ruhnke, M. 2002. Skin and mucous membrane infections, p. 307-325. In R. A. Calderone (ed.), Candida and candidiasis. American Society for Microbiology, Washington, D.C.
- 35.San Millán, R., P. Ezkurra, G. Quindós, R. Robert, J. M. Senet, and J. Pontón. 1996. Effect of monoclonal antibodies directed against Candida albicans cell wall antigens on the adhesion of the fungus to polystyrene. Microbiology 142:2271-2277. [DOI] [PubMed] [Google Scholar]
- 36.San Millán, R., N. Elguezabal, P. Regúlez, M. D. Moragues, G. Quindós, and J. Pontón. 2000. Effect of salivary secretory IgA on the adhesion of Candida albicans to polystyrene. Microbiology 146:2105-2112. [DOI] [PubMed] [Google Scholar]
- 37.Smail, E. H., and J. M. Jones. 1984. Demonstration and solubilization of antigens expressed primarily on the surfaces of Candida albicans germ tubes. Infect. Immun. 45:74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umazume, M., E. Ueta, and T. Osaki. 1995. Reduced inhibition of Candida albicans adhesion by saliva from patients receiving oral cancer therapy. J. Clin. Microbiol. 33:432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidotto, V., I. Vieta, P. Fisicaro, S. Conti, M. Gerloni, C. Cantelli, J. Pontón, and L. Polonelli. 1997. Influence of glucose and ammonium on the reactivity in vitro of Candida albicans heat shock mannoproteins with secretory IgA. J. Mycol. Med. 4:201-204. [Google Scholar]
- 40.Vudhichamnong, K., D. M. Walker, and H. C. Ryley. 1982. The effect of secretory immunoglobulin A on the in vitro adherence of the yeast Candida albicans to human oral epithelial cells. Arch. Oral Biol. 27:617-621. [DOI] [PubMed] [Google Scholar]
- 41.Young, R. A., and T. J. Elliot. 1989. Stress proteins, infection, and immune surveillance. Cell 59:5-8. [DOI] [PubMed] [Google Scholar]