Abstract
Aims
It has been assumed that both plasma cholinesterase (EC 3.1.1.8) and oxidative enzymes are needed for optimum formation of the bronchodilator terbutaline from its biscarbamate prodrug bambuterol. The present study aimed at investigating the fate of bambuterol in subjects with deficient plasma cholinesterase but with normal oxidative (CYP2D6) capability.
Methods
The pharmacokinetics of bambuterol and terbutaline were studied in four healthy subjects (two men and two women) being homozygous for the atypical gene for plasma cholinesterase. Their oxidative metabolism was apparently good as they were all rapid metabolizers of debrisoquine. Bambuterol hydrochloride 20 mg was given orally once daily for 10 days, and plasma and urine samples were taken for 1.5 days (plasma) and 4.5 days (urine) after administration of the last dose.
Results
The pharmacokinetic parameters in the present study were grossly similar to those found in a study of bambuterol in subjects with normal plasma cholinesterase activity (N). However, subjects with atypical cholinesterase had a shorter terminal half-life of bambuterol (a measure of uptake rate), 4.8–12.6 h vs 8.3–22.3 h in N, and slightly higher plasma concentrations of bambuterol (average concentrations 1.9–3.7 nmol l−1vs 1.5–3.1 nmol l−1 in N). Peak/trough terbutaline plasma concentrations ratios (2.1–3.2) were somewhat increased, but average plasma concentrations (8.3–14.5 nmol l−1) and terminal half-life (16.5–21.8 h) of terbutaline did not differ.
Conclusions
In Caucasian populations, one subject out of 2500 is homozygous for the atypical gene for plasma cholinesterase. The atypical enzyme has a much lower affinity for bambuterol than the normal enzyme. Nevertheless, the subjects with atypical cholinesterase were able to produce terbutaline as efficiently as normal subjects. This might be explained by an altered uptake and metabolism in the absence of plasma cholinesterase, or the importance of this enzyme for the formation of terbutaline from bambuterol in vivo may have been overestimated.
Keywords: Bambuterol, terbutaline, pharmacokinetics, atypical plasma cholinesterase
Introduction
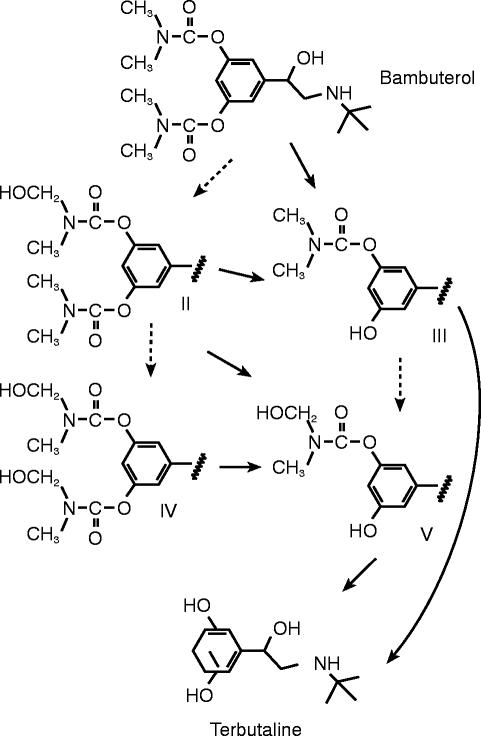
Bambuterol is the inactive bis-N,N-dimethylcarbamate prodrug to the β2-adrenoceptor agonist terbutaline. The active moiety is formed through hydrolytic and/or oxidative pathways (Figure 1). The hydrolytic reactions are catalysed mainly by plasma cholinesterase (EC 3.1.1.8) and the oxidative reactions most likely by cytochrome P450 (CYP) enzymes [1].
Figure 1.
Oxidative (---→) and hydrolytic (→) metabolic pathways of bambuterol to terbutaline
Plasma cholinesterase (pChE) is produced by the liver and exhibits polymorphism [2]. The activity of pChE may be reduced for several reasons such as physiological variation, disease, drugs, and genetic factors. This does not seem to interfere with normal physiology but may influence the duration of action of substances dependent on pChE for their elimination, such as suxamethonium and mivacurium [2–6].
Bambuterol has a very high affinity for pChE from subjects with normal genotype (UU) or from those heterozygous for the atypical and the normal gene (UA). Much lower affinity of bambuterol is seen towards the plasma cholinesterase produced in subjects homozygous for the atypical gene (AA) and hydrolysis of bambuterol is slow in plasma from these subjects [7]. Homozygotes for the atypical gene are rare in Caucasian populations, the frequency being about 1/2500 in the Danish population [3].
In healthy subjects with normal cholinesterase genotype, the administration of a normal oral dose (about 20 mg) of bambuterol hydrochloride once daily resulted in sustained plasma concentration profiles of terbutaline with a mean peak/trough ratio of slightly less than 2.0 [8]. The pChE activities just before dosing were between 55 and 77% (mean 67%) of non-inhibited activities. One hour after dosing, the activities had dropped to between 17 and 60% (mean 40%), but 48 h after the last dose they were all at least 85% (mean 95%). Two weeks after the last dose, the subjects had regained their normal pChE activities.
In Denmark, a register has been built up about subjects with abnormal genotypes of pChE [3]. This offered a possibility to recruit a group of individuals for a study of the fate of bambuterol and the formation of terbutaline in subjects with deficient pChE but with normal oxidative (CYP2D6) capability.
Methods
The study was of an open design. It was run at Herlev Hospital, Herlev, Denmark, in accordance with the Declaration of Helsinki, approved by the Local Ethics Committee, and registered by the Danish National Board of Health and Welfare. The subjects were fully informed verbally and in writing about the nature and objective of the study. Verbal consent to participation was obtained prior to the start of the study.
Subjects
Four healthy subjects, two men and two women (sisters), participated. The subjects were homozygous for the atypical gene for pChE according to the determination of genotype made by the Danish Cholinesterase Research Unit and selected from the card index of this unit.
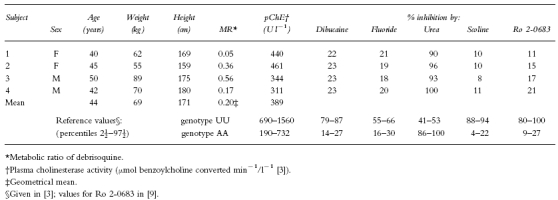
Demographic data and biochemical findings indicative of pChE genotype of the subjects are given in Table 1. The genotype determination is a result of family studies (pedigree investigations) in combination with biochemical phenotyping using a battery of selective inhibitors [3, 9]. In this study, the family investigation assured that the four subjects were homozygous for the atypical gene (AA), otherwise this genotype could not have been distinguished from that with one silent and one atypical gene (AS).
Table 1.
Demographic data and biochemical characteristics of the subjects before treatment with bambuterol
Hydrolytic capability
The subjects’ basal pChE activities measured by the hydrolysis of benzoylcholine [10] were 311–461 (mean 389) U l−1 in blood samples taken 17 days before start of treatment (Table 1). Normal range is 690–1560 U l−1 [3].
Oxidative capability
As a part of the exclusion criteria, and before entering the study, the subjects were tested for their ability to metabolize debrisoquine [11]. The metabolic ratio (MR) between debrisoquine and its metabolite 4-hydroxy-debrisoquine depends on the activity of the enzyme CYP2D6. All subjects were found to be fast metabolizers of debrisoquine (MR<12.6, Table 1) and, on average, 79% of the measured 6 h urinary excretion was the hydroxylated metabolite.
Treatment plan
The subjects were given repeated doses of bambuterol hydrochloride as rapidly dissolving tablets (Bambec®, Astra, Sweden) over a period of 10 days (days 1–10). The dose was 20 mg (50 μmol) taken in the morning after breakfast, then no food was allowed for at least 2 h.
Blood samples were obtained before treatment and up to 36 h after the last dose (days 10–11). The blood samples (10 ml) were collected into sodium-heparinized Venoject® sampling tubes into which 100 μl of a 1 mm solution of an esterase inhibitor (D2456, a bambuterol analogue [12]) had been added. The tubes were immediately centrifuged, and the plasma was stored at −20° C.
Urine was collected in tared polyethene bottles. Two initial collection periods were 6 h, then 12 h collections ensued until 108 h after the last dosing. Two 10 ml portions were stored at −20° C until analysed.
Laboratory assessments
Bambuterol was measured by gas chromatography plus mass spectrometry [12]. At concentrations of 1 nmol l−1 in plasma and 8 nmol l−1 in urine (spiked samples), the coefficient of variation (CV) was about 4% for plasma and 5.4% for urine at the time of validation of the methods (within-day variation). The lower limit of quantification was set to 0.5 nmol l−1 in plasma and to 4 nmol l−1 in urine.
Terbutaline was measured by high performance liquid chromatography with electrochemical detection. The published procedure [13] was slightly modified for analysis of the urine samples. At the time of validation of the methods, the CV (spiked samples) at 8 nmol l−1 in plasma was 2.1% within a day and 3.6% between days. In urine at concentrations of 120 and 320 nmol l−1, CV was 7.2 and 4.6%, respectively (between-day variation). The lower limit of quantification was set to 4 nmol l−1 in plasma and to 80 nmol l−1 in urine.
Plasma cholinesterase activity during and after treatment was measured at steady state on days 10–11 (0, 1, 3, 12, and 24 h after dose) and 3 weeks after the last dose of bambuterol.
Plasma and 6 h collections of urine for the determination of creatinine clearance were sampled before treatment and on day 10. The samples were taken from collections also intended for the determination of debrisoquine (before treatment) or bambuterol and terbutaline (day 10). One plasma sample was taken in the middle of the urine collection interval.
Pharmacokinetic parameters
AUC=area under the curve of plasma concentration vs time during the dosing interval, calculated by the linear trapezoidal rule.
Css=average plasma concentration, calculated as AUC divided by the length of the dosing interval (24 h).
Cmax=peak plasma concentration
Tmax=time of peak plasma concentration
Cmin=trough plasma concentration
Ae=amount excreted in urine during the dosing interval.
Renal clearance was calculated as Ae divided by AUC.
dAe/dt=urinary excretion rate, calculated as the amount excreted in a collection period divided by the length of the collection period. The calculated rate was time-located at the midpoint of the period.
ω=terminal disappearance rate constant, calculated from the slope found after linear regression of ln[dAe/dt] vs time data during the last, monoexponential phase of the curve.
Terminal half-life=ln 2/ω
Creatinine clearance was calculated by dividing dAe/dt of creatinine by plasma creatinine.
Statistical test
A two-tailed t-test for difference in means was applied to compare the terminal half-lives of bambuterol and terbutaline found in this study and the ones found in a previous study in healthy subjects with normal pChE activity [8].The test was performed on the assumption of an additive model. The level of significance was defined as P=0.05.
Results
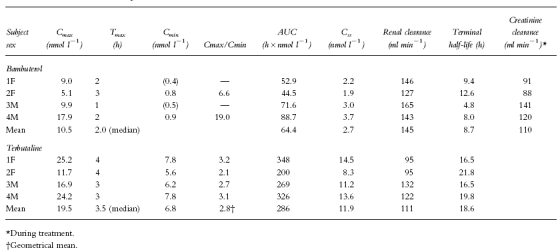
Pharmacokinetic parameters are given in Table 2.
Table 2.
Characteristics of the plasma concentration-time curves, renal clearances, and terminal half-lives of bambuterol and terbutaline
Plasma data
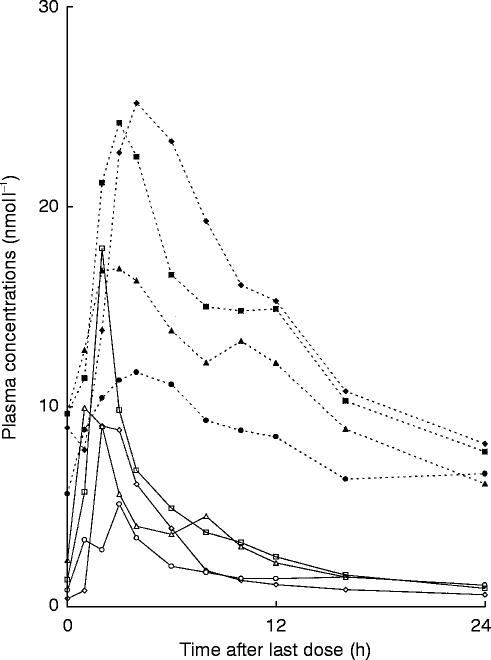
No subject had detectable plasma concentrations of bambuterol or terbutaline before entering the study. Measured plasma concentrations in the four subjects during a dosing interval after 10 days treatment are shown in Figure 2.
Figure 2.
Steady-state plasma concentrations of bambuterol and terbutaline in four subjects with atypical plasma cholinesterase who ingested 20 mg of bambuterol hydrochloride once daily for 10 days
Bambuterol was measurable in plasma during the whole steady-state dosing interval, but at 0 h in subject 1 and at 24 h in subject 3 values were slightly below the limit of quantification of 0.5 nmol l−1. Rough estimates of values were 0.4 and 0.5 nmol l−1, respectively, but, because of the uncertainty of the estimates, they were not used for calculations of Cmax/Cminratios. Such ratios were calculated only for subjects 2 and 4 (Table 2).
Mean AUC for bambuterol over the 24 h dosing interval was 64.4 (44.5–88.7) nmol l−1h, corresponding to an average plasma concentration of 2.7 nmol l−1. The lowest and the highest values were in subjects 2 and 4, respectively. Peak plasma concentration of bambuterol was reached 1–3 h (median 2.0 h) after the last dose. The mean peak concentration was 10.5 (5.1–17.9) nmol l−1.
Terbutaline was measurable in plasma up to at least 24 h. Mean AUC for terbutaline over the dosing interval was 286 (200–348) nmol l−1. Css(mean 11.9 nmol l−1) was time-located 12–13 h (mean 12.8 h) after dose intake. The individual Css-values had the same rank order as the peak concentrations. Peak plasma concentration of terbutaline occurred 3–4 h (median 3.5 h) after the last dose. Mean peak concentration was 19.5 (11.7–25.2) nmol l−1. As with bambuterol, the lowest peak was found in subject 2. The trough concentrations of terbutaline (mean 6.8 nmol l−1) differed less than the peaks between subjects. The geometrical mean peak/trough concentration ratio was 2.8.
Urinary data
No subject had detectable urine concentrations of bambuterol or terbutaline before entry into the study. The mean urinary excretion of bambuterol over the dosing interval was 568 (338–763) nmol, corresponding to 1.14% of the administered dose. Within the interval, the major part was excreted during the initial 12 h (mean 80%). Urinary excretion data during the dosing interval reflected the plasma concentration of bambuterol with the lowest excretion in subject 2 (0.68% of the dose) and the highest excretion in subject 4 (1.53%). Mean renal clearance of bambuterol over the entire dosing interval was 145 ml min−1, which was higher than creatinine clearance in all participants. Creatinine clearance was of the same order before and during bambuterol treatment.
The mean amount of terbutaline excreted during the dosing interval was 1903 (1134–2376) nmol, corresponding to 3.81% of the dose. The individual values ranked like those found with bambuterol. About 60% of the excretion within the interval took place during the first 12 h after dosing. Mean renal clearance of terbutaline over the entire dosing interval was 111 ml min−1, similar to creatinine clearance in all participants.
Terminal half-lives
The mean terminal half-life of bambuterol was 8.7 (4.8–12.6) h, shorter than the 14.2 h previously found [8] in subjects with normal pChE activity (P=0.044). The mean terminal half-life of terbutaline was 18.6 (16.5–21.8) h, similar to the value found in the normal subjects (mean 19.7 h).
Plasma cholinesterase activity
The inhibition by the treatment was low. The greatest inhibition was found in the samples taken 3 h after the last administration of bambuterol: at the most 26% (subject 4).
Adverse events
Adverse events were recorded on study days 1–14. Participants except subject 2 experienced mild or moderate adverse events, consistent with those commonly seen with β2-adrenoceptor agonists: tremor, restlessness, headache and palpitations.
Discussion
Surprisingly, the four subjects with atypical cholinesterase were all capable of producing terbutaline from bambuterol as efficiently as subjects with normal hydrolytic and oxidative capacity [8]. We had expected to find a higher AUC of bambuterol and a diminished and slower production of terbutaline as a result of these subjects’ low basal activity of plasma cholinesterase and the poor affinity of the atypical enzyme for bambuterol. Instead, the pharmacokinetic parameters found in the present study (Table 2) were very similar to those found in a study of bambuterol in subjects with normal pChE activity given the same dose [8]. The subjects with atypical cholinesterase had a shorter terminal half-life and slightly higher plasma concentrations of bambuterol. They had a somewhat higher peak concentration of terbutaline with an increased fluctuation (Cmax/Cmin) within the dosing interval. However, the AUC values of terbutaline were the same as those found in subjects with normal pChE activity.
This contradicts partly the hypothesis (based on in vitro studies) [1] that both the oxidative and the hydrolytic pathways are required for optimum bioconversion of bambuterol to terbutaline.
Formation of terbutaline from bambuterol cannot occur without hydrolysis (Figure 1). Plasma cholinesterase is considered to be the blood enzyme predominantly catalysing the hydrolysis. However, the quantitative importance of hydrolysis of bambuterol in plasma relative to hydrolysis in tissues like intestine and liver has hitherto not been determined. Liver microsomes from various species have been tested for their capacity to hydrolyse bambuterol [1]. Microsomes from human livers had intermediate activity and those from rat liver no activity, while liver microsomes from guinea pigs had the highest activity. The enzymes catalysing these processes may be non-specific carboxyesterases known to be very active in liver microsomes or nonspecific cholinesterases probably present in the microsomal preparations [1]. In the absence of plasma cholinesterase, these enzymes may contribute to a greater extent to the hydrolytic conversion of bambuterol to its active metabolite. Also, the hydroxylated metabolites (II, IV, and V in Figure 1) have been shown to be converted partially to N-demethylated compounds which hydrolyse spontaneously with half-lives of a few hours at body temperature and physiological pH [14].
Debrisoquine was chosen as a marker of oxidative metabolism in the present study, because bambuterol has a molecular structure that fulfils requirements for being a substrate for CYP2D6 [15, 16]. Indeed, preliminary experiments showed disappearance of bambuterol and/or rise of hydroxylated metabolites when bambuterol was incubated with cDNA-expressed CYP2D6 (Gentest Corp, Woburn, MA, USA; G Hallström, G Jönsson, personal communication). The prestudy debrisoquine test revealed that our four subjects were markedly extensive metabolizers [17]. As about 90% of the Danish population are characterized as fast metabolizers of debrisoquine/sparteine, this finding might be incidental [18]. However, it cannot be excluded that a high oxidative metabolism in the present subjects might have compensated for their low plasma cholinesterase activity by promoting formation of intermediary metabolites less stable towards hydrolysis. The oxidative pathway of bambuterol metabolism may then be quantitatively more important in vivo than previously believed. Further analysis of intermediate metabolites would be helpful to determine the relation between oxidative and hydrolytic metabolism and to identify the enzymes involved.
Bambuterol terminal half-life after oral administration is a measure of uptake rather than elimination [8]. Therefore, the shorter terminal half-life of bambuterol and the higher peak concentrations of bambuterol and terbutaline found in the four atypical subjects as compared with genetically normal subjects indicate a faster absorption or bioavailability rate of the prodrug. It is not known how much bambuterol is biotransformed by pChE or by enzymes other than cholinesterases during passage through the intestinal mucosa. The normal pChE enzyme may act as a limiting factor regarding the fraction of the absorbed dose that reaches the blood and subsequently the liver intact. In the four subjects studied, the lower activity of pChE and its low affinity for bambuterol might have allowed a larger intact fraction to pass the intestinal mucosa into the portal blood, thereby also promoting a fast oxidative metabolism in the liver.
In conclusion, after repeated oral administration of 20 mg bambuterol hydrochloride, four subjects, homozygous for the atypical gene for plasma cholinesterase, had average plasma concentrations of bambuterol and its active metabolite terbutaline which were grossly similar to those seen in subjects with normal plasma cholinesterase activity. This might be explained by an altered uptake and metabolism in the absence of plasma cholinesterase, or the importance of this enzyme for the formation of terbutaline from bambuterol in vivo may have been overestimated.
References
- 1.Svensson L-Å, Tunek A. The design and bioactivation of presystemically stable prodrugs. Drug Metab Rev. 1988;19:165–194. doi: 10.3109/03602538809049622. [DOI] [PubMed] [Google Scholar]
- 2.Whittaker M. Plasma cholinesterase variants and the anaesthetist. Anaesthesia. 1980;35:174–197. doi: 10.1111/j.1365-2044.1980.tb03800.x. [DOI] [PubMed] [Google Scholar]
- 3.Viby-Mogensen J. Cholinesterase and succinylcholine. Dan Med Bull. 1983;30:129–150. [PubMed] [Google Scholar]
- 4.Bang U, Viby-Mogensen J, Wirén J-E, Theil Skovgaard L. The effect of bambuterol (carbamylated terbutaline) on plasma cholinesterase activity and suxamethonium-induced neuromuscular blockade in genotypically normal patients. Acta Anaesthesiol Scand. 1990;34:596–599. doi: 10.1111/j.1399-6576.1990.tb03152.x. [DOI] [PubMed] [Google Scholar]
- 5.Bang U, Viby-Mogensen J, Wirén J-E. The effect of bambuterol on plasma cholinesterase activity and suxamethonium-induced neuromuscular blockade in subjects heterozygous for abnormal plasma cholinesterase. Acta Anaesthesiol Scand. 1990;34:600–604. doi: 10.1111/j.1399-6576.1990.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 6.Østergaard D, Kristensen RW, Bang U, Pedersen NA, Viby Mogensen J. Influence of bambuterol on the pharmacokinetics and pharmacodynamics of mivacurium. Anesthesiology. 1995;83:899. [Google Scholar]
- 7.Tunek A, Hjertberg E, Viby-Mogensen J. Interactions of bambuterol with human serum cholinesterase of the genotypes EuEu (normal), EaEa (atypical) and EuEa. Biochem Pharmacol. 1991;41:345–348. doi: 10.1016/0006-2952(91)90530-i. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg L, Rosenborg J, Weibull E, Jönsson S, Kennedy B-M, Nilsson M. Pharmacokinetics of bambuterol in healthy subjects. Br J Clin Pharmacol. 1998;45:471–478. doi: 10.1046/j.1365-2125.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen FS, Skovgaard LT, Viby-Mogensen J. Identification of human plasma cholinesterase variants in 6688 individuals using biochemical analysis. Acta Anaesthesiol Scand. 1995;39:157–162. doi: 10.1111/j.1399-6576.1995.tb04035.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalow W, Lindsay HA. A comparison of optical and manometric methods for the assay of human serum cholinesterase. Can J Biochem Physiol. 1955;33:568–574. [PubMed] [Google Scholar]
- 11.Steiner E, Iselius L, Alván G, Lindsten J, Sjöqvist F. A family study of genetic and environmental factors determining polymorphic hydroxylation of debrisoquine. Clin Pharmacol Ther. 1985;38:394–401. doi: 10.1038/clpt.1985.193. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg C, Jönsson S, Paulsson J. Determination of bambuterol, a prodrug of terbutaline in plasma and urine by gas chromatography/mass spectrometry. Biomed Environ Mass Spectrom. 1990;19:218–224. doi: 10.1002/bms.1200190403. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy B-M, Blomgren A, Edholm L-E, Roos C. Quantitative determination of terbutaline in human plasma after administration of bambuterol using coupled columns and electrochemical detection. Chromatographia. 1987;24:895–899. [Google Scholar]
- 14.Lindberg C, Roos C, Tunek A, Svensson LÅ. Metabolism of bambuterol in rat liver microsomes: identification of hydroxylated and demethylated products by liquid chromatography mass spectrometry. Drug Metab Disp. 1989;17:311–322. [PubMed] [Google Scholar]
- 15.Islam SA, Wolf CR, Lennard MS, Sternberg MJE. A three-dimensional molecular template for substrates of human cytochrome P450 involved in debrisoquine 4-hydroxylation. Carcinogenesis. 1991;12:2211–2219. doi: 10.1093/carcin/12.12.2211. [DOI] [PubMed] [Google Scholar]
- 16.Strobl GR, von Kruedener S, Stöckigt J, et al. Development of a pharmacophore for inhibition of human liver cytochrome P-450, 2D6: Molecular modeling and inhibition studies. J Med Chem. 1993;36:1136–1145. doi: 10.1021/jm00061a004. [DOI] [PubMed] [Google Scholar]
- 17.Steiner E, Bertilsson L, Säwe J, et al. Polymorphic debrisoquin hydroxylation in 757 Swedish subjects. Clin Pharmacol Ther. 1988;44:431–435. doi: 10.1038/clpt.1988.176. [DOI] [PubMed] [Google Scholar]
- 18.Brøsen K, Otton SV, Gram LF. Sparteine oxidation polymorphism in Denmark. Acta Pharmacol Toxicol. 1985;57:357–360. doi: 10.1111/j.1600-0773.1985.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 19.Madsen H, Kramer-Nielsen K, Brøsen K. Imipramine metabolism in relation to the sparteine and mephenytoin oxidation polymorphisms – A population study. Br J Clin Pharmacol. 1995;39:433–439. doi: 10.1111/j.1365-2125.1995.tb04473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]