Abstract
Aims
Endothelin is a peptide produced by endothelial cells with many biological properties. In the human skin microcirculation endothelin induces neurogenic vasodilation associated with burning pruritus. We investigated the mechanisms involved in this response.
Methods
The effects of prolonged pretreatment with capsaicin, a specific inhibitor of polimodal nociceptor fibres, and of the nitric oxide synthase inhibitor l-NMMA on endothelin-1-induced vasodilation were studied in 15 human subjects. Furthermore, we investigated the effects of the ETA-selective antagonist PD147953 on bradykinin-induced vasodilation.
Results
After local injection, endothelin-1 caused vasoconstriction at the injection site and a profound vasodilation in the surrounding area (flare reaction, P<0.01). This response was specific and not induced by saline, albumin, acetylcholine or an ET-antagonist. Prolonged capsaicin pretreatment inhibited endothelin-1 induced vasodilation in the area surrounding the injection site, but not the central vasoconstriction at the injection site. Bradykinin also induced a marked vasodilation in the area surrounding the injection site; this was not inhibited by an ETA-selective antagonist, while the flare reaction was. l-NMMA applied at the site of the flare reaction prevented endothelin-1-induced vasodilation.
Conclusions
Endothelin-1 in the human skin microcirculation stimulates polimodal nociceptor fibres leading to the release of nitric oxide. This response may play a pathophysiological role in inflammatory processes in the human skin.
Keywords: endothelin-1, nitric oxide, human skin, nociceptive fibres
Introduction
Endothelin is a 21-amino acid peptide produced by endothelial cells with potent vasoconstrictor properties [1]. However, endothelin also influences embryonic development [2], acts as a mitogen [3] and has important central nervous system effects. Endothelin is expressed in human neurons and can activate the sympathetic nervous system after intracisternal infusion [4].
In the human skin microcirculation endothelin-1 not only induces potent vasoconstriction at the injection site, but profound vasodilation in the surrounding area [5]. Endothelin-3, which has a higher affinity to ETB-receptors, induces a weaker vasodilation in the skin microcirculation [5]. This endothelin-induced vasodilation is prevented by co-administration of lignocaine [5] suggesting a neurogenic mechanism. Administration of an ETA-receptor antagonist prevents not only endothelin-induced vasoconstriction, but also neurogenic vasodilation [5]. An ETA-/ETB-receptor antagonist has no additional effect [5, 6].
The human skin protects the body from a variety of potentially dangerous influences. Therefore, a nocifensor system has been postulated which—through the release of neuronal mediators and autacoids—is responsible for the so-called axon-reaction [7]. Serotonin, bradykinin and histamine induce neurogenic local inflammatory reactions. Some of these effects seem to be mediated by small myelinated (Aδ) or unmyelinated (C-type) fibres. Experimentally, these nociceptor fibres can be stimulated by mechanical, thermal and chemical stimuli such as serotonin, bradykinin and substance P [8]. Capsaicin is a neurotoxin, which after prolonged application selectively blocks polimodal nociceptor fibres [9].
To elucidate the mechanisms of endothelin-induced neurogenic vasodilation in the human skin microcirculation, we studied the effects of prolonged capsaicin application on endothelin-1 induced vasodilation in human subjects. As in vitro endothelin stimulates the release of nitric oxide from endothelial cells [10, 11] and peripheral neurons release it [12], we assessed the effects of nitric oxide synthase inhibition on endothelin-induced vasodilation.
Methods
Study population
Experiments were performed in 15 male healthy volunteers (age 18–29 years) who were investigated repeatedly. Subjects were in supine position during the experiment with the forearm fixed to avoid artifacts. The study was approved by the Ethics Committee of the Inselspital, Bern, Switzerland. All subjects gave their written informed consent prior to experiments.
Experimental setup
Room temperature was kept between 19–22° C. In all subjects stability of skin blood flow during the experiment was confirmed at a site where no injection was made. After a resting period of 20 min, injection site and the measurement point at 8 mm distance were marked with a template on the volar forearm. Not more than four injection sites were allowed per forearm. After baseline measurements, agents were injected with 0.4 mm needles (Omnikan® 30, B. Braun, Melsungen/FRG). Two injections (10 μl each) were performed at the same site. First either saline, l-NMMA (10−6 mol) or the ET-antagonist (10−8 mol) were injected followed by either endothelin-1 (10−12 mol), acetylcholine (10−8 mol), bradykinin (10−12 mol) or noradrenaline (10−10 mol). Injections were made strictly intradermally producing a symmetrical wheal without visible spreading outside the wheel. If these criteria were not fulfilled the injection site was excluded from analysis. In l-NMMA experiments, the second injection (endothelin or saline) was made 20 min after injection of l-NMMA, as pilot experiments had shown that this period was necessary to inhibit NO-synthase. Injections of l-NMMA were performed in the flare area, i.e. at 8 mm distance from the injection site of endothelin. Control experiments were performed with noradrenaline injected at the same site, i.e. at 8 mm distance from the injection of endothelin. For the capsaicin experiments, a 0.25% capsaicin ointment was applied for 12 h on a 4×4 cm area of the volar surface of the forearm; before injection of endothelin-1, absolute blood flow was assessed. Endothelin injection was performed under control conditions as well as 3 h after removal of the capsaicin ointment. Control experiments with saline were performed with the same modalities of injection. A Periflux laser Doppler flowmeter (Perimed®, Sweden) with a probe holder was used to assess skin blood flow [13, 14]. The resulting voltage output, expressed in arbitrary ‘perfusion units’, is an index of blood flow [13]. Blood flow was measured before and 5, 10, 15, 20, 25 and 30 min after injection at the two marked sites [5, 6].
Drugs
Endothelin-1, l-nitro-monomethyl-arginine (l-NMMA), bradykinin, acetylcholine (all Clinalfa), PD147953 (Parke-Davis), noradrenaline, 0.9% NaCl solution, 0.25% capsaicin ointment was used (all Hospital Pharmacy, Inselspital Bern, Switzerland). Solutions of 10−7 mol l−1 of endothelin-1 were prepared corresponding to 10−12 mol/10 μl. For endothelin antagonist and l-NMMA, a solution of 10−6 mol/10 μl was prepared. For bradykinin and acetylcholine a dilution of 10−9 and 10−8 mol/10 μl was used, respectively. Solutions were prepared immediately before use to avoid loss of efficacy [5].
Analysis and statistics
The mean between minimum and maximum value of a 20 s reading was calculated. Values were registered and analysed using Stat View 4.5 (ABACUS Inc). Results are expressed as differences from baseline and control (Δ) in mean±s.e. mean; the significance of differences was calculated by multiple analysis of variances with the factors drug and time (95% confidence intervals). A Bonferroni post-hoc test with correction for multiple comparisons was used to identify the statistical differences of the drug used.
Results
Endothelin-1
At the injection site, endothelin-1 induced marked vasoconstriction (−65±16 PU; P<0.001), but it increased blood flow within the area of axon reaction, i.e. at a 8 mm distance from the injection site (Figures 1 and 2; P<0.001 vs saline). In all subjects, endothelin-1 injection induced a burning pruritus.
Figure 1.
Experiment showing endothelin-1 induced vasodilation in the area surrounding the injection site before (a) and after (b) prolonged pretreatment with capsaicin ointment (0.25%; 12 h). Note the marked flush spreading in the area surrounding the injection, which was markedly blunted after capsaicin treatment
Figure 2.
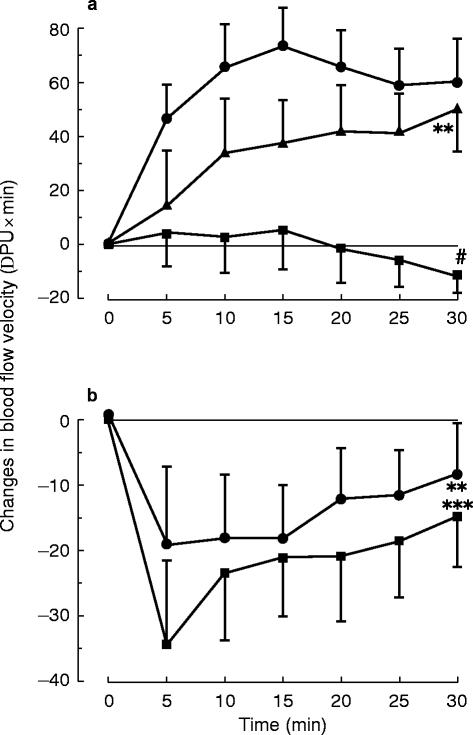
Effect of capsaicin on endothelin-1 induced neurogenic vasodilation in the human skin microcirculation in vivo: Subjects were treated locally with capsaicin ointment. Treatment with capsaicin (0.25% ointment over 12 h; □) markedly reduced endothelin-1 (10−12 mol; •) induced neurogenic vasodilation. ***P<0.001 vs saline, #P<0.02vs control
Bradykinin
At the injection site, bradykinin did not significantly change blood flow. In contrast, bradykinin increased blood flow within the area of axon reaction (+99±46 PU; P<0.01). Bradykinin injection induced a similar burning pruritus as endothelin-1.
Acetylcholine
At the injection site, acetylcholine markedly increased blood flow (+52±12 PU; P<0.01). This vasodilation at the injection site was prevented by l-NMMA (10−6 mol; P<0.05 vs acetylcholine). Acetylcholine did not induce a flare reaction. No pruritus or burning occurred after acetylcholine injection.
Noradrenaline
At the injection site, noradrenaline (10−10 mol) induced marked vasoconstriction (Figure 3 [right panel]). However, noradrenaline had no effect on endothelin-induced vasodilation (Figure 3 [left panel]). Noradrenaline (10−10 mol) did not induce a flare reaction. There was no pruritus after the injection of noradrenaline.
Figure 3.
a) Effect of l-NMMA (10−6 mol, ▪) and noradrenaline (10−10 mol, ▴) on endothelin-1 (10−12 mol, •) induced vasodilation in the human skin microcirculation in vivo. Injection of l-NMMA into endothelin-induced flare prevented vasodilation, whereas noradrenaline had no effect. b) Control experiments with l-NMMA (10−6 mol, •) and noradrenaline (10−10 mol, ▪). Both substances induced similar vasoconstriction. Data are changes to control. **/***P<0.01/0.001 vs saline, #P<0.02 vs control
Capsaicin
After prolonged local capsaicin pretreatment (0.25% ointment for 12 h), baseline blood flow before endothelin-1 injection was similar to control conditions (NS). Also, pretreatment with capsaicin did not influence endothelin-1 induced vasoconstriction at the injection site (NS). In contrast, prolonged pretreatment with capsaicin (0.25% ointment for 12 h) markedly inhibited endothelin-1 induced vasodilation within the area of axon reaction (Figures 1 and 2).
Endothelin-antagonist
The ETA-receptor antagonist PD147953 did not change blood flow within the area of axon reaction, i.e. 8 mm from injection site, but induced a vasodilation at the injection site (data not shown); furthermore and in contrast to the response to ET-1 [5, 6], PD147953 did not significantly influence bradykinin-induced vasodilation in the surrounding area (NS vs bradykinin alone).
l-nitro-monomethyl-arginine (l-NMMA)
l-NMMA did not induce a flare reaction nor any pruritus. Injection of l-NMMA (10−6 mol) alone decreased blood flow to a similar degree as norepinephrine in the central area (Figure 3 [right panel]). Nevertheless, l-NMMA, in contrast to noradrenaline, when injected into the area of axon reaction induced by endothelin-1 fully prevented endothelin-induced vasodilation (Figure 3 [left panel]).
Discussion
This study demonstrates, that in the human skin microcirculation endothelin-1 causes neurogenic vasodilation associated with burning pruritus mediated by polimodal nociceptor fibres. Indeed, prolonged application of capsaicin, which specifically inhibits these fibres by depletion of their neurotransmitters, markedly reduced endothelin-induced vasodilation under these conditions. Furthermore, this study shows that the stimulation of these fibres by endothelin via ETA-receptors [5] leads to the local release of nitric oxide, as the inhibitor of nitric oxide production l-NMMA specifically prevented endothelin-induced vasodilation.
Endothelin is known to cause vasodilation by activating endothelial ETB-receptors and release of nitric oxide and prostacyclin [10, 11]. The vasodilation of the skin microcirculation described in this study, however, differs from this response for the following reasons: 1) this vasodilation involves ETA-receptors, rather than ETB-receptors [5]; 2) the time course differs, as the vasodilation follows (15–30 s) rather than preceeds the vasoconstriction; 3) the flare response occurs at a distance of 1–2 cm from the injection site and 4) lignocaine [5] as well as prolonged pretreatment with capsaicin inhibits this vasodilation demonstrating that neural mechanisms, i.e. polimodal nociceptor fibres, are involved.
The effects of endothelin in the human skin microcirculation are specific and not related to the injection itself. Indeed, several other substances such as saline, albumin, noradrenaline, l-NMMA, acetylcholine, lignocaine and endothelin-antagonists do not cause a flare reaction, although they share the same injection trauma [5, 6].
Endothelin-1 induced vasodilation in the area surrounding the injection site can be prevented by an ETA-selective antagonist [5]. Furthermore, endothelin-3, which has a higher affinity to ETB-receptors, induces a weaker vasodilation [5]. Thus, in the human skin microcirculation, endothelin-1 acts mainly through ETA-receptors.
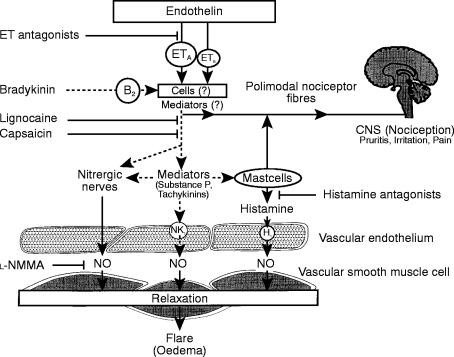
The fact, that endothelin-1 induced vasodilation in the surrounding area can be prevented by lignocaine [5] and markedly inhibited by prolonged treatment with capsaicin, demonstrates, that neural mechanisms are involved. However, neurons predominantly express the ETB-receptor [15, 16], whereas the effects of endothelin-1 in the human skin microcirculation are mediated by ETA-receptors. In addition to vascular smooth muscle cells [17], ETA-receptors have been shown to be present on other cells such as macrophages [18], skin and cardiac fibroblasts [19], human keratinocytes [20], and platelets [21], although the mentioned cells partially express also ETB-receptors [18, 22]. Thus, endothelin-1 induced vasodilation in the human skin microcirculation is possibly mediated via release of mediators from cells of the connective tissue (Figure 4); indeed, endothelin in vitro can stimulate mast cells of several species to release mediators such as histamine and cytokines [23], although it does not stimulate human mast cells in vitro [24]. However, to elucidate which cells and mediators are involved in this response, goes beyond the scope of the present in vivo study.
Figure 4.
Hypothesis of mechanisms of endothelin-induced vasodilation in the human skin microcirculation in vivo: Endothelin via ETA-receptors stimulates polimodal nociceptor fibres or activates cells (i.e. mastcells, fibroblasts) which stimulate nociceptor fibres. This leads to centripetal message to the brain (pruritus, burning) and locally to release of nitric oxide and vasodilation. Dashed lines: speculative mechanisms of the hypothesis
Endothelin-1 releases nitric oxide through the stimulation of these polimodal nociceptor fibres (Figure 4). In isolated blood vessels, endothelin releases nitric oxide from endothelial cells [10, 11]. In the human skin microcirculation, however, a novel neuronal mechanism of interaction between endothelin and nitric oxide exists, as the release of nitric oxide from endothelial cells is mediated via ETB- rather than ETA-receptors [10, 11]. Neurons of nitrergic nerves also release nitric oxide in the brain, blood vessels and corpora cavernosa [25–27]. Hence, it is likely, that polimodal nociceptor fibres either release nitric oxide themselves or are interconnected with nitrergic fibres innervating skin microvessels.
The fact that the endothelin antagonist could not prevent bradykinin-induced flare reaction shows that bradykinin does not act through the release of endogenous endothelin. If endogenous endothelin plays a role in the axon reaction, it possibly acts through other mechanisms or acts at an earlier stage of the cascade of mediators present in the skin microcirculation (Figure 4). It is likely, that the flare reaction induced by bradykinin leads to the release of nitric oxide, too; however, it was beyond the scope of the present study to investigate this effect.
Besides the local effects of the axon-reaction, an important mechanism of the nocifensor system is to transmit local irritation to central nervous centres mediated by these fibres. Polimodal nociceptor fibres react to heat, chemical and mechanical stimuli; this causes vasodilation, and a centripetal message to the central nervous system (pruritus irritation, pain) probably mediated in part through the release of histamine [8]; in some cases also capillary leakage leading to local oedema is induced (Figure 4). In this study, endothelin led to marked pruritus and burning, which was not seen with several other substances. Since endothelin is involved in this axon reaction it may play a role in the nocifensor system, which helps to protect the body from dangerous environmental impacts (Figure 4).
If endogenous endothelin is involved in neurogenic inflammation, this could have important clinical implications. Indeed, in several skin diseases such as diabetic neuropathy [28–31], herpes zoster [32, 33], psoriasis [34], and (solar) pruritus [34], but also in arthritis [35], cluster headache [36] and erythromelalgia [37], involvement of capsaicin-sensitive fibres leading to neurogenic inflammation contributes to the development and/or maintenance of these diseases. Loss of nociception and axon-reflex vasodilation occurs in diabetic neuropathy [28].
In summary, the present study reports a new mechanism of endothelin-1 in the human skin microcirculation, which involves polimodal nociceptor fibres and release of nitric oxide. Future studies will elucidate the role of endogenous endothelin in neurogenic inflammation and whether endothelin-antagonists could be useful in the treatment of diseases in which this reaction is involved.
Acknowledgments
This study was supported by the Swiss National Foundation (No. 32-32655.91 and No. 32-35591.92 [SCORE]). René R. Wenzel was recipient of a stipend of the German Research Association (Deutsche Forschungsgemeinschaft, No. WE 1772/1-1).
References
- 1.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara Y, Kurihara H, Suzuki H, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 3.Takuwa N, Takuwa Y, Yanagisawa M, Yamashita K, Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989;264:7856–7861. [PubMed] [Google Scholar]
- 4.Kawano Y, Yoshida K, Yoshimi H, Kuramochi M, Omae T. The cardiovascular effect of intracerebroventricular endothelin in rats. J Hypertens. 1989;7(suppl):522–523. doi: 10.1097/00004872-198900076-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RR, Noll G, Lüscher TF. Endothelin receptor antagonists inhibit endothelin in human skin microcirculation. Hypertension. 1994;23:581–586. doi: 10.1161/01.hyp.23.5.581. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel RR, Duthiers N, Noll G, Bucher J, Kaufmann U, Lüscher TF. Endothelin and calcium antagonists in the skin microcirculation of patients with coronary artery disease. Circulation. 1996;94:316–322. doi: 10.1161/01.cir.94.3.316. [DOI] [PubMed] [Google Scholar]
- 7.Lewis T. Experiments relating to cutaneous hyperalgesia and its spread through somatic nerves. Clin Sci. 1935;2:373–423. [Google Scholar]
- 8.Pierau FK, Szolcsanyi J. Neurogenic inflammation: Axon reflex in pigs. Agents and Actions. 1989;26:231–232. doi: 10.1007/BF02126621. [DOI] [PubMed] [Google Scholar]
- 9.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and pretreatment with capsaicin. Br J Pharmacol Chemother. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Nucci G, Thomas R, D’Orleans JP, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohi Y, Lüscher TF. Endothelin in hypertensive resistance arteries. Intraluminal and extraluminal dysfunction. Hypertension. 1991;18:543–549. doi: 10.1161/01.hyp.18.4.543. [DOI] [PubMed] [Google Scholar]
- 12.Bredt DS, Synder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 13.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254:56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson GE, Tenland T, Öberg PA. A new instrument for continuous measurement of tissue blood flow by light beating spectroscopy. IEEE Trans Biom Eng. 1980:12–19. doi: 10.1109/TBME.1980.326686. BME-27, No. 1. [DOI] [PubMed] [Google Scholar]
- 15.Baynash AG, Hosoda K, Giaid A, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 16.Takimoto M, Inui T, Okada T, Urade Y. Contraction of smooth muscle by activation of endothelin receptors on autonomic neurons. FEBS Lett. 1993;324:277–282. doi: 10.1016/0014-5793(93)80134-g. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- 18.Kishino J, Hanasaki K, Kato T, Arita H. Endothelin-induced intracellular Ca2+ mobilization through its specific receptors in murine peritoneal macrophages. FEBS Lett. 1991;280:103–106. doi: 10.1016/0014-5793(91)80214-n. [DOI] [PubMed] [Google Scholar]
- 19.Fujisaki H, Ito H, Hirata Y, et al. Natriuretic peptides inhibit angiotensin II-induced proliferation of rat cardiac fibroblasts by blocking endothelin-1 gene expression. J Clin Invest. 1995;96:1059–1065. doi: 10.1172/JCI118092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagnato A, Venuti A, Di Castro V, Marcante ML. Identification of the ETA receptor subtype that mediates endothelin induced autocrine proliferation of normal human keratinocytes. Biochem Biophys Res Commun. 1995;209:80–86. doi: 10.1006/bbrc.1995.1473. [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM, Lariviere R, Schiffrin EL. Endothelin influences pHi of human platelets through protein kinase C mediated pathways. Thromb Res. 1995;78:55–65. doi: 10.1016/0049-3848(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 22.Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 23.Uchida Y, Ninomiya H, Sakamoto T, et al. ET-1 released histamine from guinea pig pulmonary but not peritoneal mast cells. Biochem Biophys Res Commun. 1992;189:1196–1201. doi: 10.1016/0006-291x(92)92331-q. [DOI] [PubMed] [Google Scholar]
- 24.Brain SD, Thomas G, Crossman DC, Fuller R, Church MK (1992) Endothelin-1 induces a histamine-dependent flare in vivo, but does not activate human skin mast cells in vitro. Br J Clin Pharmacol. 1992;33:117–120. doi: 10.1111/j.1365-2125.1992.tb04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasakov L, Cellek S, Moncada S. Characterization of nitrergic neurotransmission during short- and long-term electrical stimulation of the rabbit anococcygeus muscle. Br J Pharmacol. 1995;115:1149–1154. doi: 10.1111/j.1476-5381.1995.tb15017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 27.Rand MJ, Li CG. Modulation of acetylcholine-induced contractions of the rat anococcygeus muscle by activation of nitrergic nerves. Br J Pharmacol. 1993;110:1479–1482. doi: 10.1111/j.1476-5381.1993.tb13988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nature Medicine. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer M, Ross D, Schrage J, et al. A highly successful and novel model for treatment of chronic painful diabetic peripheral neuropathy. Diabetes Care. 1993;16:1103–1115. doi: 10.2337/diacare.16.8.1103. [DOI] [PubMed] [Google Scholar]
- 30.Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Arch Intern Med. 1991;151:2225–2229. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- 31.Tandan R, Lewis G, Badger G, Fries T. Topical capsaicin in painful diabetic neuropathy. Effect on sensory function. Diabetes Care. 1992;15:15–18. doi: 10.2337/diacare.15.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Peikert A, Hentrich M, Ochs G. Topical 0.025% capsaicin in chronic post-herpetic neuralgia: efficacy, predictors of response and long-term course. J Neurol. 1991;238:452–456. doi: 10.1007/BF00314653. [DOI] [PubMed] [Google Scholar]
- 33.Watson C, Tyler K, Bickers D, Millikan L, Smith S, Coleman E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther. 1993;15:510–526. [PubMed] [Google Scholar]
- 34.Ellis C, Berberian B, Sulica V, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438–442. doi: 10.1016/0190-9622(93)70208-b. [DOI] [PubMed] [Google Scholar]
- 35.Deal C, Schnitzer T, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383–395. [PubMed] [Google Scholar]
- 36.Fusco B, Marabini S, Maggi C, Fiore G, Geppetti P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain. 1994;59:321–325. doi: 10.1016/0304-3959(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 37.Muhiddin K, Gallen I, Harries S, Pearce V. The use of capsaicin cream in a case of erythromelalgia. Postgrad Med J. 1994;70:841–843. doi: 10.1136/pgmj.70.829.841. [DOI] [PMC free article] [PubMed] [Google Scholar]