Abstract
To determine whether maternal filariasis influences the risk of infection by and immunity to Wuchereria bancrofti in children, we performed a cross-sectional study in an area of Kenya where filariasis is endemic. Residents of 211 households were enrolled; 376 parents and 938 of their offspring between the ages of 2 and 17 years were examined for filarial infection status as determined by blood-borne microfilariae and filarial antigenemia. Children of infected mothers had a three- to fourfold increased risk of filarial infection, as ascertained by circulating filarial antigen, relative to children of uninfected mothers (P < 0.001). Paternal infection did not correlate with childhood infection status, indicating a specific maternal effect. Peripheral blood mononuclear cells from children of filaria-infected mothers (n = 33) had higher levels of constitutive interleukin-5 (IL-5) and IL-10, increased microfilarial antigen-specific IL-5 production, and diminished microfilarial antigen-driven lymphocyte proliferation than cells from children of uninfected mothers (n = 46; P < 0.05). In contrast, there were no differences between the two groups in adult worm antigen-driven gamma interferon, IL-2, IL-4, IL-5, and IL-10 production and lymphocyte proliferation. These data indicate that maternal filarial infection increases childhood susceptibility to W. bancrofti and skews filaria-specific immunity toward a Th2-type cytokine response. The results support the notion that in utero exposure to filarial antigens affects the natural history of filariasis during childhood.
Lymphatic filariasis caused by Wuchereria bancrofti or Brugia malayi is a parasitic infection in which adult-stage worms dwell in afferent lymphatic vessels and first-stage larvae or microfilariae (Mf) circulate in the bloodstream. Women commonly harbor filarial infections during their childbearing years, raising the possibility that the developing fetus may be exposed to filarial antigens in utero and thereby have altered immunity and susceptibility to infection during early childhood. Animal models of intravascular helminthic infections support this hypothesis, as these have shown that offspring of infected pregnant rodents are immunologically less responsive to parasite antigens, have less pathology, and are more susceptible than offspring of uninfected mothers (9, 17, 21, 32, 36). Analogous studies of humans are inherently more complex and difficult to interpret because of heterogeneities in exposure to infective-stage parasites, genetic differences among various study populations, and prior treatment with antifilarial drugs. Nevertheless, evidence exists that in utero exposure to filarial antigen occurs in human filariasis and that children born of infected mothers are at increased risk of infection relative to offspring of uninfected mothers (3, 16, 20, 23, 24, 39). It has not, however, been resolved whether the apparent association between various prenatal risk factors and susceptibility to infection is a result of immune tolerance leading to an inability to generate protective immunity, as suggested by some (14, 20, 35), or reflects differences in exposure to mosquito-borne infective-stage larvae, as suggested by others (1-3, 33). We examined the risk of infection and T-cell and antibody responses to antigens of adult worm and larva-stage parasites in Kenyan children and their mothers with a design that attempts to control for these complexities. Mother-child pairs who participated in the study were all from the same area of endemicity, had similar ethnic backgrounds, and had no known prior use of antifilarial medications. Moreover, potential confounding effects related to local transmission heterogeneity versus in utero exposure to filarial antigen were controlled for by comparison of child and paternal infection status.
MATERIALS AND METHODS
Study population.
Study subjects resided in Darigube and Eshu villages, Kwale District, South Coast Province, Kenya, near the Tanzanian border. No systematic treatment for lymphatic filariasis had been undertaken in these communities. It is estimated that residents of the area are exposed to one infective bite from mosquitoes harboring W. bancrofti every other day (26, 28). Seventeen percent of households reported using insecticide-impregnated bed nets, although none indicated that the bed nets had recently been retreated. Children ≤17 years old (n = 938) and their parents (n = 376) in 211 households were enrolled in the study. Fathers living in 165 households (78% of the total) were examined. Based on interviews of household members, if it was suspected that children might not be biologically related to their parents, e.g., because of adoption or remarriage, etc., the household was excluded from the study. An additional 208 village residents >40 years old who were not parents of children were included to evaluate the infection burden in the community. Infected individuals were offered the standard local treatment for bancroftian filariasis (6 mg of diethylcarbamazine/kg of body weight). The Human Investigations Institutional Review Boards of Case Western Reserve University and the Kenyan Medical Research Institute approved the protocol for this study.
Determination of infection status.
Assessment of Mf status was based on collection of 200 μl of blood obtained by finger prick between 2200 and 0200 h. The sample was mixed with 800 μl of 3% acetic acid, and Mf were counted in a hemocytometer by light microscopy (6). An additional 200 μl of blood was subsequently obtained for measurement of circulating filarial antigens (CAg) by using the Og4C3 enzyme-linked immunosorbent assay (ELISA) (27) according to the manufacturer's instructions (TropbioMed, James Cook University Tropical Biotechnology Ltd., Townsville, Australia). Samples showing >32 U/ml were considered positive.
Antigens and mitogens.
B. malayi adult worm antigen (BmA) was extracted in saline from an equal number of adult male and female worms of B. malayi. Saline extracts (MFE) were prepared from thoroughly washed Mf obtained from the peritoneal cavities of jirds as described previously (11). The endotoxin level in these preparations was <0.5 ng/ml, which is less than that required for lipopolysaccharide stimulation of cytokines from human lymphocytes (4). Purified protein derivative (PPD) of Mycobacterium tuberculosis was obtained from Evan Medical Ltd. (Leatherhead, Surrey, United Kingdom). The mitogens phorbol 12-myristate 13-acetate (Calbiochem, La Jolla, Calif.) with ionomycin (Calbiochem) were used in parallel cultures.
Cytokine production.
Seventy-nine children between the ages of 2 and 17 years donated blood for immunological studies. Peripheral blood mononuclear cells (PBMC) were prepared by density gradient centrifugation on Ficoll-Hypaque from heparinized venous blood and resuspended in RPMI 1640 supplemented with 10% fetal calf serum, 4 mM l-glutamine, 25 mM HEPES, and 80 μg of gentamicin (BioWhittaker, Walkersville, Md.) per ml (C-RPMI). PBMC were cultured at a concentration of 2 × 106 cells/ml in C-RPMI in a total volume of 1 ml. BmA (10 μg/ml), MFE (1 μg/ml), PPD (10 μg/ml), or phorbol 12-myristate 13-acetate (50 pg/ml) with ionomycin (1 μg/ml) was added separately to duplicate or triplicate cultures, depending on the number of lymphocytes available. Cultures with medium alone served as controls. Cells were incubated at 37°C in 5% CO2, and supernatants were collected at 48 h for measurements of interleukin-4 (IL-4) and IL-2 and at 72 h for measurement of IL-5, IL-10, and gamma interferon (IFN-γ) and immediately frozen at −70°C. Frozen supernatants were subsequently thawed for determination of cytokine production by ELISA, which was expressed in picograms per milliliter by interpolation from standard curves based on recombinant lymphokines with antibodies. Antibody pairs for capture and detection (all biotinylated) for the cytokines studied were as follows: IL-5, TRFK5 and 5D10 (PharMingen, San Diego, Calif.); IL-4, 8D4 and 25D2 (PharMingen); IFN-γ, M-700 and M-701 (Endogen, Cambridge, Mass.); and IL-10, 18551D and 18652D (PharMingen). The limits of detection for the cytokine ELISAs were 20 pg/ml for IL-5, 16 pg/ml for IL-4, 10 pg/ml for IFN-γ, and 16 pg/ml for IL-10.
Proliferation assays.
Lymphocyte proliferation assays were performed as previously described (14). Briefly, 2 × 105 PBMC were cultured in 96-well microtiter plates with RPMI 1640, 10% human AB serum, 4 mM l-glutamine, 25 mM HEPES and 80 μg of gentamicin (BioWhittaker) per ml and stimulated for 5 days with BmA (10 μg/ml), MFE (1 μg/ml), and PPD (10 μg/ml), or with medium alone, and for 3 days with phytohemagglutinin (10 μg/ml). The cells were pulsed with 1 μCi of [3H]thymidine (New England Nuclear Dupont, Wilmington, Del.) for an additional 12 h, harvested onto a glass filter, and measured for radioactivity incorporation with a Packard Matrix beta counter.
Antibody assays.
BmA- and MFE-specific immunoglobulin G (IgG), IgG4, and IgE antibodies were measured by ELISA as previously described (16).
Statistical analysis.
Analysis of infection status in the offspring of infected compared to uninfected parents was performed by using Mantel-Haenszel chi-square statistics and Cornfield confidence intervals (CIs) of odds ratios (Epi Info version 6; Centers for Disease Control and Prevention, Atlanta, Ga.). Other data were log transformed, producing a normal distribution for most cytokines examined. In these cases the difference between mean values was determined by Student's t test. For cytokines that were not normalized by log transformation, comparisons were performed by the nonparametic Mann-Whitney U test.
RESULTS
Age- and sex-specific prevalence of W. bancrofti infection.
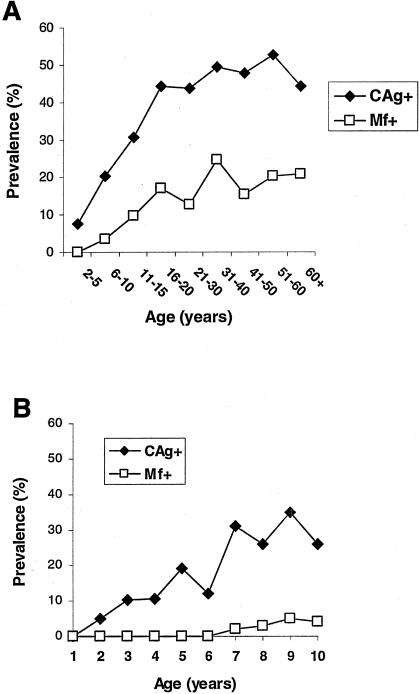
Overall, 213 of 1,511 (14.1%) and 569 of 1,522 (37.4%) people surveyed were positive for blood Mf and CAg, respectively. The prevalence of persons who were CAg+, the more sensitive of the two measures (23), increased with age during childhood and reached a plateau after age 16 years (Fig. 1A). In the subset of children ≤10 years old (n = 251), the proportion who were CAg+ increased progressively with age (Fig. 1B). For example, 5% of 2-year-old subjects and 30% of 7- to 10-year-old subjects were CAg+. In contrast, blood Mf were not detectable in children until the age of 7 years. There was no difference in prevalence of blood Mf+ or CAg+ subjects according to gender. Overall, 87 of 211 (42%) mothers in the study (ages 18 to 46 years) were infected with W. bancrofti, i.e., were either CAg+ Mf− or CAg+ Mf+.
FIG. 1.
(A) Relationship between age and prevalence of infection among all individuals residing in two adjacent villages where filariasis is endemic in the Coast Province, Kwale District, Kenya (n = 1,522). Microfilaremia was determined by collection of 200 μl of blood at night and CAg was determined based on the Og4C3 assay. (B) Prevalence of infection in children 1 to 10 years of age in the same population (n = 251).
Relationship of parental and childhood infection status.
Children ≤17 years old whose mothers were CAg+, irrespective of Mf status were 3.7-fold more likely to be CAg+ than age-matched subjects whose mothers were not infected, i.e., were CAg− Mf− (Table 1). The relative risk of childhood infection increased to 5.4-fold in children <10 years old. The association between maternal and child infection status was also evaluated according to whether the mother was CAg+ Mf− or CAg+ Mf+. This distinction was made because CAg+ Mf+ women may have a greater parasite burden, particularly with Mf in the intravascular space, than CAg+ Mf− women. The former situation may therefore more likely result in exposure of the developing fetus to filarial antigens in utero (37). The risk of childhood filarial infection (ages ≤17) was even greater for offspring of CAg+ Mf+ women (odds ratio, 4.9; CI, 2.6 to 6.9) than for offspring of CAg+ Mf− women (odds ratio, 2.6; CI, 1.3 to 5.4). By contrast, there was no significant association between paternal and childhood infection status. In summary, children with filaria-infected mothers, not fathers, were more likely to be infected themselves.
TABLE 1.
Relationship of parental W. bancrofti infection status to childhood infection status
| Parent | Infection of parenta | All children
|
Children <10 yr
|
||
|---|---|---|---|---|---|
| No. of children infecteda/total (% infected) | Odds ratio (95% CI) | No. of children infected/total (% infected) | Odds ratio (95% CI) | ||
| Mother | Yes | 150/403 (37.2) | 3.7 | 97/253 (38.3) | 5.4 |
| No | 74/535 (13.8) | (2.7-5.1) | 37/366 (10.1) | (3.6-8.4) | |
| Father | Yes | 101/359 (28.1) | 1.4 | 64/222 (28.8) | 1.5 |
| No | 72/321 (22.4) | (0.9-1.9) | 45/213 (21.1) | (0.98-2.3) | |
Infection status based on the presence of W. bancrofti CA, with or without blood Mf.
Infection and antibody responses in children, grouped according to maternal infection status.
A total of 938 children were eligible to donate blood for immunological studies. Based on informed consent of parents and logistical constraints, 100 children were recruited and 79 agreed to participate in the study. Children were divided into two groups based on maternal CAg status. The median ages of children with infected (CAg+) and uninfected (CAg−) mothers were the same (7 years). The proportion of CAg+ children was higher in the group with infected mothers than in the group with uninfected mothers (33 and 17%, respectively), although this difference did not reach statistical significant (P = 0.1 by the chi-square test).
The filaria-specific IgG4 isotype was examined because it has been shown to be associated with the presence of active infection (19) and to compete for binding of the same epitopes as IgE (12). Parasite-specific IgE potentially correlates with levels of acquired resistance in helminth infections (8). Seventy-three percent of children of infected mothers had elevated BmA-specific IgG4, compared to 43% of children of uninfected mothers (P < 0.01) (Table 2). The proportions of children with BmA-specific IgG and IgE antibody isotypes were equivalent in the two groups. The ratio of filaria-specific IgG4 to IgE was greater among children of filaria-infected than among children of uninfected women. Consistent with the notion that cumulative exposure to infective mosquitoes was similar for the two groups, the proportions of children with IgE antibodies to infective third-stage larvae (L3) were 52 and 50% in the two groups (Table 2).
TABLE 2.
Infection rates and filaria-specific antibody responses in children of infected and uninfected women
| Parameter | Valuea for children with the following maternal infection status:
|
|
|---|---|---|
| Infectedb | Uninfected | |
| n | 33 | 46 |
| Median age, yr (range) | 7 (2-17) | 7 (2-17) |
| No. (%) infected | 11 (33) | 8 (17) |
| No. (%) BmA-specific IgG4 positivec | 24 (73) | 20 (43) |
| Geometric mean BmA IgG4 levels among responders (U/ml) | 1,860 | 1,166 |
| No. (%) BmA-specific IgG positived | 25 (76) | 29 (63) |
| No. (%) BmA-specific IgE positivee | 20 (61) | 29 (63) |
| Geometric mean BmA IgE levels among responders (U/ml) | 562 | 886 |
| No. (%) L3-specific IgE positivef | 17 (52) | 23 (50) |
| BmA-specific IgG4/IgE ratio | 4.7 | 0.9 |
Values in boldface indicate statistically significant differences between the two groups (P < 0.05).
Twenty-four mothers were CAg+ Mf+ and nine were CAg+ Mf−.
A positive value was considered >230 U/ml for IgG4, based on the mean plus two standard deviation of values in sera obtained from age-matched children residing in the Turkana region of Kenya, where lymphatic filariasis is not endemic and children are heavily infected with intestinal helminths.
A positive value was considered >297 U/ml. This cutoff value was established in the same fashion as for BmA-specific IgG4.
A positive value was considered >89 U/ml.
A positive value was considered >121 U/ml.
Filarial antigen-driven cytokine and lymphocyte proliferation responses in infected versus uninfected children.
Because children of infected mothers were more likely to be infected themselves than children of uninfected mothers (Table 2), it is possible that childhood infection status per se, independent of maternal infection, is a major variable that determines filarial immunity (30). We therefore first determined whether there were differences in cytokine responses according to the infection status of the child (Table 3). Constitutive, MFE-, BmA-, and mitogen-driven IL-5, IFN-γ, IL-2, IL-4, and IL-10 responses were similar among groups of infected (CAg+ Mf+ or CAg+ Mf−) and uninfected (CAg− Mf−) children in overall levels of cytokine production and frequency of responders (data not shown). Infected children had diminished lymphocyte proliferation responses to MFE but not BmA. Spontaneous lymphocyte proliferation was similar for the two groups. Because filaria-specific IgG4 indicates early or light infection (19), the frequency and levels of cytokine responses in IgG4+ and IgG4− children were compared. No differences were observed between the two groups. Overall, these observations show that the infection state of the child does not correlate with changes in cytokine production but does correlate with decreased proliferation.
TABLE 3.
Cytokine responses in infected versus uninfected children
| Antigen | Cytokine or lymphocyte response | Cytokine concn (pg/ml) or cpm ina:
|
|
|---|---|---|---|
| Infected childrenb (n = 19) | Uninfected childrenb (n = 60) | ||
| BmA | IL-5 | 54 ± 162 | 74 ± 430 |
| IFN-γ | 27 ± 165 | 44 ± 708 | |
| IL-2 | 8 ± 41 | 8 ± 163 | |
| IL-4 | 4 ± 47 | 4 ± 19 | |
| IL-10 | 12 ± 96 | 12 ± 112 | |
| Lymphocyte proliferation | 3,234 ± 811 | 5,129 ± 936 | |
| MFE | IL-5 | 4 ± 13 | 18 ± 44 |
| IFN-γ | 28 ± 204 | 14 ± 33 | |
| IL-2 | 7 ± 43 | 4 ± 14 | |
| IL-4 | 2 ± 95 | 3 ± 39 | |
| IL-10 | 119 ± 158 | 24 ± 180 | |
| Lymphocyte proliferation | 627 ± 184 | 2,178 ± 398* | |
Net geometric mean ± 95% CI cytokine production in PBMC culture supernatants or mean (± SD) counts per minute (for lymphocyte proliferation). *P < 0.05.
Infected, CAg+; uninfected, CAg−.
Relationship of childhood cytokine and lymphocyte proliferation responses to maternal filarial infection status.
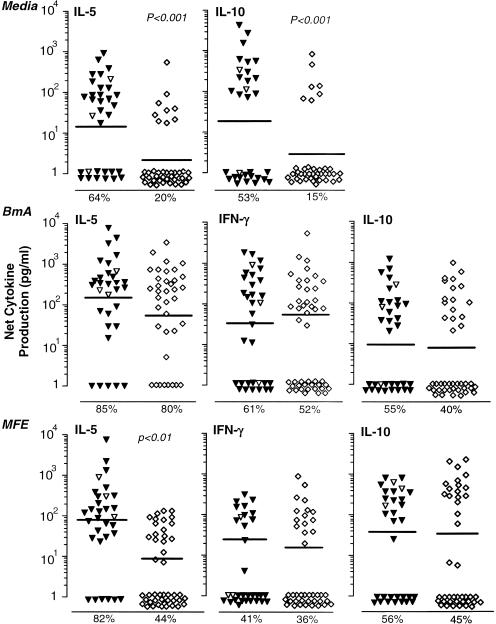
All study subjects had robust mitogen-driven cytokine production and lymphocyte proliferation responses indicating viable PBMC. Mitogen-driven cytokine production and lymphocyte proliferation were equivalent for PBMC for the groups of children with infected versus uninfected mothers (data not shown). The proportion of individuals with constitutive IL-5 and IL-10 production, however, was greater for children of infected mothers than for those of uninfected mothers (Fig. 2, upper panel). The proportion and levels of constitutive IL-2, IL-4, or IFN-γ production (data not shown) and spontaneous lymphocyte proliferation responses (Fig. 3) did not differ for the two groups. Similarly, the net BmA-driven cytokine production (spontaneous cytokine production subtracted from antigen-driven cytokine production) (Fig. 2, middle panel) and lymphocyte proliferation (Fig. 3) were equivalent for children of infected and uninfected mothers. In contrast to the case for BmA, MFE-stimulated IL-5 production was ninefold greater for PBMC of children of infected versus uninfected mothers (Fig. 2, lower panel). Among individuals who responded to MFE, the level of MFE-driven IL-5 was higher (geometric mean = 257 pg/ml) in children with infected mothers than in children of uninfected mothers (geometric mean = 63 pg/ml) (P < 0.05). Equivalent levels of net MFE-driven IFN-γ and IL-10 (Fig. 2, lower panel) and IL-2 and IL-4 (data not shown) were produced by PBMC from children of infected versus uninfected mothers.
FIG. 2.
Cytokine production by PBMC from children of infected (CAg+) versus uninfected (CAg−) mothers. (Upper panel) Constitutive cytokine production. (Middle panel) Net (antigen-driven minus spontaneous) BmA-driven cytokine production. (Lower panel) Net MFE-induced cytokine production. Each point represents the mean for duplicate cultures from a single individual. Bars represent geometric means. Triangles, offspring of infected mothers; open triangles, samples that were further examined for CD4+ cell depletion; diamonds, offspring of uninfected mothers. The percentage of individuals showing a positive response is shown below each panel. Significant differences between groups, based on chi-square analysis (media) or Student's t test of log-transformed data (BmA- and MFE-driven responses) are indicated.
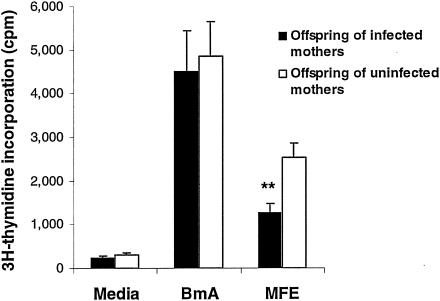
FIG. 3.
Lymphocyte proliferation responses of PBMC from children of infected and uninfected mothers. Bars indicate means ± SEM of counts per minute determined in triplicate for each individual. For studies of spontaneous and BmA-driven lymphocyte proliferation, PBMC from 33 children of infected mothers and 46 children of uninfected mothers were examined. Since childhood infection affected proliferation responses to MFE, responses to this antigen preparation were limited to 22 children of infected mothers and 38 children of uninfected mothers. **, P < 0.01.
Because MFE-stimulated lymphocyte proliferation was lower for infected children than for uninfected children (Table 3), infected children were excluded in the comparison of MFE-induced lymphocyte proliferation responses stratified according to maternal infection status. In this subset of 60 children, MFE-driven lymphocyte proliferation was reduced in offspring of infected mothers versus offspring of uninfected mothers (Fig. 3). Similar to observations shown in Fig. 2, MFE-driven IL-5 production was also increased in uninfected children with infected mothers (geometric mean ± standard error of the mean [SEM] = 103 ± 374 pg/ml) compared to those with uninfected mothers (geometric mean ± SEM = 10 ± 11 pg/ml) (P < 0.01) in this subset of children. BmA-driven cytokine production was also equivalent in those with infected compared to uninfected mothers (data not shown). In summary, these data show that the infective state of the mother correlates with increased larval antigen-driven IL-5 production and decreased lymphocyte proliferation.
CD4+ T cells are required for filarial antigen-driven cytokine production in PBMC.
Depletion of CD4+ T cells from PBMC of three children who were responsive to filarial antigen resulted in the complete loss of constitutive, BmA-, and MFE-driven IL-5, IL-10, IFN-γ, and IL-2 production. Filarial antigen-driven cytokine responses in these three children in the absence of CD4+ cell depletion is shown in Fig. 2. In two of the subjects, CD4+ cells were enriched by immunomagnetic positive selection and stimulated with both BmA and MFE in the presence of 5% antigen-presenting cells, as previously described (23), to show maximal BmA-driven cytokine production for each of the two subjects as follows: net IL-5 release, 177 and 435 pg/ml; net IL-10 release, 0 and 741 pg/ml; net IFN-γ release, 589 and 146 pg/ml; and net IL-2 release, 211 and 0 pg/ml.
DISCUSSION
These data show that Kenyan children whose mothers are infected with the filarial parasite W. bancrofti have an increased risk of filarial infection compared with children whose mothers are not infected. This increased risk was most pronounced for children younger than 10 years old and for those of mothers with both blood-borne Mf and filarial antigenemia as opposed to filarial antigenemia alone. Consistent with reports from other areas where filarial infection is endemic (20, 24), childhood filarial infections were not significantly associated with paternal infection status, suggesting that maternal infection during the child's gestation accounted for the observed pattern of mother-child infections.
Earlier studies have proposed that exposure to filarial antigens transported from the maternal circulation during gestation results in immunological tolerance of the unborn fetus. Pertinent data supporting this include diminished filarial antigen-specific lymphocyte proliferation and cytokine production (20, 35), which are believed to result in increased susceptibility to infection. If in utero exposure to filarial antigens uniformly results in tolerance through anergy or deletion of filaria-specific T cells, filaria-specific cord blood T-cell responses by newborns of filaria-infected women would be expected to be absent or weak, with hyporesponsiveness that persists during early childhood. Data from several studies showing the existence of newborn filaria-specific T- and B-cell responses (10, 16, 23, 34) that persist into infancy (22), however, suggest that this is not the case, at least for a subset of children of filaria-infected mothers. In the present study, we evaluated lymphocyte cytokine and proliferation responses to filarial antigens prepared from adult worms and Mf in children. Whereas there was no difference in adult worm-driven lymphocyte responses among children of infected versus uninfected mothers, a greater proportion of children of infected mothers had constitutive IL-5 and IL-10 production, enhanced levels of MFE-driven IL-5, and diminished MFE-specific lymphocyte proliferation. These observations support previous observations of fetal priming of filaria-specific T cells in some offspring of infected mothers that persist and expand with repeated exposure to filariasis during childhood (reference 22 and unpublished data). Taken together, these observations are consistent with the hypothesis that excretory or secretory products of intravascular Mf cross the placenta to induce partial tolerance or otherwise alter the fetal immune response so that the subsequent susceptibility to natural infection is increased.
The failure to observe reduced filarial antigen-driven IL-2 and IFN-γ among infected compared to uninfected subjects contrasts with most published studies (reviewed in reference 29). These reports generally examined adults and not children. We also examined T-cell responses in parents of the study children and other adults in the same population and found that filaria-specific lymphocyte proliferation and cytokine production declined with age in infected but not in uninfected individuals, suggesting development of peripheral tolerance (unpublished data). These age-related changes do not affect our conclusions of differences in cytokine production and lymphocyte proliferation between offspring of infected and uninfected women, because the two groups were similar in age (Table 1).
Explanations other than prenatal antigen exposure may account for an increased risk for infection in children of filaria-infected women. The degree of exposure to mosquito-borne L3 may differ for children of infected versus uninfected mothers. For example, mosquitoes are more likely to ingest Mf and subsequently transmit infection to other members of a given household if one or more individuals are Mf+ (25). Several observations argue against differences in exposure as a significant variable in the present study. First, children with infected fathers living in the same household were not at increased risk of infection compared with children in households where the father was not infected. Second, assuming that filarial antibodies reflect exposure to infective mosquitoes, the frequencies and levels of filaria-specific IgG and IgE antibodies were similar for children of infected versus uninfected mothers. Studies in Haiti and Tanzania found a similar association of childhood infection with maternal but not paternal infection status (20, 24), while reports from Papua New Guinea (1), Brazil (2), India (3), and East Africa (33) showed that both maternal and paternal infection status correlated with infection in children. The latter data suggest that exposure is a major risk factor for filarial infection in children. Interpretations put forth in these various studies are not mutually exclusive, and the observed differences in the association with paternal infection status may result from differences in study design, human and vector behavior, and transmission intensities. Although within-household exposure to infective mosquitoes was not directly measured in the present study or any other study, parents living in the same household are unlikely to experience levels of exposure that differ significantly from those of their children. Most fathers in the study villages do not work outside the community for extended periods and customarily sleep in the family household. The principal vectors of W. bancrofti in the study villages, Anopheles gambiae and Anopheles funestus, are highly anthrophilic and take their blood meals at night when families are asleep in their domicile (28). It is also possible that some children may be genetically predisposed to filarial infection. The lack of association between paternal and childhood infection status argues against this explanation.
The precise mechanism by which prenatal exposure to filarial antigens might lead to increased susceptibility to W. bancrofti infection after birth is not clear. The diminished lymphocyte proliferation responses to MFE that we observed in children is consistent with earlier studies of young adults in the Cook Islands, where transmission of filariasis had nearly been eliminated over the period of 17 to 19 years since the time of their birth (35). Alternatively, enhanced Th2-type responses that develop as a consequence of prenatal exposure to parasite antigens, a phenomenon that has been observed in children of mothers with onchocerciasis (7), may modulate development of protective immunity. Since IFN-γ, IL-2, and enhanced lymphocyte proliferation responses have been proposed to contribute to partial immunity in lymphatic filariasis (5), such responses could be down-regulated by IL-10 (15) or other Th2-type cytokines, thereby rendering such individuals more susceptible to infection. This is not likely to be the case in the present study, since IFN-γ and IL-2 production was similar for children of infected versus uninfected mothers. With respect to role of antibody, mean levels of filaria-specific IgG4 were higher in children of infected mothers than in those of uninfected mothers. This difference may reflect greater infection burdens in the former group, since filaria-specific IgG4 has been taken as a marker for active infection (18, 19). By contrast, although filaria-specific IgE levels were equivalent, the ratio of BmA-specific IgG4 to IgE antibodies was significantly higher in children of filaria-infected mothers than in children of uninfected mothers. It has previously been suggested that IgG4 blocks the effector function of IgE in human filariasis (13), and there is reasonable evidence that in human schistosomiasis parasite-specific IgE correlates with the level of acquired resistance (8). As is true for any cross-sectional study, caution should be exercised in drawing inferences regarding the impact of prenatal sensitization on immunity and susceptibility to infection. The infection status of the mother at the time of birth is not known in such studies, and even if it were, the impact on the fetus may be to stimulate an immune response, induce tolerance, or have no effect. Distinguishing among these different outcomes can be accomplished only by examining cord blood responses and their correlation with infection in a longitudinal cohort study.
Prenatal determinants on the subsequent host immune responses may also affect the clinical outcome of parasitic infections. This is highlighted by the more severe clinical pathology and exaggerated immunological responses observed in expatriate populations infected with lymphatic filariasis relative to endemic populations (31, 38). Finally, a practical implication is the impact that prenatal antigenic experience may have on vaccines administered at birth or during infancy (18). These and similar findings may influence whether community-based control programs for helminth infections should target women of childbearing age for treatment.
Acknowledgments
We appreciate the cooperation of the residents of Darigube and Eshu and the assistance of the technical staff at the Msambweni District Hospital and Division of Vector Borne Diseases field station. We thank Lynn Elson for help with epidemiological and immunological aspects of the study. The Director of the Kenyan Medical Research Institute, Nairobi, Kenya, provided permission for this study.
National Institute of Allergy and Infectious Diseases grant AI33061 provided support for this study.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alexander, N. D., J. W. Kazura, M. J. Bockarie, R. T. Perry, Z. B. Dimber, B. T. Grenfell, and M. P. Alpers. 1998. Parental infection confounded with local infection intensity as risk factors for childhood microfilaraemia in bancroftian filariasis. Trans. R. Soc. Trop. Med. Hyg. 92:23-24. [DOI] [PubMed] [Google Scholar]
- 2.Braga, C., M. F. Albuquerque, H. C. Schindler, M. R. Silva, A. Maciel, A. Furtado, A. B. Carvalho, W. Souza, and R. A. Ximenes. 1998. Risk factors for the occurrence of bancroftian filariasis infection in children living in endemic areas of northeast of Brazil. J. Trop. Pediatr. 44:87-91. [DOI] [PubMed] [Google Scholar]
- 3.Das, P. K., A. Sirvidya, P. Vanamail, K. D. Ramaiah, S. P. Pani, E. Michael, and D. A. Bundy. 1997. Wuchereria bancrofti microfilaraemia in children in relation to parental infection status. Trans. R. Soc. Trop. Med. Hyg. 91:677-679. [DOI] [PubMed] [Google Scholar]
- 4.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimock, K. A., M. L. Eberhard, and P. J. Lammie. 1996. Th1-like antifilarial immune responses predominate in antigen-negative persons. Infect. Immun. 64:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhard, M. L., and P. J. Lammie. 1991. Laboratory diagnosis of filariasis. Clin. Lab. Med. 11:977-1010. [PubMed] [Google Scholar]
- 7.Elson, L. H., A. Days, M. Calvopina, W. Paredes, E. Araujo, R. H. Guderian, J. E. Bradley, and T. B. Nutman. 1996. In utero exposure to Onchocerca volvulus: relationship to subsequent infection intensity and cellular immune responsiveness. Infect. Immun. 64:5061-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 9.Haque, A., and A. Capron. 1982. Transplacental transfer of rodent microfilariae induces antigen-specific tolerance in rats. Nature 299:361-363. [DOI] [PubMed] [Google Scholar]
- 10.Hitch, W. L., M. L. Eberhard, and P. J. Lammie. 1997. Investigation of the influence of maternal infection with Wuchereria bancrofti on the humoral and cellular responses of neonates to filarial antigens. Ann. Trop. Med. Parasitol. 91:461-469. [DOI] [PubMed] [Google Scholar]
- 11.Hussain, R., and E. A. Ottesen. 1985. IgE responses in human filariasis. III. Specificities of IgE and IgG antibodies compared by immunoblot analysis. J. Immunol. 135:1415-1420. [PubMed] [Google Scholar]
- 12.Hussain, R., and E. A. Ottesen. 1986. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J. Immunol. 136:1859-1863. [PubMed] [Google Scholar]
- 13.Hussain, R., R. W. Poindexter, and E. A. Ottesen. 1992. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J. Immunol. 148:2731-2737. [PubMed] [Google Scholar]
- 14.King, C. L., V. Kumaraswami, R. W. Poindexter, S. Kumari, K. Jayaraman, D. W. Alling, E. A. Ottesen, and T. B. Nutman. 1992. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaremic state. J. Clin. Invest. 89:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Invest. 92:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, C. L., I. Malhotra, P. Mungai, A. Wamachi, J. Kioko, J. H. Ouma, and J. W. Kazura. 1998. B cell sensitization to helminthic infection develops in utero in humans. J. Immunol. 160:3578-3584. [PubMed] [Google Scholar]
- 17.Klei, T. R., D. P. Blanchard, and S. U. Coleman. 1986. Development of Brugia pahangi infections and lymphatic lesions in male offspring of female jirds with homologous infections. Trans. R. Soc. Trop. Med. Hyg. 80:214-216. [DOI] [PubMed] [Google Scholar]
- 18.Kurniawan, A., M. Yazdanbakhsh, R. van Ree, R. Aalberse, M. E. Selkirk, F. Partono, and R. M. Maizels. 1993. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 150:3941-3950. [PubMed] [Google Scholar]
- 19.Kwan-Lim, G., K. Forsyth, and R. Maizels. 1990. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J. Immunol. 145:4298-4305. [PubMed] [Google Scholar]
- 20.Lammie, P. J., W. L. Hitch, E. M. Walker Allen, W. Hightower, and M. L. Eberhard. 1991. Maternal filarial infection as risk factor for infection in children. Lancet 337:1005-1006. [DOI] [PubMed] [Google Scholar]
- 21.Lewert, R. M., and S. Mandlowitz. 1969. Schistosomiasis: prenatal induction of tolerance to antigens. Nature 224:1029-1030. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra, I., P. Mungai, A. Wamachi, J. Kioko, J. H. Ouma, J. W. Kazura, and C. L. King. 1999. Helminth- and bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 162:6843-6848. [PubMed] [Google Scholar]
- 23.Malhotra, I., J. Ouma, A. Wamachi, J. Kioko, P. Mungai, A. Omollo, L. Elson, D. Koech, J. W. Kazura, and C. L. King. 1997. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J. Clin. Invest. 99:1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyrowitsch, D. W., P. E. Simonsen, and W. H. Makunde. 1995. Bancroftian filariasis: analysis of infection and disease in five endemic communities of north-eastern Tanzania. Ann. Trop. Med. Parasitol. 89:653-663. [DOI] [PubMed] [Google Scholar]
- 25.Michael, E., K. D. Ramaiah, S. L. Hoti, G. Barker, M. R. Paul, J. Yuvaraj, P. K. Das, B. T. Grenfell, and D. A. Bundy. 2001. Quantifying mosquito biting patterns on humans by DNA fingerprinting of bloodmeals. Am. J. Trop. Med. Hyg. 65:722-728. [DOI] [PubMed] [Google Scholar]
- 26.Michael, E., P. E. Simonsen, M. Malecela, W. G. Jaoko, E. M. Pedersen, D. Mukoko, R. T. Rwegoshora, and D. W. Meyrowitsch. 2001. Transmission intensity and the immunoepidemiology of bancroftian filariasis in East Africa. Parasite Immunol. 23:373-388. [DOI] [PubMed] [Google Scholar]
- 27.More, S. J., and D. B. Copeman. 1990. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop. Med. Parasitol. 41:403-406. [PubMed] [Google Scholar]
- 28.Mukoko, D. N. A. 2000. Assessment of the effect of permethrin impregnated bednets on the transmission of lymphatic filariasis in Kwale District, coastal region, Kenya. Ph.D. dissertation. University of Nairobi and Danish Bilharziasis Laboratory, Nairobi, Kenya.
- 29.Nutman, T. B., and V. Kumaraswami. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389-399. [DOI] [PubMed] [Google Scholar]
- 30.Ottesen, E. A., P. F. Weller, and L. Heck. 1977. Specific cellular immune unresponsiveness in human filariasis. Immunology 33:413-421. [PMC free article] [PubMed] [Google Scholar]
- 31.Partono, F., and Purnomo. 1978. Clinical features of timorian filariasis among immigrants to an endemic area in West Flores, Indonesia. Southeast Asian J. Trop. Med. Public Health 9:338-343. [PubMed] [Google Scholar]
- 32.Schrater, A. F., A. Spielman, and W. F. Piessens. 1983. Predisposition to Brugia malayi microfilaremia in progeny of infected gerbils. Am. J. Trop. Med. Hyg. 32:1306-1308. [DOI] [PubMed] [Google Scholar]
- 33.Simonsen, P. E., D. W. Meyrowitsch, W. G. Jaoko, M. N. Malecela, D. Mukoko, E. M. Pedersen, J. H. Ouma, R. T. Rwegoshora, N. Masese, P. Magnussen, B. B. Estambale, and E. Michael. 2002. Bancroftian filariasis infection, disease, and specific antibody response patterns in a high and a low endemicity community in East Africa. Am. J. Trop. Med. Hyg. 66:550-559. [DOI] [PubMed] [Google Scholar]
- 34.Soboslay, P. T., S. M. Geiger, B. Drabner, M. Banla, E. Batchassi, L. A. Kowu, A. Stadler, and H. Schulz-Key. 1999. Prenatal immune priming in onchocerciasis-onchocerca volvulus-specific cellular responsiveness and cytokine production in newborns from infected mothers. Clin. Exp. Immunol. 117:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steel, C., A. Guinea, J. S. McCarthy, and E. A. Ottesen. 1994. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens. Lancet 343:890-893. [DOI] [PubMed] [Google Scholar]
- 36.Storey, N., J. C. Kee, J. M. Behnke, and D. Wakelin. 1988. Prenatal sensitisation in experimental filariasis: observations on Acanthocheilonema viteae infections in mice. Trop. Med. Parasitol. 39:299-303. [PubMed] [Google Scholar]
- 37.Tisch, D. J., F. E. Hazlett, W. Kastens, M. P. Alpers, M. J. Bockarie, and J. W. Kazura. 2001. Ecologic and biologic determinants of filarial antigenemia in bancroftian filariasis in Papua New Guinea. J. Infect. Dis. 184:898-904. [DOI] [PubMed] [Google Scholar]
- 38.Wartman, W. 1947. Filariasis in American armed forces in World War II. Medicine 26:334-352. [DOI] [PubMed]
- 39.Weil, G. J., R. Hussain, V. Kumaraswami, S. P. Tripathy, K. S. Phillips, and E. A. Ottesen. 1983. Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J. Clin. Invest. 71:1124-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]