Abstract
Aims
To investigate the dose-response relationship and contribution of verapamil SR and trandolapril given in combination once a day for the treatment of essential hypertension.
Methods
A randomized, double-blind, placebo controlled, factorial, 12 arm parallel group comparison with placebo, verapamil SR (120, 180 mg), trandolapril (0.5, 1.0, 2.0 mg) covering all combinations of both drugs. A 4 week placebo run-in period followed by 6 weeks of treatment. Four hundred and fifty-six patients from office practice (22 centres) with mild to moderate hypertension enrolled and 426 with diastolic pressure ≥100 mm Hg at the end of run-in period were randomized. Main outcome measures were reduction in sitting systolic (SBP) and sitting diastolic (DBP) blood pressure.
Results
The combination of verapamil SR and trandolapril, particularly verapamil SR 180 mg and trandolapril 0.5 or 1.0 mg was significantly superior to both monocomponents at the same dose (P<0.05). For these combinations, the adjusted mean reductions in DBP from baseline to last visit were 14.1 and 16.0 mm Hg, respectively. Response surface analysis provided further evidence that these combinations were optimal for antihypertensive efficacy. All treatments were well tolerated. The incidence of adverse events did not differ significantly between treatment groups; the profile of adverse events on combination therapy was mild and consistent with that of each monocomponent.
Conclusions
All dosage combinations of verapamil SR and trandolapril produced significantly greater reduction of blood pressure than the monotherapy at the same dosage. However, verapamil SR 180 mg in combination with trandolapril 1.0 mg was the dosage with the greatest blood pressure reduction and had the greatest effects compared with the monocomponents.
Keywords: verapamil, trandolapril, combination therapy, essential hypertension, multifactorial design, dose-response study
Introduction
Treatment of hypertension is usually initiated with a low dose of a single agent and titrated to a higher dose as required [1, 2]. Up to 60% [3] of patients may require the addition of a second agent to achieve satisfactory blood pressure control. The addition of a second drug is often better tolerated and more effective than titration to higher doses of the single agent.
ACE inhibitors [4] and calcium antagonists are effective antihypertensive drugs [2]. Both are well tolerated and lack undesirable metabolic effects [5]. The combination of the calcium antagonist, verapamil SR (sustained release), and the long-acting lipophilic ACE inhibitor, trandolapril [6], may be a useful management strategy for patients with hypertension poorly controlled after monotherapy.
The aim of this study was to investigate the dose-response relation for combination therapy with verapamil SR and trandolapril, and the contribution of each monocomponent in the combination in patients with mild to moderate essential hypertension.
Methods
Four hundred and eighty patients with newly diagnosed or established mild to moderate hypertension, from 23 general practices or hospital outpatients clinics, were enrolled in the study. Patients with severe or malignant hypertension, major organ dysfunction, clinically significant renal, hepatic or gastrointestinal impairment, immunological or connective tissue disorders, a history of recent myocardial infarction (within 3 months), cerebrovascular accident (within 12 months) or hypertensive encephalopathy, or any of the known contraindications to either verapamil or ACE inhibitor therapy, were excluded. Women who were pregnant, lactating, or not using adequate contraceptive measures were also excluded. Concomitant antihypertensive medication was not permitted. The study and protocol were approved by the Ethics Committees (the primary-Landesärztekammer Rheinland Pfalz and the other states Landesärztekammern) and was conducted in accordance with the Declaration of Helsinki. Each patient consented to the study subsequent to full explanation of what was involved in the study and written consent was obtained.
Following a wash-out period of 1 week (for patients previously treated with antihypertensive therapy), patients were enrolled into a 4-week single-blind, placebo run-in period. At the end of the run-in period, patients with a consistently elevated sitting DBP within the range of 100–115 mm Hg, which differed by less than 10 mm Hg from that observed on the previous visit, were randomized to double-blind, placebo-controlled, factorial design, for 6 weeks one of 12 treatment groups: verapamil SR monotherapy (0, 120, 180 mg), trandolapril monotherapy (0, 0.5, 1, 2 mg), or all possible dose combinations of the two drugs. All patients received two capsules (verapamil SR plus trandolapril) once-daily with or immediately after breakfast during the treatment period. Patients were seen at weekly intervals during the run-in period and every 2 weeks during the treatment period.
Compliance and the stability of hypertension were assessed during the run-in period. Blood pressure and heart rate were measured in the sitting and standing positions before the daily dose at trough on the morning of each visit. Blood pressure was measured in the same arm throughout the study using the mercury sphygmomanometer. After resting for 10 min, sitting blood pressure was measured three times at 2 min intervals and the measurement was repeated after standing for 2 min. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were taken as phase I and phase V, respectively, of the Korotkoff sounds. Heart rate was measured from the ECG. Routine haematological, biochemical and urinary investigations were undertaken at the end of the run-in and treatment periods. An ECG at rest was performed at the end of the placebo run-in period and at 2-weekly intervals during the treatment period. Spontaneously reported and observed adverse events were recorded.
Statistical analysis
The reduction in sitting DBP from baseline (defined as the end of the run-in period) at the last available measurement during the treatment period was evaluated using analysis of covariance with verapamil, trandolapril and verapamil-by-trandolapril-interaction and centre effects as factors and baseline sitting DBP as the covariate.
The superiority of combination therapy over either monotherapy was assessed using previously described statistical methods [7–10]. Two kinds of superiority were defined, weak superiority and strong superiority. Weak superiority was assessed by comparing all combination groups containing a specific dosage of one of the monocomponents with all other monocomponent groups simultaneously. Testing started with the higher dose, and only if this was statistically significant (one-sided tests, 5% level) were the lower-dose combinations tested. Variability of sample size in the different treatment groups was accounted for by testing of linear contrasts based on analysis of covariance. Testing for strong superiority was undertaken only if weak superiority was confirmed. Strong superiority was established if combination therapy at a specific combination dosage produced a significantly greater reduction in blood pressure than each component at the same dose (one-sided test for each condition, 5% level) [10]. Analysis of covariance with partial F-tests was used to compare all six combination therapies with the corresponding monotherapies. The reduction in DBP with either monotherapy was also compared with that achieved on matching placebo (one-sided test). Response surface analysis based on a quadratic model, incorporating effects of both monocomponents, the interaction between these and with baseline DBP as a covariate, was used to investigate the dose-response relationship of the combination.
Normalization and responder rates were analysed using a logistic regression model including both monocomponents, the interaction between these and the centre as factors. Safety data were analysed descriptively.
Results
Four hundred and eighty patients were enrolled into the placebo run-in period. There were discrepancies in source data and case report forms in one centre, and all 24 patients enrolled at this centre were excluded from analysis. A further 30 patients were withdrawn during the run-in period of the study for non-medical reasons, and another two patients who were randomized but failed to attend during the treatment period were also excluded from the efficacy analysis. Consequently 424 patients (30 placebo, 59 verapamil monotherapy, 85 trandolapril monotherapy and 250 verapamil SR/trandolapril combination therapy) were evaluable for efficacy. The treatment groups were comparable in baseline characteristics. As regards gender, age, and baseline blood pressure, 234 patients (55.2%) had received antihypertensive therapy prior to entry, most frequently with β-adrenoceptor blockers, calcium antagonists or ACE-inhibitors.
The adjusted mean reduction in DBP from baseline to the last evaluable was most pronounced in the verapamil SR 180 mg/trandolapril 1 mg group (16.0 mm Hg) (Table 1). All the combinations of verapamil SR/trandolapril produced a greater mean reduction in DBP and SBP than the corresponding monotherapy and significantly greater reductions in blood pressure than placebo (analysis of covariance, P<0.005). Testing for weak superiority showed a clear contribution of each monocomponent in the combination. The adjusted mean reduction in DBP with trandolapril monotherapy (all doses, 11.8 mm Hg) was significantly less than that achieved when given in combination with verapamil SR 180 mg (15.1 mm Hg) or 120 mg (14.5 mm Hg); P=0.0015 and 0.0073, respectively. Similarly, the adjusted mean reduction in DBP with verapamil monotherapy (all doses, 10.1 mm Hg) was significantly less than that achieved when given in combination with trandolapril 2 mg (15.1 mm Hg, P=0.00005), trandolapril 1 mg (15.0 mm Hg, P=0.0001) or trandolapril 0.5 mg (14.3 mm Hg, P=0.0016).
Table 1.
Absolute and adjusted mean change in blood pressure (systolic/diastolic blood pressure, mm Hg) from baseline to the last visit on double-blind treatment
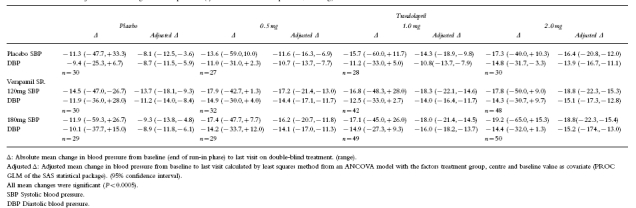
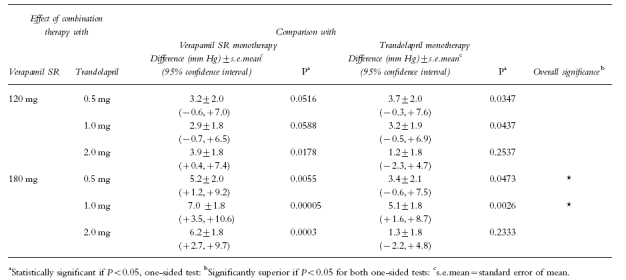
Testing for strong superiority showed that the combination of verapamil SR 180 mg with either trandolapril 0.5 or 1 mg was significantly superior to both mono-components at the same dose (P<0.05, one-sided tests). The combination of verapamil SR 180 mg/trandolapril 1 mg was also significantly superior to the corresponding monotherapy after alpha correction according to Bonferroni (adjusted mean reduction in DBP 16.0 mm Hg; P<0.05). The gain in therapeutic efficacy in terms of reduction in DBP was 5.2 mm Hg for verapamil SR/trandolapril 0.5 mg and 7.0 mm Hg for verapamil SR/trandolapril 1 mg compared with the verapamil component, and 3.4 mm Hg and 5.1 mm Hg respectively compared with the trandolapril component (Table 2). Although the reduction in SBP was greater on combination therapy than on monotherapy, it was not possible to demonstrate a statistically significant strong superiority of the combination therapy over both monotherapies.
Table 2.
Comparison of the effect of combination therapy with the individual agents on the reduction in sitting diastolic blood pressure (n=424)
The response surface analysis for DBP for the verapamil SR component, the model showed progressive reduction in DBP over the dose range 120 to 180 mg. For the trandolapril component, the model showed a progressive reduction in DBP over the dose range 0.5 to 1 mg, with plateauing at around a dose of 1.5 mg. In all treatment groups, except verapamil SR 120 mg once daily, pulse rate was reduced by 0.2 to 3.5 beats×min−1.
The incidence of adverse clinical events was similar during both periods of the study, occurring in 47 of 426 patients (11.0%) during the run-in period and 63 of 426 patients (14.8%) during the double-blind treatment period. During the treatment period the incidence of adverse events did not differ significantly between treatment groups: 17% on placebo, 14–17% on verapamil monotherapy, 15–23% on trandolapril monotherapy and 9–26% on combination therapy. There was no increase in the frequency of reporting of adverse events on higher doses of the component therapies.
The profile of adverse events with the combination was consistent with the adverse event profile of each mono-component. By far the most commonly reported adverse event in each group was headache or pressure in the head, the incidence ranging from 3.3% on verapamil SR monotherapy to 10% on placebo. In the majority of these patients, the event was considered to be at least possibly related to treatment.
During double-blind treatment 13 patients dropped out or were prematurely withdrawn. Out of these seven patients (five on combination therapy, and one each on trandolapril monotherapy or placebo) were withdrawn because of adverse events; however, in only three of these patients were the events considered to be possibly related to study medication. The percentage of patients who dropped-out and/or were withdrawn during double-blind treatment was comparable for all treatment regimens (3.3–3.6%), except of verapamil SR monotherapy (0.0%).
There was no evidence of any clinically relevant trends for changes in any of the routine laboratory safety variables or in the resting ECG during the treatment phase.
Discussion
This study demonstrates the combination of various dosages of verapamil (SR 180 mg) and trandolapril (0.5 or 1 mg) are significantly superior to both monocomponents at the same dosages in regard to blood pressure lowering effects (adjusted mean reduction in DBP 14.1 and 16.0 mm Hg, P<0.05 for both one-sided tests). Response surface analysis also confirmed that this combination is optimal for blood pressure reduction. The results of this study agree with previous findings which have indicated the suitability of combination therapy with verapamil SR and an ACE inhibitor in mild to moderate hypertension [11].
The combination of verapamil SR and trandolapril has recently [12] been compared with atenolol/chlorothiazide, lisinopril/hydrochlorothiazide and placebo. The low dose combination verapamil SR/trandolapril 180/2 mg once a day showed a similar response as atenolol/chlorothiazide 100/25 mg once daily or lisinopril/hydrochlorothiazide 20/12.5 mg once daily. The combination of half doses of verapamil SR (120 mg) and trandolapril (1 mg) is more effective in reducing blood pressure than a full dose of either drug [13].
Combination therapy can offer advantages over monotherapy for the patient with poorly controlled hypertension: superior efficacy, improved tolerability. A fixed combination may promote compliance, and acceptability of treatment.
In this study, combination therapy with verapamil SR/trandolapril was generally well tolerated with the overall incidence and frequency of adverse events comparable with those observed with monotherapy with either component.
In conclusion, the antihypertensive effect of combination therapy with verapamil SR/trandolapril was shown to be significantly superior to monotherapy with either component in the group of patients studied. The dose with the most pronounced blood pressure lowering effect was verapamil SR 180 mg plus trandolapril 1 mg. Verapamil SR/ trandolapril combination therapy was well tolerated.
Acknowledgments
This study was financially supported by Knoll A. G., Ludwigshafen, Germany
References
- 1.The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNCV) Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 2.Guidelines Sub-Committee. 1993 Guidelines for the management of mild hypertension: memorandum from a World Health Organisation/International Society of Hypertension meeting. J Hypertension. 1993;11:905–918. doi: 10.1097/00004872-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 3.James IM. Which hypertensive? Br J Clin Pract. 1990;44:102–105. [PubMed] [Google Scholar]
- 4.Scholze J. on behalf of the executive committee of the German hypertension society. ACE-inhibitors as first line choice agents for the monotherapy in hypertension. Nieren- und Hochdruckkrankheiten. 1995;24:12–15. [Google Scholar]
- 5.Lithell H. Insulin resistance and cardiovascular drugs. Clin Exp Hypertens. 1993;14:151–162. doi: 10.3109/10641969209036178. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman LR, McTavish D. Trandolapril. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in essential hypertension. Drugs. 1994;48:71–90. doi: 10.2165/00003495-199448010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hung HMJ, Ng T-H, Chi GYH, Lipicky RJ. Response surface and factorial designs for combination antihypertensive drugs. Drug Information J. 1990;24:371–378. [Google Scholar]
- 8.Hung HMJ, Ng T-H, Chi GYH, Lipicky RJ. Testing for the existence of a dose combination beating its components. Proceedings of the Biopharmaceutical Section of the American Statistical Association. 1989 [Google Scholar]
- 9.Ng TH, Hung HMJ, Chi GYH. A new statistical method for testing a combination therapy's superiority to both of its components. Proceedings of the Biopharmaceutical Section of the American Statistical Association. 1989 [Google Scholar]
- 10.Laska EM, Meissner MJ. Testing whether an identified treatment is best. Biometrics. 1989;45:1139–1151. [PubMed] [Google Scholar]
- 11.Peto R. Clinical trial methodology. Biomedicine Special Issue. 1978;28:24–36. [PubMed] [Google Scholar]
- 12.de Leeuw PW, Notter T, Zilles P. Comparison of different fixed antihypertensive combination drugs. A double blind, placebo-controlled parallel group study. J Hypertension. 1997;15:87–91. doi: 10.1097/00004872-199715010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Nalbantgil I, Önder R, Nalbantgil S. Verapamil SR and trandolapril and their combination in the treatment of obese hypertensive subjects, a double blind study. Curr Ther Res. 1996;57:990–997. [Google Scholar]


