Abstract
We have produced two synthetic genes that code for the F2 domain located within region II of the 175-kDa Plasmodium falciparum erythrocyte binding antigen (EBA-175) to determine the effects of codon alteration on protein expression in homologous and heterologous host systems. EBA-175 plays a key role in the process of merozoite invasion into erythrocytes through a specific receptor-ligand interaction. The F2 domain of EBA-175 is the ligand that binds to the glycophorin A receptor on human erythrocytes and is therefore a target of vaccine development efforts. We designed synthetic genes based on P. falciparum, Escherichia coli, and Pichia codon usage and expressed recombinant F2 in E. coli and Pichia pastoris. Compared to the expression of the native F2 sequence, conversion to prokaryote (E. coli)- or eukaryote (Pichia)-based codon usage dramatically improved the levels of recombinant protein expression in both E. coli and P. pastoris. The majority of the protein expressed in E. coli, however, was produced as inclusion bodies. The protein expressed in P. pastoris, on the other hand, was expressed as a secreted, soluble protein. The P. pastoris-produced protein was superior to that produced in E. coli based on its ability to bind to red blood cells. Consistent with these observations, the antibodies generated against the Pichia-produced protein prevented the binding of recombinant EBA to red blood cells. These antibodies recognize EBA-175 present on merozoites as well as in sporozoites by immunofluorescence. Our results suggest that the Pichia-based EBA-F2 vaccine construct has further potential to be developed for clinical use.
Despite various efforts at intervention, malaria continues to be on the rise globally. In addition to the loss of human life, malaria is also a serious economic burden, being responsible for a “50% decrease in the per capita GDP of malaria endemic countries in comparison to non-endemic countries” (9). There is, therefore, an increased focus on developing newer methods, in addition to using conventional ones, to decrease the burden of the disease.
Evidence from field studies suggests that a reduction in parasite load is sufficient to reduce malaria-related morbidity and mortality. In areas where malaria is endemic, children and adults have been shown to acquire a certain level of clinical immunity following multiple infections, in that they do not exhibit symptoms of disease despite the presence of low levels of circulating parasites (2). There is strong evidence to suggest that antibodies are the primary mediators of this type of immunity, as demonstrated by a decrease in parasite burden in children following passive transfer of antibodies from immune adults (7, 27). Information from natural immunity can be utilized to develop a vaccination strategy to actively induce immunity to blood stage antigens in order to achieve protection from clinical disease. Of the several antigens expressed during the erythrocytic stages of the parasite, those involved in the process of merozoite invasion into erythrocytes can serve as potential vaccine candidates. The goal of such an intervention would be to reduce the rate of merozoite invasion, leading to a decrease in the overall parasite burden and thus causing a reduction in severe disease and death from malaria.
The 175-kDa erythrocyte binding antigen of Plasmodium falciparum, EBA-175, is one of the most extensively studied malarial antigens. EBA-175 has been shown to be a parasite ligand that binds to glycophorin A on the surface of erythrocytes. This process is thought to be one of the early events that lead to the entry of merozoites into erythrocyte. Studies conducted over the last several years show that EBA-175 belongs to the Ebl family of proteins (1), members of which are involved in invasion and share motifs with the Var family of proteins involved in cytoadherence (20, 30). The sequence (18) and domain structure of this molecule are conserved and bear similarity to those of other molecules involved in invasion, except for a duplication within the 5′ cysteine-rich region II (RII) in EBA-175 of P. falciparum (1). Of the two domains in RII, designated F1 and F2, the red blood cell binding function is located primarily within the F2 domain (29). By definition, RII, as well as F2, is a highly cysteine-rich molecule. The number and position of the cysteines are conserved not only within the EBAs from various strains but also among the members of the Ebl/Var family, suggesting their potential involvement in receptor recognition.
There is evidence to show that EBA-175 is a target of a protective immune response. Antibodies generated against baculovirus-produced recombinant F2, fused to glutathione S-transferase, prevented the binding of EBA-RII to erythrocytes (24). In in vitro assays, antibodies generated against bacterially produced and refolded F2 domain of EBA-175 (26), and baculovirus-produced RII (23), inhibited merozoite invasion of P. falciparum into erythrocytes in a strain-independent manner. In an in vivo challenge study in Aotus monkeys, Jones et al. (13) observed partial protection with a DNA-protein prime boost immunization approach using EBA-RII but no protection with protein alone. Seroepidemiological studies indicate that antibodies to RII of EBA-175 are associated with protection against clinical disease (25). Together, these data indicate that EBA is a strong candidate for a vaccine against malaria and needs to be developed further to test its vaccine potential in monkeys and humans.
A major requirement for a successful recombinant protein-based vaccine is the ability to produce biologically active protein that can be easily scaled up for mass production. Research shows that production of a biologically active recombinant protein depends upon (i) the microenvironment within the host cell used for expression and (ii) the compatibility of codon usage between the native gene sequence and that of the expression host. Historically, Escherichia coli has been used for the expression of proteins in large amounts. However, due to the lack of a proper redox environment within the cytoplasmic compartment of E. coli, it is often difficult to produce correctly folded molecules that require the formation of disulfide bonds. In recent years Pichia pastoris, a eukaryotic expression host, has been developed as an alternate to the E. coli expression system, and it has the advantage of being able to produce complex molecules that are difficult to express and/or properly fold in bacteria. P. falciparum presents an additional challenge in that its genome is about 82% A+T rich, the highest A+T content of any known organism (9). This results in the use of different sets of codons for a given amino acid compared to other hosts (32), making it difficult to clone and express some of the malarial antigens in a heterologous system.
This study was undertaken to select an expression system to produce high levels of a conformationally folded, biologically active F2 domain of EBA-175 that required the least amount of postexpression manipulation to acquire native structure. In order to accomplish this, we determined the effects of codon modification on the expression of EBA-F2 in E. coli and P. pastoris. We also evaluated the effects of homologous and heterologous codon use in the two expression hosts on the conformation of recombinant F2, as assessed by red blood cell binding function, and their ability to induce antibodies that inhibit the binding of red blood cells to recombinant EBA. Our results show that compared to the native sequence, codon optimization significantly improved the expression of F2 in both the prokaryotic and eukaryotic systems. The protein produced in Pichia, however, was superior in terms of its expression levels, solubility, and biological activity.
MATERIALS AND METHODS
Construction of native and synthetic F2 genes.
F2 gene constructs were made based on the native P. falciparum codons (GenBank accession number U32207), E. coli codons, and Pichia codons (www.kazusa.or.jp). The native P. falciparum construct was similar to the wild-type sequence in all respects except that asparagine105 was changed to glutamine105 in order to disrupt a potential N-linked glycosylation site. The E. coli codon-optimized construct was designed by using the most frequently used codons for E. coli. The glycosylation site of this construct was not modified. The Pichia codon-optimized construct was based on the predicted most frequently used codons for Pichia. However, codons for four of the most frequently used amino acids (lysine, aspartic acid, glutamic acid, and asparagine) were altered to prevent the potential depletion of those tRNAs due to high usage. The glycosylation site of this construct was also disrupted by changing asparagine105 to glutamine105. All constructs were designed with appropriate restriction sites and a carboxyl-terminal His6 tag to enable purification. Synthetic genes were constructed and assembled by Retrogen Inc. (San Diego, Calif.). The synthetic genes were cloned into the PCR-blunt vector (Invitrogen, Carlsbad, Calif.).
Cloning and expression. (i) E. coli constructs.
The native P. falciparum F2 gene was cloned into the pQE60 vector (Qiagen, Valencia, Calif.) by utilizing the bacteriophage T5 promoter transcription-translation machinery. The resultant plasmid was designated pQE60-Pf-F2. The E. coli codon-optimized gene was cloned into a modified version of pQE60. The vector was modified to introduce a kanamycin gene and a lacI gene that constitutively expresses the lac repressor protein to enable tight regulation of foreign protein expression. The modified plasmid, designated pQE60-AKI, contained the ColE1 origin of replication, expressed the foreign protein under control of the T5 promoter system, and was selectable under either ampicillin or kanamycin or under dual pressure. The plasmid carrying the E. coli codon-optimized gene was designated pQE60-AKI-eF2, or AKI-eF2 for short. The plasmids were transformed into E. coli XL-1 Blue (Stratagene, La Jolla, Calif.) cells, and after screening, recombinant clones were further transformed into the BL21(DE3) (Novagen, Madison, Wis.) host for expression.
(ii) F2 expression in E. coli.
For bacterial expression of the native P. falciparum gene, MA21, a plasmid carrying RII of EBA-175 from the 3D7 strain of P. falciparum (24) was used as a template to amplify the F2 domain with KlenTaq polymerase (BD Biosciences, San Jose, Calif.). The gene was cloned via compatible restriction sites into the pQE60 vector and expressed in the BL21(DE3) host. The E. coli codon-optimized gene was synthesized with the required restriction sites and was cloned into the pQE60-AKI plasmid and also expressed in BL21(DE3) cells. Both clones were grown in Superbroth with phosphates and 0.8% glycerol in a BioFlow3000 10L fermentor (New Brunswick Scientific, Edison, N.J.). Cells were grown at 37°C and induced at an A600 of between 4 and 6 with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Based on the optimal expression condition for each clone, the cells were harvested at 2 h (pQE60-Pf-F2) or 4 h (AKI-eF2) postinduction. The cell paste was frozen at −70°C until processing.
(iii) P. pastoris constructs.
PicZα (Invitrogen), a zeocin-selectable plasmid, was used for cloning and expression in P. pastoris. The plasmid contains an alcohol oxidase 1 promoter from P. pastoris fused to the α-mating factor from Saccharomyces cerevisiae for directing the protein to the secretory pathway. Upon induction with methanol, the protein is expressed under control of the alcohol oxidase 1 promoter and secreted into the culture medium. E. coli XL-1 Blue (Stratagene) was used to maintain the plasmids. A histidine auxotroph, protease-deficient strain of the methylotrophic yeast P. pastoris, SMD1168 (Invitrogen), was used for expression.
(iv) F2 expression in P. pastoris.
PCR-blunt plasmids carrying the synthetic F2 constructs were amplified, and the inserts were digested and cloned into the appropriately digested PicZα vector. E. coli XL-1 blue cells were transformed with the constructs, and zeocin-resistant clones were screened for the insert by PCR and restriction digestion. Positive clones were digested with restriction enzyme SstI, and the linearized plasmid was used to transform P. pastoris SMD1168 with the Easy Comp kit (Invitrogen). The transformation mixture was plated on yeast-peptone-dextrose-sorbitol plates containing 125 μg of zeocin/ml. Mut+ clones were grown in YPD medium. For expression, the clones were grown in buffered glycerol medium (1% yeast extract, 2% peptone, 1.34% yeast nitrogen base, 4 × 10−5% biotin, 100 mM potassium phosphate [pH 6.0], 1% glycerol) for ∼24 h. The cells were pelleted and induced with fresh medium containing 1% methanol for another 24 h. Supernatants were tested for expression by enzyme-linked immunosorbent assay (ELISA) or Western blotting with EBA-specific antibodies. All constructs used in the study are listed in Table 1.
TABLE 1.
Constructs used
| Expression host | Gene description | Construct designation |
|---|---|---|
| E. coli | P. falciparum codon-based gene | pQE60-Pf-F2 |
| E. coli codon-based gene | AKI-eF2 | |
| P. pastoris | P. falciparum codon-based gene | Pf-F2 |
| E. coli codon-based gene | eF2 | |
| Pichia codon-based gene | yF2 |
Protein purification.
The E. coli cell pastes were disrupted by microfluidization in a 110s microfluidizer (Microfluidics Corp., Newton, Mass.). The samples were spun at 15,000 rpm for 30 min. The pQE60-Pf-F2 protein was purified from the soluble fraction. Briefly, the supernatant was loaded onto Ni-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen) in the presence of 0.5% Tween 80 and 40 mM imidazole. The column was washed extensively, and the protein was eluted with 1 M imidazole. AKI-eF2 was purified from the insoluble fraction by using 0.5% N-lauryl sarcosine (Sarkosyl; Sigma Chemical Co., St. Louis, Mo.). The protein was purified over Ni-NTA-superflow, and the Sarkosyl was replaced by 0.1% Tween 80. The protein was eluted in the presence of 200 mM imidazole and 0.1% Tween 80 in phosphate buffer.
The Pichia-expressed protein yF2 was purified from culture supernatant. The supernatant was dialyzed extensively against phosphate-buffered saline (PBS) to remove all medium components. The sample was then loaded over Ni-NTA agarose and eluted with 200 mM imidazole in phosphate buffer.
Antibodies.
Monoclonal antibodies (MAbs) generated against a baculovirus-produced RII of EBA-175 (A. Barbosa et al., unpublished) or polyclonal mouse sera against the recombinant F2 domain were used as the primary antibodies. The two MAbs used in the study were MAb RII.10, which recognizes a functional epitope on RII, and MAb RII.19, which recognizes a nonconformational epitope on RII. Polyclonal anti-F2 antibodies were generated by immunizing BALB/c mice four times with 10 μg (per dose) of nonreduced or reduced and alkylated E. coli-produced AKI-eF2 and nonreduced yF2 in Montanide IS270 (Sigma) adjuvant at 2-week intervals. Blood samples were collected 10 weeks following the primary immunization, and sera were stored at −20°C until use.
ELISA.
Immulon 2HB plates (Dynatech, Alexandria, Va.) were coated overnight at 4°C with 50 μl (per well) of baculovirus-produced RII of EBA-175, at a concentration of 1 μg/ml, diluted with PBS (pH 7.4). After removal of the unbound antigen by washing the plates four times with PBS containing 0.1% Tween 20 (PBS-T), plates were blocked with PBS-casein (Pierce, Rockford, Ill.) to prevent nonspecific binding. Serum diluted in PBS-T was added and left for 2 h at 37°C. After washing, horseradish peroxidase-labeled anti-mouse immunoglobulin G (IgG) (Kirkegaard and Perry, Gaithersburg, Md.) was added to the plates and left for an hour at room temperature. Plates were washed, the reaction was developed with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)], and the A405 was read after 30 min.
Western blotting.
Native EBA-175 purified from culture supernatants (described below) or purified recombinant proteins were separated on Bis-Tris sodium dodecyl sulfate-4 to 20% polyacrylamide gels (Invitrogen) and run under nonreducing conditions with the MES (morpholineethanesulfonic acid) buffer system. Samples were transferred electrophoretically onto nitrocellulose membranes (Invitrogen). The membranes were blocked for up to 1 h with nonfat milk in PBS-T. After being washed with PBS-T, the blots were incubated overnight at 4°C with primary antibody diluted in PBS-T. Alkaline phosphatase-labeled anti-mouse IgG (Kirkegaard and Perry) was added after the primary antibody was washed off. The blots were developed with nitroblue tetrazolium-5-bromo-4 chloroindol-3-yl phosphate solution (Promega, Madison, Wis.).
Immunofluorescence.
Sporozoites obtained from mosquitoes infected with P. falciparum or Percoll-purified schizonts were applied to slides, air dried, and fixed with methanol. The slides were blocked with bovine serum albumin (BSA) diluted to 1% in PBS (PBS-BSA) for 30 min. Primary antibodies diluted in PBS-BSA were added to the wells, and the slides were incubated in a humidified chamber for 1 h at room temperature. The slides were washed with PBS, and fluorescein isothiocyanate-labeled goat anti-mouse antibody (Promega) was added and left for 30 min at room temperature. Slides were washed and mounted in Vectashield (Vector Labs, Burlingame, Calif.) and viewed on an Olympus (Melville, N.Y.) microscope.
Preparation of native EBA.
Normal human erythrocytes were used to maintain the 3D7 clone from the NF54 strain, the FVO strain, and the ItG strain of P. falciparum in culture in the presence of 10% human AB serum (12, 31). Native EBA-175 was purified according to the method described by Camus and Hadley (4) with slight modifications. Briefly, synchronized parasites were concentrated over Percoll-alanine to obtain over 95% pure schizonts. The enriched parasites were washed in RPMI 1640 and put back in culture, in complete medium (RPMI 1640 containing 10% normal human serum and 25 mM HEPES), at a concentration of 4 × 107 parasitized erythrocytes/ml. Culture supernatant, collected after overnight incubation at 37°C, was used to prepare EBA-175. The culture supernatant was diluted in RPMI 1640 and incubated with washed O-positive erythrocytes for an hour at room temperature with continuous mixing. The cells were washed in RPMI 1640, and bound EBA-175 was eluted by incubating with 1 M NaCl in 50 mM Tris (pH 7.4) with 2 mM phenylmethylsulfonyl fluoride for 10 min at room temperature.
Assays of erythrocyte binding and inhibition of erythrocyte binding.
Two different erythrocyte binding assays were performed. For a direct binding assay, 50 μl of a packed suspension of erythrocytes was incubated with recombinant protein, diluted in RPMI, for 45 min at 37°C. The sample was spun down, and the supernatant (or unbound fraction) was incubated with fresh erythrocytes. The process was repeated for a total of five times. The results were analyzed by assessing the depletion of antigen in the supernatant as well as by eluting the bound protein from erythrocytes. For the former, supernatant was analyzed for depletion of protein (compared to the starting sample) by Coomassie blue staining. For further confirmation by Western blotting, the erythrocytes were washed with RPMI and the bound protein was eluted from the erythrocytes with 1 M NaCl. The eluted samples were run on a sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose paper, and probed with polyclonal anti-yF2 antibodies.
An indirect plate binding assay was performed to determine the red blood cell binding of yF2 as well as to assess the inhibitory potential of anti-F2 sera. Anti-EBA MAb RII.19 was used as a primary capture antibody. Plastic petri dishes were coated with 20 μg of MAb RII.19/ml and incubated overnight at 4°C. After washing, the spots were blocked with 1% BSA diluted in PBS for 30 min. The BSA solution was aspirated and replaced with recombinant yF2 diluted in 0.1% BSA for 2 h at room temperature. To assess the ability of anti-yF2 or normal mouse serum to inhibit the binding of erythrocytes to yF2, the spots were washed and incubated with serum diluted in 0.1% BSA for an hour at room temperature. Control wells did not receive any serum. After washing, the spots were incubated with erythrocytes diluted to a final hematocrit of 2% in 0.1% BSA for 30 min at room temperature. The plates were washed gently with PBS and then fixed with 2% glutaraldehyde diluted in PBS. Results were scored qualitatively based on visual binding. Wells with no serum served as a positive control.
Assay of inhibition of receptor (glycophorin A) binding.
Glycophorin A (Sigma) was biotinylated (Amersham Biosciences, Piscataway, N.J.) and gel purified on Sephadex G-25 to remove unincorporated biotin. Immulon 2HB plates were coated overnight with 3 μg of baculovirus RII diluted in PBS (pH 7.4)/ml. The unbound antigen was washed off with PBS-T, and the nonspecific sites were blocked with 1% BSA diluted in PBS (pH 7.4). The plates were incubated with different dilutions of normal mouse serum or sera from mice immunized with reduced or nonreduced AKI-eF2 or with yF2. Sera were diluted in PBS-T-0.1% BSA and incubated for 90 min at room temperature. The plates were washed and incubated for 1 h with biotinylated glycophorin A diluted to 0.25 μg/ml in PBS-T-BSA. After washing, streptavidin labeled with alkaline phosphatase (Amersham Biosciences) was added and left for 30 min at room temperature. Plates were developed with p-nitrophenylphosphate, and the A405 was read kinetically for 30 min.
Nucleotide sequence accession numbers.
The sequences of the E. coli and Pichia codon-optimized constructs have been submitted to GenBank under accession numbers AY311508 and AY311509, respectively.
RESULTS AND DISCUSSION
Effect of codon optimization and host cells on expression.
Due to the extreme A+T richness of the Plasmodium genome and the resultant disparity in codon usage with commonly used expression systems, it is sometimes difficult to optimally express malarial genes in heterologous systems. We compared the effects of codon modification on the expression of the F2 domain of EBA-175 of P. falciparum in E. coli and P. pastoris. EBA-175 serves as the parasite ligand that is involved in binding to human red blood cells through a defined, highly cysteine-rich domain termed F2. We designed three F2 constructs based on the native P. falciparum codons, E. coli codons, and Pichia codons (see Materials and Methods). The E. coli codon-based gene was synthesized first and was based on the most frequently used E. coli codons. Subsequently, due to the facts that P. falciparum lacks N- and O-linked glycosylation (10) and Pichia is known to potentially glycosylate proteins, we decided to mutate the single potential glycosylation site from N105xT107 to Q105xT107 in the remaining two constructs. Thus, the F2 genes based on the P. falciparum codons and the Pichia codons were altered from the native genes to mutate the single potential glycosylation site. The synthetic gene based on Pichia codons was based on the most frequently used codons, with the exception of four amino acids (out of 314), for which the codons were altered from those used most frequently in order to prevent the potential depletion of tRNAs encoding the same (14). All constructs were made as C-terminal His6 fusion proteins to enable purification of full-length protein. Expression in E. coli was induced with IPTG, and that in P. pastoris was induced with methanol.
For expression in E. coli, we compared the native F2 gene to a synthetic gene based on E. coli codons. Recombinant proteins expressed by both constructs were recognized by a conformational MAb, RII.10, in a Western blot (Fig. 1A). However, a comparison of the levels of expression, as estimated by a densitometric scan (Imagequant 5.0; Molecular Dynamics) of the Western blot, revealed that the E. coli codon-optimized construct expressed approximately fourfold more protein. The yield of purified protein from the wild-type construct (pQE60-Pf-F2) was very low (data not shown) and was not deemed suitable for further scale-up for a vaccine. The E. coli codon-optimized construct expressed significantly higher levels of recombinant protein. However, the protein was insoluble and required extraction with Sarkosyl, a strong anionic detergent. In contrast, the majority of the recombinant F2 encoded by the native codon-based gene was expressed as a nonionic-detergent-extractable protein (data not shown). While not all soluble proteins are correctly folded, it is known that incorrectly folded proteins tend to form insoluble aggregates in the form of inclusion bodies (5). We observed that a large percentage of the Ni-NTA-purified, E. coli codon-optimized protein tended to form reduction-sensitive aggregates (Fig. 1B), and only a portion of the protein was recognized by the conformational MAb in comparison to the polyclonal antibody (Fig. 1C). That only a portion of this protein was folded in the correct conformation was also evident from the fact that in a red blood cell binding assay, a critical test for determining the conformation of EBA, a population of the protein did not bind to red blood cells even on sequential incubation with fresh red blood cells (data not shown). The presence of a heterogeneous population of correctly and incorrectly folded protein also affects the quality of antibodies generated against it (discussed below). The difference in the solubilities of the proteins encoded by the P. falciparum codons versus the E. coli codons could be due to the fact that use of the most frequently used E. coli codons speeds up the translational machinery and thus influences the folding of the recombinant protein. Protein folding is known to be determined by several factors, including the availability of appropriate tRNAs and the amount of protein being expressed. The presence of low-abundance codons is a mechanism used by host cells to slow down protein translation to enable the protein to acquire the domain architecture needed for attaining its final conformation (19). High expression levels achieved by the codon-optimized construct in E. coli may have contributed to incorrect folding of the recombinant protein. Thus, while altering the codons can lead to an increase in levels of expression, it also compromises the quality of protein in terms of achieving its native conformation. Obtaining the correct conformation of a recombinant molecule in vitro involves refolding by using complex multistep processes that lead to a significant decrease in the final yield of protein (28). In order to avoid these postexpression manipulations, we explored the feasibility of using a eukaryotic expression system.
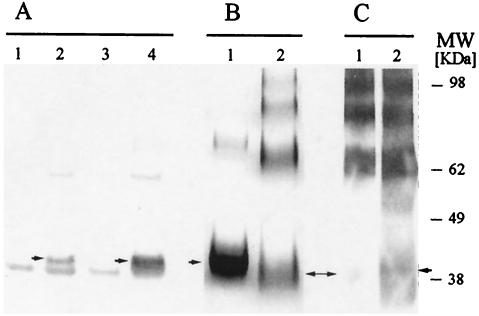
FIG. 1.
Expression of recombinant F2 in E. coli. (A) Recognition of pQE60-Pf-F2 (native codons) and AKI-eF2 (E. coli codons) by a conformational MAb, MAb RII.10, in a Western blot. Lanes 1 and 2, uninduced and induced pQE60-Pf-F2, respectively; lanes 3 and 4, uninduced and induced AKI-eF2, respectively. Monomeric F2 is indicated by the arrows. (B) Coomassie blue-stained polyacrylamide gel comparing the reduced (lane 1) and nonreduced (lane 2) Ni-NTA-purified AKI-eF2, revealing that the protein tends to form aggregates. (C) Western blot analysis revealing that only a small proportion of the monomeric form of AKI-eF2 is recognized by a conformational MAb (lane 1) compared to the polyclonal anti-EBA antibodies (lane 2).
We next looked at the expression of F2 in P. pastoris, a unicellular eukaryote that has many similarities to E. coli in terms of ease of cloning foreign genes, as well as having a tightly controlled inducible expression in cultures that are easy to handle. Being a eukaryote, P. pastoris is capable of several posttranslational modifications, the most important being its ability to form disulfide bonds that enable proper folding of molecules. In addition, yeast mitochondrial DNA bears a remarkable similarity to plasmodial DNA in that it is 82% A+T and contains very A+T-rich spacers that separate A+T-rich genes (32). Therefore, it may be possible to express some, though not all, A+T-rich malarial genes in yeast. Indeed, while some proteins are expressed in yeast with their native plasmodial sequences (16, 21, 22), changing the codons has been shown to increase the expression of some other malarial proteins (3, 15, 17). We compared the expression in P. pastoris of F2 encoded by the native, E. coli, and Pichia codons.
All recombinant clones were tested for expression by ELISA and/or by Western blotting at 24-h intervals for up to 96 h. Expression data at 24 h for one clone of each of the three synthetic genes are shown in Fig. 2. The native F2 gene did not show any detectable expression by Western blotting or ELISA (data not shown). The eF2 and yF2 clones expressed protein that was detectable by Western blotting (Fig. 2) and ELISA (data not shown). However, densitometric analysis showed that the yF2 clone expressed protein at levels that were up to ninefold higher than those expressed by eF2. Representative E. coli and Pichia codon-based clones, designated eF2 and yF2, respectively, were grown in a shake flask to compare their expression levels. The yF2 clone produced a large amount of protein that was detectable by Coomassie blue staining of the culture supernatant run on a polyacrylamide gel (Fig. 3A). eF2, while detected by Western blotting, could be seen on a Coomassie blue-stained gel only following concentration over an Ni-NTA column (data not shown). Both the eF2 and yF2 proteins were conformational as determined by the fact that both were recognized by a conformational MAb (data not shown). Unlike the E. coli-produced protein (see above), the proteins made in P. pastoris showed very little aggregation. The protein showed a shift in mobility upon reduction with 2-mercaptoethanol, indicating the presence of disulfide bonds (Fig. 3B). Furthermore, polyclonal anti-yF2 and functional anti-EBA MAb RII.10 showed similar immunoblot profiles in a Western blot (Fig. 3C), suggesting that the majority of recombinant F2 expressed in P. pastoris is biologically functional. Reduced yF2 was recognized by polyclonal antiserum but was not recognized by MAb RII.10 (Fig. 3C).
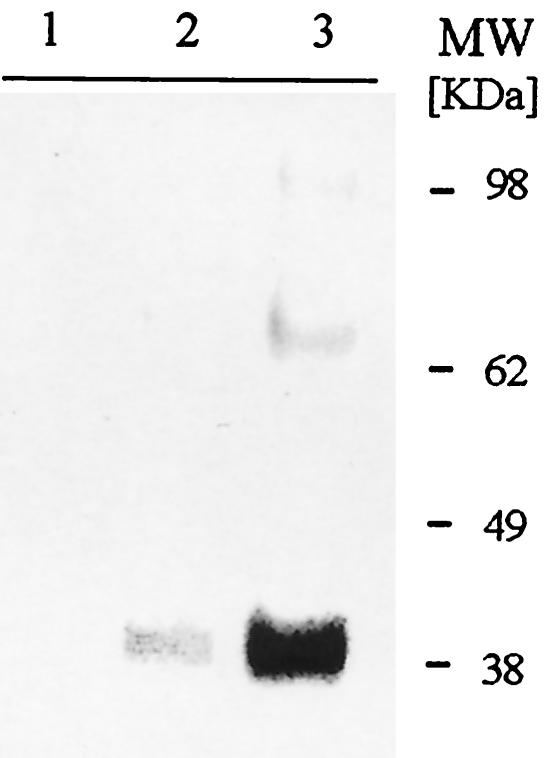
FIG. 2.
Expression of recombinant F2 in P. pastoris. Western blot analysis revealed that Pf-F2 (native codons) did not show any detectable expression (lane 1). eF2 (E. coli codons) (lane 2) and yF2 (Pichia codons) (lane 3) expressed protein recognized by a conformational MAb.
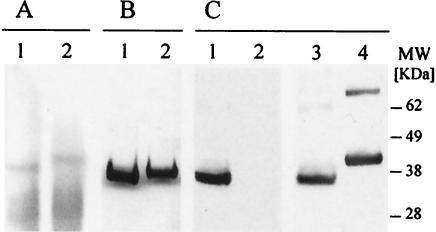
FIG. 3.
Expression of yF2 (Pichia codon-optimized protein) in P. pastoris. (A) yF2 was detectable in reduced (lane 1) and nonreduced (lane 2) culture supernatants upon Coomassie blue staining. (B) Coomassie blue staining of yF2 purified over Ni-NTA, showing the presence of the monomeric form of the protein (lane 1), which had a reduced mobility following reduction (lane 2). (C) Nonreduced yF2 was recognized to an equal extent by a conformational MAb (lane 1) and polyclonal anti-yF2 antiserum (lane 3). Reduced yF2 was recognized by the polyclonal antibodies (lane 4) but was not recognized by the MAb (lane 2).
Our results show that as with E. coli, there is an effect of codons on the expression of EBA-F2 in P. pastoris. The A+T contents of the three constructs were 72% for native F2, 62% for E. coli F2, and 72% for Pichia F2. The differences in expression levels, therefore, could not be explained on the basis of the overall A+T content. They could possibly be a result of the choice of codons used by the three organisms and their ability to provide a suitable expression machinery for the optimal codons. Analysis of the native and Pichia codon-optimized genes showed that despite their similar A+T contents, codons for 47% of the amino acids were altered to fit the Pichia codon usage. Additionally, it is possible that the P. falciparum F2 sequence forms regions that are recognized as, as-yet-poorly defined, polyadenylation sites or transcriptional termination sites causing premature truncation of protein in Pichia. The presence of such transcription termination sequences has been described recently for Schizosaccharomyces pombe (6). In this study, occurrence of the sequence AAUAAA in foreign genes serves as an early transcription terminator. Nonetheless, we observe that a synthetic gene based on the yeast codons is capable of producing antigen that is expressed in large amounts. The yF2 clone expressed approximately 250 μg of protein/ml in a shake flask. We believe that these amounts can be scaled up even further during fermentation to produce sufficient amount of protein for clinical trials.
Functional characterization of yF2.
EBA-175 has the unique advantage of having a defined functional role. The molecule has been shown to bind erythrocytes, and this binding is sensitive to removal of sialic acid residues from its receptor, glycophorin A, by treatment with neuraminidase. To determine if the recombinant protein produced in P. pastoris was biologically active, we tested its ability to bind to erythrocytes. yF2 bound to normal erythrocytes (Fig. 4). The Coomassie blue-stained gel showed depletion of yF2 following incubation with erythrocytes. Although there was some protein in the unbound supernatant after the first pass over red blood cells, we were able to deplete all protein after two passes (Fig. 4A), demonstrating that the residual protein in the first unbound fraction was due to a limitation of the number of red blood cells used in our assay and not to the presence of a population of protein that was unable to bind red blood cells. The specificity was confirmed by probing the washed and eluted fraction of erythrocytes with polyclonal anti-F2 antibodies (Fig. 4B). No protein was eluted from erythrocytes incubated with supernatant after three incubations. Similar to the case for native EBA-175, yF2 did not bind to neuraminidase-treated erythrocytes. The entire protein was recovered in the unbound fraction (Fig. 4A), and the eluted fraction showed no reactivity to anti-EBA antibodies (Fig. 4B). These results show that all of the soluble recombinant yeast-produced F2 binds to red blood cells in a specific manner. In contrast, only part of the E. coli-produced protein bound to red blood cells (data not shown). This suggests that P. pastoris-produced yF2 is correctly folded and biologically active. Our results further confirm earlier observations that F2 alone is sufficient to bind to red blood cells.
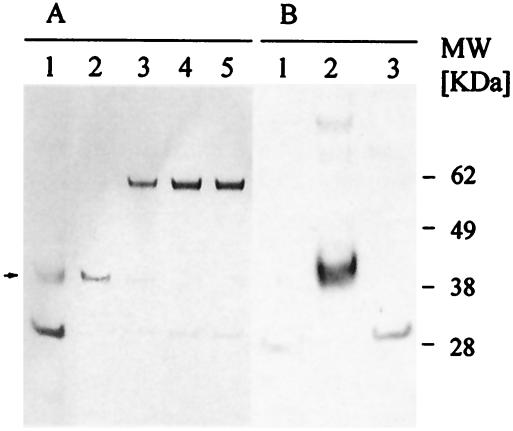
FIG. 4.
Analysis of erythrocyte binding capacity of yF2. (A) yF2 (lane 2) was incubated serially with fresh erythrocytes, and the samples were analyzed on a Coomassie blue-stained gel. Coomassie blue staining revealed depletion of yF2 in the supernatant following incubation with normal human erythrocytes. After two serial incubations, there was complete depletion of antigen (lanes 3 and 4). Lane 5, normal erythrocytes incubated with medium alone. Removal of sialic acids upon neuraminidase treatment abolished the ability of yF2 to bind to erythrocytes (lane 1). (B) The erythrocytes described for panel A were washed and eluted with salt to confirm the specificity. In a Western blot analysis, no antigen was detected in the eluted fraction of the neuraminadase-treated erythrocytes (lane 1). F2 was detected in the eluate of normal erythrocytes after the first round of incubation with yF2 (lane 2). No protein was eluted from the cells after the third passage of unbound supernatant (lane 3), confirming that all of the antigen was depleted after two serial incubations with fresh erythrocytes.
Immunological characterization of F2 produced in E. coli and P. pastoris.
Based on reactivity to the conformational MAb and red blood cell binding characteristics, the antigen produced in P. pastoris appeared to be more closely related to native EBA-175 than the one produced in E. coli. There is, however, a population of AKI-eF2 that is recognized by the conformational MAb RII.10. To what extent does a recombinant protein need to mimic the native protein in order to produce antibodies that are functional?
In order to establish whether a protein that contains a mixed population of correctly and incorrectly folded conformers is still able to generate antibodies that are able to inhibit red blood cell binding, we immunized mice with the two recombinant F2 proteins. We also immunized mice with a reduced and alkylated protein to determine what kind of antibodies are generated following immunization with the deliberately altered antigen so as to ascertain the limits of the stringency of the conformational requirement of F2 to induce an inhibitory immune response. Thus, mice were immunized with E. coli-produced AKI-eF2 in either the reduced and alkylated (R-AKI-eF2) or the nonreduced (NR-AKI-eF2) form or with yeast-produced yF2 to assess the relative immunogenicities of F2 produced in E. coli and P. pastoris. Baculovirus-produced RII, which was shown previously to mimic native EBA-175 (24), was used as the plate antigen to ensure that the immune response measured was specific to EBA-175 and was not against contaminating host proteins present in the immunogen.
Mice immunized with either reduced or nonreduced AKI-eF2 had a higher reactivity to the reduced form of the plate antigen than the mice immunized with yF2 (Fig. 5A), suggesting that the E. coli-produced protein contains epitopes that are not present in the correctly folded molecule. Mice immunized with yF2 recognized the nonreduced plate antigen better than the nonreduced form (Fig. 5). Hence, it appears that the recombinant protein needs to closely mimic the native protein in order to produce antibodies that recognize a conformationally folded molecule.
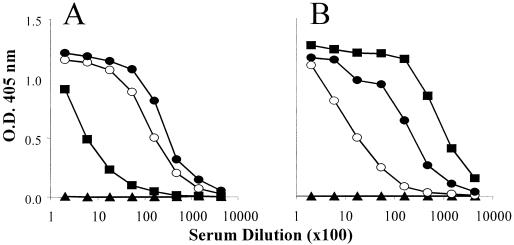
FIG. 5.
ELISA IgG titers in sera of mice immunized with reduced and nonreduced forms of F2. (A) Sera from mice immunized with reduced (○) or nonreduced (•) AKI-eF2 had better reactivity to the reduced form of EBA-RII. Sera from mice immunized with yF2 (▪) reacted poorly to the reduced plate antigen. (B) In contrast, sera from mice immunized with yF2 (▪) had high antibody titers to nonreduced EBA-RII. Sera from mice immunized with AKI-eF2 (reduced ○ and nonreduced •) did not recognize the nonreduced plate antigen well. Normal mouse serum (▴) did not react to either reduced or nonreduced plate antigen. O.D., optical density.
An advantageous prerequisite of a successful vaccine is its ability to generate antibodies that are functional. EBA-175 has been studied extensively, and its host receptor is well characterized. Sialic acid residues present on glycophorin A are the primary receptors for parasite invasion. We tested the ability of the anti-F2 antibodies described above to prevent the binding of recombinant RII to its receptor glycophorin A. In this assay, anti-yF2 sera blocked the binding of biotinylated glycophorin A to baculovirus RII (Fig. 6), while sera from mice immunized with E. coli-produced nonreduced or reduced and alkylated F2 did not generate antibodies that could prevent the binding of glycophorin A to EBA-RII. These data suggest that antibodies generated by immunization with an incorrectly folded molecule may not be able to prevent receptor-ligand interaction. Thus, among the recombinant F2s tested, yF2 was the only immunogen that induced antibodies that could prevent the binding of EBA-175 to its receptor and thus can potentially block parasite invasion into red blood cells, the essential goal of an EBA-175 based vaccine.
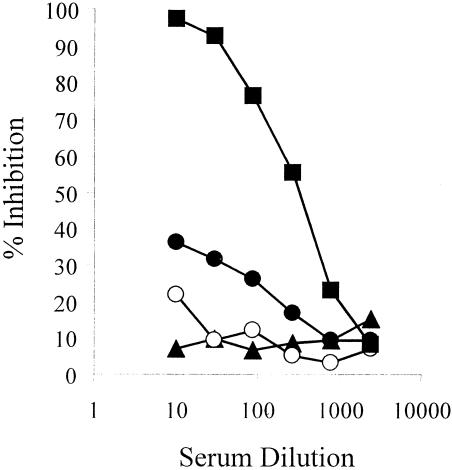
FIG. 6.
Functional characterization of anti-F2 antibodies. Sera from mice immunized with yF2 (▪) inhibited the binding of biotinylated glycophorin A to RII, while sera from mice immunized with reduced (○) and nonreduced (•) AKI-eF2 failed to inhibit this binding. Normal mouse serum (▴) served as a control.
Further characterization of anti-yF2 antibodies.
Having established that yeast-produced F2 and anti-yF2 antibodies appear to be superior to the product made in E. coli, we performed additional characterization of anti-yF2 antibodies. In addition to the inhibition of glycophorin A binding (discussed above), we also looked at the ability of anti-yF2 antibodies to prevent red blood cell interaction with EBA-RII. In an in vitro assay, anti-yF2 antibodies were able to inhibit the binding of erythrocytes to yF2, captured on petri dishes by an anti-EBA-RII MAb, in a dose-dependent manner. Anti-yF2 serum was able to block the binding of erythrocytes to yF2 up to a serum dilution of 1:160 (data not shown). These data are promising indicators of the potential of generating growth-inhibitory antibodies upon immunization of larger animals with yF2.
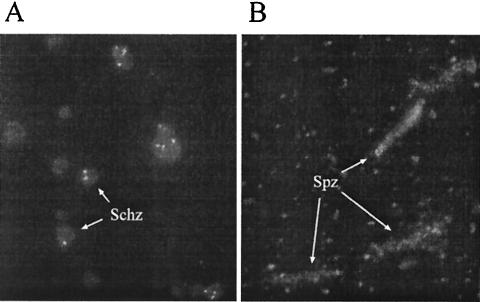
To determine if these antibodies recognized native EBA-175, we performed immunofluorescence assays (IFA) with asexual stages of the parasite. The antibodies recognized parasite-expressed EBA-175 antigen on the apical ends of parasitized erythrocytes by IFA (Fig. 7A). To test whether anti-yF2 antibodies were also capable of recognizing antigen on preerythrocytic stages, we performed an IFA with P. falciparum sporozoites. The antibodies showed a punctate recognition pattern, with a more intense staining towards the center of the sporozoite (Fig. 7B). Our data are consistent with recent reports by Gruner and colleagues (11), who demonstrated the presence of EBA-175 on the sporozoite and liver stages of the parasites. This dual recognition of antigen in the erythrocytic and preerythrocytic stages by anti-yF2 antibodies adds to the value of using this antigen as a potential vaccine that not only can target the blood stage infection but may also be able to control or limit the preerythrocytic stages as well.
FIG. 7.
(A) Mice immunized with yF2 showed a punctate fluorescence pattern on the apical end of schizonts. (B) Sporozoites also demonstrated a punctate recognition along the entire parasite.
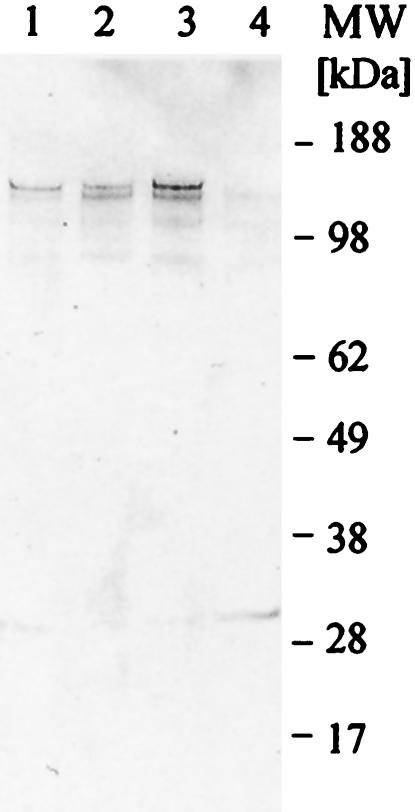
EBA-175 is highly conserved among all of the P. falciparum isolates sequenced so far, even though there are different invasion mechanisms used by various strains (4, 8). In light of the different invasion mechanisms, it is important to see whether the antibodies generated against EBA-175 from one strain recognize EBA-175 from other strains. We tested the reactivities of antibodies to yF2 from the 3D7 strain to EBA-175 prepared from culture supernatants from three different strains of parasite: FVO, a sialic acid-dependent strain; 3D7, which is only partially dependent on sialic acids; and ItG, whose sialic acid dependence has not been reported. Our results demonstrate that antibodies generated against yF2 from the 3D7 strain recognize native EBA from homologous (3D7) as well as heterologous (FVO and ItG) strains (Fig. 8). Narum et al. (23) have reported that antibodies against RII from the FVO strain prevent invasion of FVO and 3D7 parasites. Similar results were obtained by immunization with the F2 domain (25). The cross-recognition of antigen by antibodies generated against one strain with antigen present on different strains may lead to a vaccine that is panreactive against different field isolates.
FIG. 8.
Anti-yF2 antibodies recognize EBA-175 from culture supernatants from homologous (3D7) (lane 1) as well as heterologous (FVO [lane 2] and ItG [lane 3]) strains of P. falciparum. Lane 4, control lane with complete medium probed with anti-yF2 antibodies.
EBA-175 has been proposed as a vaccine candidate by virtue of its distinct role in invasion. In an Aotus trial (13), RII of EBA-175 was tested as a DNA plasmid, protein, or a combination DNA-protein vaccine in a prime-boost regimen. While none of the monkeys that received either DNA alone or protein alone showed protection, three-fourths of the monkeys that received the DNA-protein combination were able to self-resolve their infection. These results suggest that an EBA-175-based vaccine, when delivered in an optimal immunization regimen, can prevent mortality caused by malaria infection. However, the observation that the monkeys immunized with protein alone did not show any protection needs to be further analyzed. There could be several possible explanations for suboptimal protection. One explanation is that the baculovirus-produced protein was glycosylated and this glycosylation may interfere with the generation of inhibitory antibodies. In our study, we mutated the glycosylation site to eliminate the possibility of producing suboptimal quality of antibodies.
An alternative explanation could be that the production of antibodies against the F1 domain present in RII may either block the inhibitory antibodies against the F2 domain or prevent the formation of a sufficiently high titer of inhibitory antibodies against the F2 domain. We hypothesized that use of F2, the domain that is sufficient on its own to bind red blood cells, might maximize the chances of producing high-titer antibodies that prevent parasite interaction with red blood cells.
In summary, we report that codon optimization leads to enhanced expression of the F2 domain of EBA-175 in both E. coli and P. pastoris. However, protein produced in P. pastoris is superior in its biological function. Antibodies generated against yF2 are able to recognize native antigen from homologous and heterologous strains of parasite. These antibodies are functionally potent and are able to prevent receptor-ligand interaction. In addition, the ability of yF2 to generate antibodies that recognize antigen on the preerythrocytic and erythrocytic stages makes it a very strong candidate for preclinical development.
Acknowledgments
We thank Arnoldo Barbosa for providing the MAbs used in this study and for his invaluable help in making the figures. We also acknowledge Tracy LaClair for technical assistance during part of the study and Victor De Armas for help with the fermentations. We deeply appreciate David Haynes and Kathy Moch for providing the parasites. We also acknowledge Ted Hall for providing sporozoite slides for IFA, Sheetij Dutta for densitometric scanning, and Lisa Ware and Bob Bowden for reviewing the manuscript.
The views expressed here are those of the authors and do not necessarily represent those of the Department of the Army or Department of Defense.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adams, J. H., B. K. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird, J. K. 1995. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today 11:105-111. [DOI] [PubMed] [Google Scholar]
- 3.Brady, C. P., R. L. Shimp, A. P. Miles, M. Whitmore, and A. W. Stowers. 2001. High-level production and purification of P30P2MSP1(19), an important vaccine antigen for malaria, expressed in the methylotropic yeast Pichia pastoris. Protein Expr. Purif. 23:468-475. [DOI] [PubMed] [Google Scholar]
- 4.Camus, D., and T. J. Hadley. 1985. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230:553-556. [DOI] [PubMed] [Google Scholar]
- 5.Carrio, M. M., and A. Villaverde. 2002. Construction and deconstruction of bacterial inclusion bodies. J. Biotechnol. 96:3-12. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, S., B. Sarmah, N. Chakraborty, and A. Dutta. 2002. Premature truncation of RNA polymerase II mediated transcription of a seed protein in Schizosaccharomyces pombe. Nucleic Acids Res. 30:2940-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, S., G. A. Butcher, and R. B. Crandall. 1969. Action of malarial antibody in vitro. Nature 223:368-371. [DOI] [PubMed] [Google Scholar]
- 8.Dolan, S. A., J. L. Proctor, D. W. Alling, Y. Okubo, T. E. Wellems, and L. H. Miller. 1994. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 64:55-63. [DOI] [PubMed] [Google Scholar]
- 9.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gowda, D. C., and E. A. Davidson. 1999. Protein glycosylation in the malaria parasite. Parasitol. Today. 15:147-152. [DOI] [PubMed] [Google Scholar]
- 11.Gruner, A. C., K. Brahimi, F. Letourneur, L. Renia, W. Eling, G. Snounou, and P. Druilhe. 2001. Expression of the erythrocyte-binding antigen 175 in sporozoites and in liver stages of Plasmodium falciparum. J Infect. Dis. 184:892-897. [DOI] [PubMed] [Google Scholar]
- 12.Haynes, J. D., C. L. Diggs, F. A. Hines, and R. E. Desjardins. 1976. Culture of human malaria parasite Plasmodium falciparum. Nature 263:767-769. [DOI] [PubMed] [Google Scholar]
- 13.Jones, T. R., D. L. Narum, A. S. Gozalo, J. Aguiar, S. R. Fuhrmann, H. Liang, J. D. Haynes, J. K. Moch, C. Lucas, T. Luu, A. J. Magill, S. L. Hoffman, and B. K. Sim. 2001. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J. Infect. Dis. 183:303-312. [DOI] [PubMed] [Google Scholar]
- 14.Kane, J. F. 1995. Effects of rare codon clusters on high-level expression of heterologous proteins in Escherichia coli. Curr. Opin. Biotechnol. 6:494-500. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70:6948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocken, C. H., M. A. Dubbeld, A. Van Der Wel, J. T. Pronk, A. P. Waters, J. A. Langermans, and A. W. Thomas. 1999. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect. Immun. 67:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocken, C. H., C. Withers-Martinez, M. A. Dubbeld, A. van der Wel, F. Hackett, A. Valderrama, M. J. Blackman, and A. W. Thomas. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect. Immun. 70:4471-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang, H., and B. K. Sim. 1997. Conservation of structure and function of the erythrocyte-binding domain of Plasmodium falciparum EBA-175. Mol. Biochem. Parasitol. 84:241-245. [DOI] [PubMed] [Google Scholar]
- 19.Makhoul, C. H., and E. N. Trifonov. 2002. Distribution of rare triplets along mRNA and their relation to protein folding. J. Biomol. Struct. Dyn. 20:413-420. [DOI] [PubMed] [Google Scholar]
- 20.Michon, P., J. R. Stevens, O. Kaneko, and J. H. Adams. 2002. Evolutionary relationships of conserved cysteine-rich motifs in adhesive molecules of malaria parasites. Mol. Biol. Evol. 19:1128-1142. [DOI] [PubMed] [Google Scholar]
- 21.Miles, A. P., Y. Zhang, A. Saul, and A. W. Stowers. 2002. Large-scale purification and characterization of malaria vaccine candidate antigen Pvs25H for use in clinical trials. Protein Expr. Purif. 25:87-96. [DOI] [PubMed] [Google Scholar]
- 22.Morgan, W. D., B. Birdsall, T. A. Frenkiel, M. G. Gradwell, P. A. Burghaus, S. E. Syed, C. Uthaipibull, A. A. Holder, and J. Feeney. 1999. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 289:113-122. [DOI] [PubMed] [Google Scholar]
- 23.Narum, D. L., J. D. Haynes, S. Fuhrmann, K. Moch, H. Liang, S. L. Hoffman, and B. K. Sim. 2000. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect. Immun. 68:1964-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ockenhouse, C. F., A. Barbosa, D. P. Blackall, C. I. Murphy, O. Kashala, S. Dutta, D. E. Lanar, and J. R. Daugherty. 2001. Sialic acid-dependent binding of baculovirus-expressed recombinant antigens from Plasmodium falciparum EBA-175 to glycophorin A. Mol. Biochem. Parasitol. 113:9-21. [DOI] [PubMed] [Google Scholar]
- 25.Okenu, D. M., E. M. Riley, Q. D. Bickle, P. U. Agomo, A. Barbosa, J. R. Daugherty, D. E. Lanar, and D. J. Conway. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 68:5559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey, K. C., S. Singh, P. Pattnaik, C. R. Pillai, U. Pillai, A. Lynn, S. K. Jain, and C. E. Chitnis. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23-33. [DOI] [PubMed] [Google Scholar]
- 27.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 28.Sijwali, P. S., L. S. Brinen, and P. J. Rosenthal. 2001. Systematic optimization of expression and refolding of the Plasmodium falciparum cysteine protease falcipain-2. Protein Expr. Purif. 22:128-134. [DOI] [PubMed] [Google Scholar]
- 29.Sim, B. K., C. E. Chitnis, K. Wasniowska, T. J. Hadley, and L. H. Miller. 1994. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264:1941-1944. [DOI] [PubMed] [Google Scholar]
- 30.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110:293-310. [DOI] [PubMed] [Google Scholar]
- 31.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 32.Weber, J. L. 1987. Analysis of sequences from the extremely A + T-rich genome of Plasmodium falciparum. Gene 52:103-109. [DOI] [PubMed] [Google Scholar]