Abstract
Shiga toxin 2e (Stx2e), produced by host-adapted Shiga toxin-producing Escherichia coli (STEC) strains, causes edema disease in weaned pigs. Edema disease is manifested as vascular necrosis, edema, neurologic signs, and death. In this study we sought to determine the correlation between the presence of Stx2e in the blood of STEC-inoculated pigs and the disease outcome. Eleven of 15 (73%) pigs with clinical and 5 of 35 (14%) pigs with subclinical edema disease had detectable levels of Stx2e in the red-blood-cell (RBC) fraction of their blood but not in serum or plasma. The presence of Stx2e in the RBC fraction was strongly associated with the development of clinical disease (relative risk, 5.8; P < 0.0001). Subclinical pigs with Stx2e in their blood developed more-extensive vascular lesions than pigs without detectable Stx2e in their blood (average proportions of necrotic arterioles, 63 and 27.5%, respectively; P = 0.001). Variations in RBC-bound Stx2e levels could in part reflect variations in the binding capacity of RBCs. As an initial step toward addressing this possibility, assays were conducted to determine if pigs vary in the Stx2e binding capacity of their RBCs. An in vitro study of noninoculated pigs demonstrated two phenotypes based on the capacity of the RBCs to bind Stx2e. While RBCs from most of the pigs consistently bound high levels of Stx2e (high-binding phenotype), consistently low Stx2e binding was detected in RBCs from a few pigs (low-binding phenotype). The low- and high-binding phenotypes of individual pigs remained consistent throughout repeated samplings over 2 months.
Shiga toxins (Stx) are bacterial exotoxins that inhibit protein synthesis in mammalian cells. Stx are produced by many strains of Escherichia coli, by some strains of Shigella, and less frequently by other bacteria (9, 22, 25). E. coli strains can produce Stx1 and/or Stx2 or Stx2 variants (22, 23). Stx are composed of an enzymatic A subunit and five receptor binding B subunits. After binding of the B subunits to the glycolipid receptor (globotriaosyl ceramide [Gb3] or globotetraosyl ceramide [Gb4]) on mammalian cells, the A subunit is activated and cleaves a specific adenine residue on the 28S rRNA, thus inhibiting protein synthesis.
Edema disease is a disease of young pigs caused by host-adapted Escherichia coli that produces a variant of Stx2, called Stx2e (3). Porcine Stx-producing E. coli (STEC) strains colonize the lower small intestine by F18 fimbria-mediated adhesion and produce Stx2e locally (3, 8, 12). A portion of intraluminal toxin is absorbed into the blood and carried to the sensitive tissues (intestine and brain), causing vascular necrosis, edema, neurologic signs, and death (16, 17). The level of intraintestinal toxin produced is different for different individual pigs, and this variation is reflected in their fecal toxin titers (8). Although pigs with high fecal toxin titers are more likely to succumb to clinical disease (neurologic signs, edema, or death) than are those with low toxin titers, fecal toxin titers are not predictive of the disease outcome for individual pigs. Some pigs develop high fecal Stx2e titers while remaining clinically normal (8). Others, with comparatively low fecal Stx2e titers, develop clinical signs and die. Some STEC-inoculated pigs with clinical edema disease have detectable Stx2e in the red-blood-cell (RBC) fraction of their blood (but not in serum) (8). In experimental trials only 30% of STEC-inoculated pigs developed clinical signs (5, 8). Additional parameters that are thought to influence the outcome of infection (besides the amount of toxin produced within the intestine) are intestinal permeability, the amount of Stx2e transported into the blood, and the portion of Stx2e in blood that is actually available to susceptible endothelial cells (6, 8, 37). Porcine RBCs are rich in surface Gb4, a glycolipid receptor for Stx2e (6). In vitro binding experiments suggest that most Stx2e activity localizes in the RBC fraction of porcine whole-blood samples, with little or no verotoxicity detected in the plasma fraction (8). Stx2e apparently also binds to RBCs in vivo when injected intravenously in pigs (6). Binding of Stx2e to RBCs may be a means for delivery of the toxin to sensitive tissues (6, 8). Alternatively, it is conceivable that RBC binding may temporarily protect sensitive tissues from exposure to Stx by rendering the toxin less available to endothelial cells (32). Binding of toxin to porcine RBCs in vivo apparently does not protect pigs against clinical edema disease, but it may delay toxin transfer into tissues (6, 8).
In humans STEC causes a lethal systemic vascular disease, hemolytic-uremic syndrome (HUS) (15, 31). The STEC strains implicated in HUS often, but not always, belong to serogroup O157:H7, colonize the large intestine via intimin-mediated adhesion, and produce Stx1 and/or Stx2 or its variants (22, 23). The pathogenesis of HUS is not completely understood. It is assumed that Stx directly or indirectly damages glomerular capillaries, causing endothelial cell necrosis and/or apoptosis, fibrin deposition in capillaries, and microvascular thrombosis along with thrombocytopenia and hemolytic anemia. Intestinally produced Stx is apparently transported across the epithelium and delivered to the target tissues (kidney, intestine, and brain) through systemic circulation (1). Stx bind to human RBCs, monocytes, and neutrophils in vitro without causing morphological changes in these cells (4, 26, 34). Binding of Stx to human RBCs in vitro varies among individuals depending on their P blood group phenotype (4). RBCs with a P1 phenotype (determined by P1 antigen, a glycolipid with a terminal sugar residue identical to that of Gb3) bind Stx more abundantly than RBCs with a P2 phenotype. Taylor et al. have proposed that RBCs of the P1 phenotype may act as a sink to bind Stx in vivo, thus preventing or delaying toxin binding to the sensitive endothelial cells in the target tissues (32). However, epidemiological data suggest that the P1 phenotype does not protect STEC-infected patients from HUS (2, 13, 21, 24, 27).
The study reported here is composed of two parts: in vivo experiments utilizing a previously described edema disease model and in vitro experiments. The objectives of the in vivo experiments were to determine (i) the incidence and duration of Shiga toxemia in pigs experimentally inoculated with host-adapted STEC and (ii) whether the levels of Stx2e bound to RBCs in such pigs correlate with the severity of disease. In the second, in vitro part of the study, we sought to determine whether pigs, like humans, vary in the capacity of their RBCs to bind Stx2e.
MATERIALS AND METHODS
Bacterial strains.
Stx2e-producing E. coli S1191 is from a pig with edema disease (18). This strain belongs to serogroup O139 and produces F18ab fimbriae, hemolysin, and heat-stable enterotoxin B (STb). E. coli S1191 is resistant to chloramphenicol. E. coli 123 is a nonpathogenic isolate from a healthy pig (serogroup O143). Inocula were prepared as described elsewhere (29).
In vivo experiments. (i) Experimental design.
The design used here for reproduction of edema disease in STEC-inoculated pigs was essentially that utilized previously (5, 8, 20). All animal experiments were carried out in accordance with the protocol approved by the Iowa State University Animal Care and Use Committee. Fifty pigs (weaned at the age of 14 to 16 days) in a total of five replicates were orally inoculated with 1010 CFU of STEC strain S1191. Fourteen control pigs were inoculated with 1010 CFU of the nonpathogenic E. coli strain 123. Room temperature was maintained at 24 to 27°C, and heat lamps were available. Pigs were fed an antibiotic-free diet, with a protein content of ≥21%, ad libitum throughout the study (5). Pigs were inoculated 8 to 10 days after weaning. STEC shedding status was assessed as previously described (8).
Throughout the experiments pigs were observed three times a day for signs of edema disease (subcutaneous edema, neurologic impairment). Pigs that developed clinical signs of edema disease were designated clinical pigs. STEC-inoculated pigs that remained free of clinical signs until the termination of the experiments, 13 to 14 days postinoculation (p.i.), were designated subclinical pigs. Pigs were euthanized with intravenous barbiturate. At necropsy, pigs were examined for gross lesions indicative of edema disease or concurrent diseases.
(ii) Histopathology.
Samples from two ileum sites (1 and 2 m proximal from the ileocecal junction) and the brain stem (medulla oblongata) were collected in 10% neutral buffered formalin from all pigs at necropsy. Tissue samples were embedded in paraffin, sectioned at 5 μm, and processed for staining with hematoxylin and eosin. Slides were coded such that the pathologist was blinded to the treatment of the pigs. Slides were examined microscopically for vascular lesions (20). The extent of vascular lesions in subclinical pigs from four replicates (n = 25) was assessed by determining the percent necrotic arteriolar profiles per tissue section. Results from all sites examined were averaged. Slides from all clinical pigs and from the subclinical pigs from one replicate were evaluated qualitatively. For these pigs, a section was considered positive if two or more vascular profiles were necrotic.
(iii) Vero cell assay for Stx2e in the feces and blood.
Fecal samples from 14 control and 30 STEC-inoculated pigs were collected every other day from 2 to 10 or 3 to 11 days p.i. Fecal samples were stored at 4°C until they were processed as described elsewhere (8).
Blood samples for the Stx2e assay were collected (in EDTA) from 50 STEC-inoculated and 14 control pigs every other day from 2 to 10 or 3 to 11 days p.i. Serum samples were also collected from some pigs 2 to 11 days p.i. Pigs that developed clinical edema disease were bled upon the onset of clinical signs (if the signs were mild) and/or prior to euthanasia. Although pigs were monitored three times daily for clinical signs of edema disease, some STEC-inoculated pigs were found dead without previously noted clinical disturbances.
Blood samples (in EDTA) were centrifuged at 1,500 × g for 10 min, the buffy coat layer was discarded, and the RBC and plasma fractions were collected. Serum samples were centrifuged at 1,500 × g for 10 min, and supernatants were collected. RBC, plasma, and serum fractions were stored at 4°C for 1 to 4 days before being assayed on Vero cells.
Assays of the blood fractions (RBC, plasma, and serum) and feces for Stx2e were carried out on monolayers of Vero cells as described previously (10, 11). Twofold dilutions of fecal supernatants and of plasma, serum, and RBC fractions were made in Hanks' balanced salt solution (HBSS) without Ca2+, Mg2+, or phenol red. The toxin titer was expressed as the log2 of the reciprocal of the highest dilution that caused the death of ≥50% of Vero cells. Some control pigs had low titers (4 to 5) of nonspecific Vero cell toxicity (not neutralized by an anti-Stx2e antibody) in their RBCs, plasma, and feces. Therefore, samples were considered to be positive for Stx2e if the Vero cell titer was ≥6 and the sample (or another sample of the same type from the same pig) was neutralized by a bovine polyclonal antibody against Stx2e (36) but not by fetal calf serum. Samples were considered neutralized if the polyclonal antibody reduced the toxin titer ≥4-fold (14).
In vitro analysis. (i) Fluorescence-activated cell sorter (FACS) analysis.
Blood samples were collected (in heparin) from 4 adult and 30 weaned pigs over a period of 2 months at 1- to 3-week intervals. Samples from each adult pig were collected on weeks 1, 2, 3, and 6. Samples from each weaned pig were collected on weeks 1, 2, 4 (n = 30), and 7 (n = 8). Immediately after collection, blood samples were centrifuged (at 1,100 × g and 4°C for 6 min), and the plasma and buffy coat were discarded. RBCs were washed in HBSS (without Ca2+, Mg2+, or phenol red), and the centrifugation was repeated. The purity of such RBC preparations obtained from three animals was assessed with a Coulter cell counter. On average, the resultant cell pellet consisted of 99.2% RBCs, 0.04% white blood cells (including 0.016% neutrophils), and 0.8% platelets (data not shown). Processed RBC fractions were stored at 4°C until they were assayed (within 1 week of sample collection).
Purified Stx2e (0.1 μg/ml; Vero cell toxicity, 106 50% cytotoxic doses [CD50]/ml) or semipurified Stx2e (Stx2e-SP) with a similar level of Vero cell toxicity was used to inoculate RBC samples (28). Stx2e-SP was produced as described for purified Stx2e except that chromatofocusing was not done (28). Ten microliters of the packed RBC fraction from each pig was incubated with 100 μl (105 Vero cell CD50) of Stx2e (for adult pigs) or Stx2e-SP (for weaned pigs) at 37°C for 1 h. Samples were then centrifuged (at 1,000 × g for 6 min in a microcentrifuge), and the supernatant was discarded. RBCs were suspended in FACS buffer (0.1% [wt/vol] NaN3 and 1.0% [wt/vol] bovine serum albumin fraction V diluted in HBSS) to a concentration of 107 to 108 cells/ml. Two samples of the RBC suspension per pig, 50 μl each (approximately 106 RBCs), were transferred to FACS tubes (Fisher Scientific, Pittsburgh, Pa.). One sample was incubated with 10 μl of primary bovine anti-Stx2e immunoglobulin G (IgG; 20 μg/ml [36]) for 30 min at 4°C (test sample). The other sample was incubated with 10 μl of bovine control IgG (10 μg/ml; Sigma-Aldrich, St. Louis, Mo.) under the same conditions (control sample). After incubation, samples were washed twice with 2 ml of FACS buffer. After the second wash, the supernatant was discarded and 10 μl of a fluorescein isothiocyanate-conjugated secondary rabbit anti-bovine IgG (heavy and light chains, 730 μg/ml; ICN, Costa Mesa, Calif.) was added to the RBC fractions of test and control samples. These suspensions were then incubated for 30 min at 4°C. Samples were washed twice, and 400 μl of FACS buffer was added to the RBC fraction after the second wash. Samples were stored in the dark at 4°C for <24 h until analysis with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). The fluorescence intensity of fluorescein isothiocyanate was measured with excitation at 488 nm and emission at 530 nm. Mean fluorescence intensities (MFI) were expressed as geometric means. The MFI for the sample from each pig was calculated by subtracting the MFI of the control sample from the MFI of the test sample. The MFI of control samples were similar to those of RBCs alone (MFI, 4 to 7).
(ii) Saturation of RBCs with Stx2e.
FACS analysis showed that pigs could be grouped into two populations according to their Stx2e binding patterns: a population with a low-binding (LB) phenotype and one with a high-binding (HB) phenotype. To test whether these two populations of pigs also differed in the amount of biologically active Stx2e that would bind to the RBC fraction, RBC suspensions in HBSS were incubated with saturating amounts of Stx2e (for adult pigs) or Stx2e-SP (for weaned pigs), and the Vero cell toxicity of the supernatant was then measured. Pigs with the LB phenotype were expected to have higher toxin titers in the supernatant, since their RBCs could bind less toxin.
Stx2e (104 Vero cell CD50) was added to 200 μl of the RBC pellet. HBSS was then added to the RBC-Stx2e mixture to make a final 30% (vol/vol) suspension of RBCs. RBC-Stx2e suspensions were incubated under the same conditions as the samples prepared for FACS analysis (1 h at 37°C). After incubation, RBCs were separated from the test supernatant by centrifugation at 1,000 × g for 4 min in the microcentrifuge. The RBC pellet was washed once with HBSS, and the centrifugation was repeated. Twofold dilutions of washed RBCs and test supernatants were made in HBSS prior to assay of these fractions on Vero cell monolayers. Samples collected in two different weeks from 12 pigs (4 adult pigs and 8 weaned pigs) were assayed.
Statistics.
The Wilcoxon signed rank test was used to compare Stx2e titers in the blood and feces of clinical and subclinical pigs on individual days and to analyze the extent of vascular necrosis in pigs with and without Stx2e in the RBC fraction. The Mann-Whitney test was used to compare Stx2e titers in the in vitro RBC saturation assay. The prevalence of the LB phenotype was estimated using a confidence interval based on the F distribution. Variation among samples was expressed as the standard error of the mean (for parametric data). Results were interpreted as significantly different if the P value was <0.05. Whenever numerous tests of significance were done for a single data set, the significance level was normalized by Bonferroni's method.
RESULTS
In vivo experiments. (i) Clinical disease.
None of the 14 control pigs developed edema disease. Fifteen of 50 STEC-inoculated pigs developed clinical edema disease. The mean incidence of clinical edema disease among the five replicates was 30% (range, 0 to 60%). Clinical disease occurred 4 to 9 days p.i.
(ii) Stx2e titers in feces and blood.
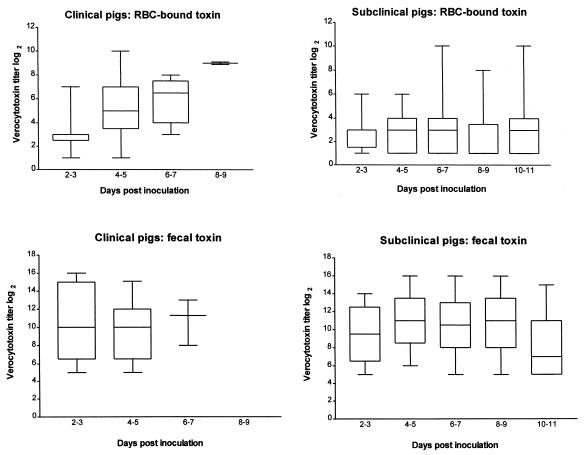
Fecal samples were collected from 14 control and 30 STEC-inoculated pigs. Two of four control pigs in one replicate developed diarrhea at days 9 to 11 p.i., and Stx2e was detected in the fecal samples from these two pigs at this time. The E. coli colonies isolated from these two pigs resembled those of the inoculum strain but were not further characterized. We assumed that this represented a late spontaneous STEC infection in the controls. Stx2e was not detected in feces from any of the other control pigs. In contrast, it was detected in the feces from all infected pigs from day 2 to day 11 p.i. The fecal Stx2e titers of clinical pigs were not different from those of subclinical pigs (Fig. 1).
FIG. 1.
Box plot depiction of daily fecal and blood verocytotoxin titers in pigs inoculated with Stx2e-producing E. coli. Fifty pigs were assayed for verocytotoxin in the RBC fraction of their blood (35 subclinical pigs; 15 clinical pigs on days 2 to 3 p.i., 15 on days 4 to 5 p.i., 8 on days 6 to 7 p.i., and 3 on days 8 to 9 p.i.). Blood samples were collected from each pig every other day from day 2 to 10 or day 3 to 11 p.i. Thirty pigs were assayed for fecal Stx2e on the same days (21 subclinical pigs; 9 clinical pigs on days 2 to 3 p.i., 8 on days 4 to 5 p.i., and 4 on days 6 to 7 p.i.). Horizontal lines inside boxes represent medians. The lower and upper borders of the boxes represent the 25th and 75th percentiles, respectively. Error bars indicate lowest and highest values (range). Some lines overlap.
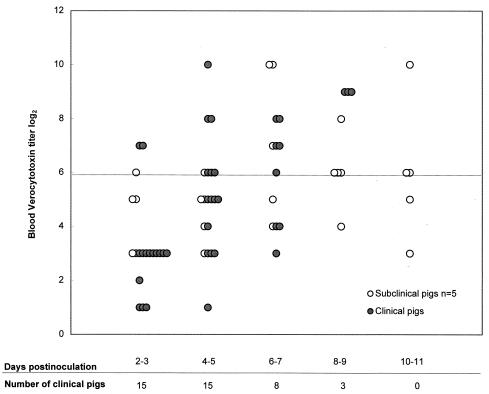
Stx2e was not detected in any of the blood fractions (RBC, serum, or plasma) of control pigs sampled every other day (n = 14 pigs for the RBC fraction; n = 5 pigs for the serum fraction; n = 4 pigs for the plasma fraction). Stx2e was detected in the RBC fractions of 11 of 15 (73%) clinical and 5 of 35 (14%) subclinical STEC-inoculated pigs. Stx2e was not detected in the serum or plasma fraction in any of the samples collected every other day from 10 STEC-inoculated pigs. These included five pigs that had Stx2e in their RBC fractions, two of which were clinical pigs. The peak verocytotoxin titer in the RBC fractions of the 15 clinical pigs was significantly higher (median, 7; range, 3 to 10; P = 0.0001) than that for the 35 subclinical pigs (median, 4; range, 1 to 10). Median daily verocytotoxin titers in the RBC fractions of clinical and subclinical pigs are shown in Fig. 1. Verocytotoxin titers in the RBC fractions of clinical pigs were significantly higher than those for subclinical pigs in samples collected at days 4 to 5 (P < 0.001) and 6 to 7 (P < 0.05) p.i. The median verocytotoxin titer of clinical pigs was also higher than that of subclinical pigs on days 8 to 9 p.i., but the difference was not statistically significant, probably because only three pigs remained in the clinical group. Stx2e titers in the RBC fractions of clinical pigs tended to peak at the time of clinical illness (Table 1). Stx2e was detected during routine (every other day) blood collection in the RBC fractions of three of six pigs that died suddenly. The remaining nine pigs exhibited clinical signs of edema disease, and Stx2e was detected in the blood of eight pigs at that time. Stx2e was detected 1 to 3 days prior to the onset of clinical signs in five of these eight pigs. The time between the detection of clinical signs (subcutaneous edema, ataxia, head tilt, circling, and recumbency) and death or euthanasia was less than 24 h. Stx2e was detected in the RBC fraction as early as day 2 p.i. (first sampling time) in both clinical and subclinical pigs (Fig. 2). Stx2e was detected in the RBC fraction in as many as three or four consecutive samples collected every other day from clinical and subclinical pigs, respectively. Examples of daily blood Stx2e titers in selected clinical and subclinical pigs are shown in Table 1.
TABLE 1.
Extent of vascular necrosis and log2 verocytotoxin titers in the RBC fractions of selected clinical and subclinical pigs
| Pig no. | Group | Extent of vascular necrosis (%)a | Log2 verocytotoxin titerb on the following days p.i.:
|
||||
|---|---|---|---|---|---|---|---|
| 2-3 | 4-5 | 6-7 | 8-9 | 10-11 | |||
| 1227 | Clinical | ND | 7 | 6* | |||
| 1220 | Subclinical | 64 | 6 | 5 | 7 | 4 | 3 |
| 1126 | Clinical | ND | 3 | 1 | 7 | 9* | |
| 1230 | Subclinical | 63 | 5 | 5 | 10 | 6 | 5 |
| 1132 | Clinical | ND | 1 | 6 | 8 | 9* | |
| 1229 | Subclinical | 53 | 5 | 6 | 10 | 6 | 6 |
Percent necrotic arteriolar profiles per tissue section. ND, not determined for clinical pigs.
Stx2e titers above the nonspecific range are boldfaced. An asterisk indicates that the pig died or was euthanized.
FIG. 2.
Log2 verocytotoxin titers in the RBC fractions of clinical (n = 15) and selected subclinical (n = 5) pigs inoculated with Stx2e-producing E. coli. All five subclinical pigs had at least one blood sample positive for verocytotoxin. A sample was considered to be positive for Stx2e if the Vero cell titer was ≥6 log2 units and the sample (or another sample of the same type from the same pig) was neutralized by a bovine polyclonal antibody against Stx2e but was not neutralized by fetal calf serum. The line indicates the maximum nonspecific verocytotoxicity of blood.
(iii) Relative risk for detectable Stx2e titers in blood and development of clinical disease.
Pigs that had the highest fecal Stx2e titers did not consistently have the highest levels of Stx2e in the RBC fraction. Furthermore, neither fecal nor RBC fraction toxin titers were predictive for the clinical outcome of STEC infection for individual animals. However, high fecal Stx2e levels were associated with an increased incidence of Stx2e in the RBC fraction: 5 of 8 (63%) pigs with peak Stx2e titers of ≥15 in feces had Stx2e in their RBC fractions, while only 6 of 22 (27%) pigs with peak fecal Stx2e titers of ≤14 had Stx2e in their RBC fractions. The relative risk for detectable levels of Stx2e in the RBC fraction if the peak fecal Stx2e titer was ≥15 was 2.3 (95% confidence interval [95% CI], 1.0 to 5.5; P = 0.1). Four of eight (50%) pigs with peak fecal toxin titers of ≥15 developed clinical disease (relative risk, 2.2; 95% CI, 0.8 to 0.2; P = 0.2). The presence of Stx2e in the RBC fraction was strongly associated with the development of clinical disease. Sixteen pigs had Stx2e in their RBC fractions, and 11 of these 16 pigs (69%) developed clinical edema disease (relative risk, 5.8; 95% CI, 2.2 to 15.5; P < 0.0001).
(iv) Vascular lesions.
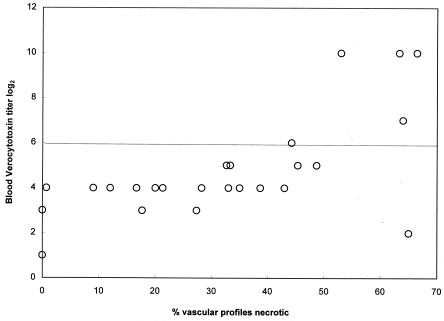
Vascular necrosis was not detected in any of the control pigs. Vascular necrosis (two or more necrotic blood vessels per tissue) was detected in 14 of 15 clinical pigs and 31 of 35 subclinical pigs. The extent of vascular necrosis (quantity) was not assessed for clinical pigs. The extent of vascular necrosis among subclinical pigs tended to be most severe in those pigs that had Stx2e in their blood RBC fractions (Fig. 3). Subclinical pigs with Stx2e in the blood (n = 5) had on average 63% of vascular profiles necrotic (median; range, 44 to 66%). Subclinical pigs without detectable Stx2e in the blood (n = 20) had 27.5% of vascular profiles necrotic (median; range, 0 to 65%). The difference between the two groups was significant (P = 0.001). While the vascular lesions in subclinical pigs with Shiga toxemia were significantly more extensive than those in pigs without Shiga toxemia, there was no direct correlation between the magnitude of Shiga toxemia and the extent of vascular lesions (Fig. 3; Table 1).
FIG. 3.
Extent of vascular necrosis (percent necrotic arteriolar profiles per tissue section) in relation to peak verocytotoxin titer in subclinical pigs inoculated with STEC (n = 25). Samples were considered to be positive for Stx2e if the Vero cell titer was ≥6 log2 units and the sample (or another sample of the same type from the same pig) was neutralized by a bovine polyclonal antibody against Stx2e but was not neutralized by fetal calf serum. The line indicates the maximum nonspecific verocytotoxicity of blood.
In vitro analysis. (i) FACS.
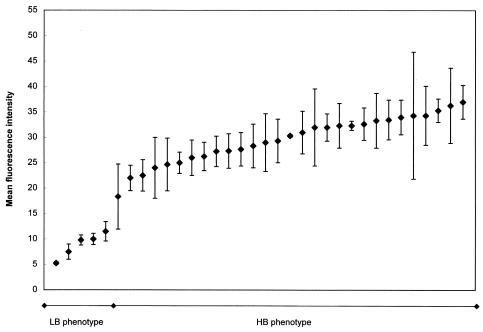
The MFI of RBC samples inoculated with Stx2e in vitro varied widely among pigs and among samples (Fig. 4). However, a few pigs (1 of 4 adult pigs and 4 of 30 weaned pigs) had consistently low MFI (mean, 8.8 ± 1.4) at all sampling times. These pigs were designated LB phenotype pigs. All the other pigs had mean MFI of ≥18 and were designated HB phenotype pigs. The mean MFI for the HB group was 29.6 ± 0.9. The low MFI of pigs with the LB phenotype was due to the fact that a comparatively low proportion of RBCs bound Stx2e. Although Stx2e bound to significantly fewer RBCs in the LB pigs, the MFI per RBC that bound Stx2e were similar for LB and HB phenotype pigs. For example, 73% of gated RBCs from a pig with the HB phenotype bound Stx2e, while only 16% of gated RBCs from a pig with the LB phenotype bound Stx2e. The MFI per individual RBC that bound Stx2e was 76 for the HB phenotype pig and 79 for the LB phenotype pig.
FIG. 4.
FACS analysis of Stx2e binding to porcine RBCs in vitro. Three to four samples per pig (collected over a period of 2 months at 1- to 3-week intervals) were analyzed and results were averaged. This analysis was performed for 5 pigs with the LB phenotype (MFI range, 5 to 12) and 29 pigs with the HB phenotype (MFI range, 18 to 37). Bars, standard errors of the means.
The MFI for adult pigs with the HB phenotype were not different from those for weaned pigs of the same phenotype, indicating that there was not an apparent age effect on the capacity of RBCs to bind Stx2e in vitro. HB and LB phenotypes were found among both adult and weaned pigs, and the particular binding phenotype of a pig was consistent throughout the sampling period. The prevalence of pigs with the LB phenotype was 15% (95% CI, 5 to 31%).
(ii) Saturation of RBCs with Stx2e.
RBCs from Stx2e-inoculated pigs with LB and HB phenotypes were further characterized by using the Vero cell assay. Stx2e titers of ≥9 were found in the RBC fractions of the Stx2e-inoculated samples. The median Stx2e titer in the test supernatant fractions of the LB phenotype pigs was significantly higher than that for the HB phenotype pigs (P = 0.0051). The corresponding median Stx2e titers in the test supernatant fractions from LB phenotype and HB phenotype pigs were 7.5 (range, 6.5 to 8.0) and 6 (range, 4.0 to 6.5), respectively.
DISCUSSION
In this study Stx2e was repeatedly detected in the RBC fraction of the blood in most of the clinically ill pigs. This result confirms previous findings and further supports the concept that Stx is transported to the sensitive tissues via blood (8). Stx2e was not detected in any serum and/or plasma samples of STEC-inoculated pigs, including those that were collected from clinical pigs with detectable Stx2e titers in the RBC fractions of their blood, indicating that Stx2e is rapidly bound to RBCs and/or leukocytes or endothelial cells. Stx2e detection in the RBC fraction of blood was associated with a high relative risk for the development of clinical disease. Presumably, all clinical pigs had at least a brief period of Shiga toxemia, and our intermittent sampling was unable to detect it in some. Stx2e was detected in the blood as early as day 2 p.i., and Shiga toxemia lasted for several days in some pigs. Toxin titers in blood tended to be highest at the time of clinical illness. Previous experiments have shown that Stx2e binding to RBCs appears to delay toxin transfer into tissues (6). However, since most pigs with detectable Stx2e in the blood became terminally ill, it appears that Stx2e binding to RBCs was transient and that the toxin was transferred to endothelial cells. The appearance of Stx2e in the blood only a few days after inoculation with STEC suggests that early events in STEC pathogenesis are critical to disease outcome. This conclusion is also supported by the observation that systemic intervention with an anti-Stx2e antibody within the first few days after inoculation with STEC can prevent the development of clinical edema disease (19).
We demonstrated that some subclinical pigs also had detectable levels of Stx2e in their RBC fractions. The levels of Stx2e in the blood, as well as the duration of Shiga toxemia in these five subclinical pigs, were comparable to those of clinical pigs. This suggests that additional factors must play a role in determining the outcome of STEC infection in these pigs. One of the factors that may influence the pathogenesis of STEC infection is the rate of Stx transport across the intestinal mucosa into the bloodstream. One bolus of Stx1 (100 ng/kg of body weight) given intravenously to baboons causes lethal acute renal failure; however, four divided doses of Stx1 (25 ng/kg × 4) given at 12-h intervals do not result in clinical or histologic features of HUS (30). Presumably, Stx2e is continuously transferred across the intestinal mucosa after pigs are infected with STEC. Pigs that developed clinical signs of edema disease tended to have significantly higher levels of Stx2e in the RBC fraction of blood earlier (days 4 to 5 p.i.) than subclinical pigs (Fig. 1 and 2), indicating that the rate of toxin transfer from the intestinal lumen into the bloodstream may be higher in clinical pigs than in subclinical pigs. For a few subclinical pigs, however, Shiga toxemia was nearly identical in magnitude, duration, and timing to that observed for clinical pigs (Table 1), indicating that factors other than the rate of toxin transfer into the blood play a role in these pigs. Alternatively, it is possible that blood Stx2e titers do not reflect the total amount of toxin absorbed into systemic circulation and that some portion of toxin may bind directly to tissue receptors without prior binding to RBCs.
Subclinical pigs with Shiga toxemia had extensive vascular lesions, indicating that the lack of clinical disease was not due to the lack of vascular receptors for Stx2e. However, there was no consistent direct correlation between the magnitude and/or duration of Shiga toxemia and the extent of vascular lesions in individual subclinical pigs. For example, pig 1229 had 53% of blood vessels necrotic, and Stx2e titers in its blood were 6, 10, 6, and 6 log2 units on days 5 to 11 p.i. (Table 1). Pig 1230 attained similar levels of Stx2e but had a shorter duration of Shiga toxemia, yet its vascular necrosis was more extensive, affecting 63% of blood vessels. While it is possible that these numbers are within the range of experimental variation, these differences may reflect individual animal variability in susceptibility to systemic Stx2e effects.
The first part of our study demonstrated that binding of in vivo-produced Stx2e to RBCs was detectable in most (11 of 15) clinical pigs and some (5 of 35) subclinical pigs. Therefore, we hypothesized that pigs may differ in the capacity of their RBCs to bind Stx2e when exposed to toxin in vitro. Two methods were used to test this hypothesis: FACS analysis to demonstrate binding and a Vero cell assay to measure the amount of biologically active RBC-bound toxin. Processing of blood samples ensured that 99% of cells incubated with Stx2e were RBCs. The results of FACS and Vero cell assays correlated with one another and demonstrated that two binding phenotypes exist in the pig population: pigs whose RBCs bind variably large amounts of Stx2e (HB phenotype) and pigs whose RBCs consistently bind little Stx2e (LB phenotype). Toxin bound to RBCs was biologically active, causing Vero cell death, presumably because Gb4 receptors on Vero cells bound Stx2e more avidly than receptors on RBCs, resulting in the transfer of the toxin from RBCs to Vero cells. RBC suspensions from LB phenotype pigs had significantly larger amounts of biologically active Stx2e in the supernatants than RBC suspensions from HB phenotype pigs.
Our in vitro findings indicated that pigs with the LB phenotype may be similar to humans with the rare P2 blood group, who bind only comparatively small amounts of Stx to their RBCs (4). Pig RBCs have been shown to be rich in Gb4, and it is assumed that Stx2e binds to this receptor (6). LB phenotype pigs had a markedly reduced portion of RBCs that bound Stx2e; however, the small proportion of RBCs that did bind Stx2e in LB phenotype pigs bound toxin at the same level as the RBCs in HB phenotype pigs. These findings suggest that only a small proportion of RBCs in LB phenotype pigs express Gb4 or another Stx2e binding receptor. The LB phenotype was stable; pigs maintained it throughout the sampling period (2 months), indicating that this phenotype is not age dependent.
It is not known whether the LB phenotype is a risk factor for the development of clinical edema disease. Reduced binding of Stx2e to the RBCs could theoretically result in increased binding of absorbed Stx2e directly to the toxin receptors on the endothelial cells, causing rapid, simultaneous saturation of receptors and leading to clinical edema disease. A similar hypothesis has been proposed for P blood types in human patients (32). However, a recent study refuted this possibility by showing that the P1 blood group was equally represented in healthy controls and E. coli O157:H7-infected children regardless of whether patients had uncomplicated illness or HUS (13), confirming the findings of previous studies that failed to associate P1 expression with diminished risk for the development of HUS after infection with E. coli O157:H7 (2, 21, 24, 27).
Alternatively, the LB phenotype could be associated with enhanced resistance against edema disease if reduced expression of Stx2e receptors on the RBCs in these pigs is reflected in reduced tissue receptor expression generally. It would be useful to determine if the binding phenotype of pigs affects their susceptibility to disease. In a pilot study, 10 randomly selected weaned pigs were inoculated with STEC S1191 as described above, and their binding phenotypes were determined prior to and after inoculation (I. Matise, unpublished data). All pigs had the HB phenotype, and this phenotype was maintained throughout the study. One pig developed clinical edema disease, indicating that pigs with the HB phenotype are susceptible to clinical edema disease. Experiments to determine if clinical disease incidence varies in groups of pigs representing each binding phenotype equally are warranted.
In humans, Stx also binds to blood neutrophils, monocytes, and platelets in vitro (7, 26, 34). Stx has also been recently demonstrated in the blood of STEC-infected human patients (33, 35). Stx2 bound to neutrophils was detected 3 to 14 days after the onset of hemorrhagic diarrhea, in some patients repeatedly, with 5 days between sampling times. In vivo binding to other blood cells (platelets, RBCs, or lymphocytes) was not observed; however, limited binding to monocytes was detected. Neutrophil-bound Stx was detected both in acutely infected patients with hemorrhagic diarrhea and HUS and in their asymptomatic household members (33). Studies to determine whether Stx2e binds to porcine white blood cells are needed.
Acknowledgments
This work was financed by NIH grant AI41328.
We thank Sheridan Booher, Megan Black, Amy Irlbeck, Pedro Navaro, Dawn Wiarda, Joe Spoo, and Nathan Eslick for their commitment and excellent technical assistance in animal experiments and laboratory procedures. Donghui Cheng provided expertise and guidance in the development of the FACS assay. We also thank Tammy Benson for assistance with statistical analysis.
Editor: V. J. DiRita
REFERENCES
- 1.Acheson, D. W., L. L. Lincicome, M. Jacewicz, and G. T. Keusch. 1998. Shiga toxin interaction with intestinal epithelial cells, p. 140-147. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 2.Ashida, A., K. Matsui, T. Chizaki, K. Yoshikawa, N. Kawamura, K. Suzuki, and H. Tamai. 1999. Erythrocyte P1 group antigen expression in VTEC-associated hemolytic uremic syndrome. Clin. Nephrol. 51:73-76. [PubMed] [Google Scholar]
- 3.Bertschinger, H. U., and C. L. Gyles. 1994. Oedema disease of pigs, p. 193-219. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, Conn.
- 4.Bitzan, M., S. Richardson, C. Huang, B. Boyd, M. Petric, and M. A. Karmali. 1994. Evidence that verotoxins (Shiga-like toxins) from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect. Immun. 62:3337-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosworth, B. T., J. E. Samuel, H. W. Moon, A. D. O'Brien, V. M. Gordon, and S. C. Whipp. 1996. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect. Immun. 64:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd, B., G. Tyrrell, M. Maloney, C. Gyles, J. Brunton, and C. Lingwood. 1993. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targetting and results in a verotoxin 1-like disease in pigs. J. Exp. Med. 177:1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooling, L. L. W., K. E. Walker, T. Gille, and T. A. W. Koerner. 1998. Shiga toxin binds human platelets via globotriaosylceramide (Pk antigen) and a novel platelet glycosphingolipid. Infect. Immun. 66:4355-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornick, N. A., I. Matise, J. E. Samuel, B. T. Bosworth, and H. W. Moon. 2000. Shiga toxin-producing Escherichia coli infection: temporal and quantitative relationships among colonization, toxin production, and systemic disease. J. Infect. Dis. 181:242-251. [DOI] [PubMed] [Google Scholar]
- 9.Feng, P. 1995. Escherichia coli serotype O157:H7: novel vehicles of infection and emergence of phenotypic variants. Emerg. Infect. Dis. 1:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentry, M. K., and J. M. Dalrymple. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon, V. M., S. C. Whipp, H. W. Moon, A. O'Brien, and J. E. Samuel. 1992. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect. Immun. 60:485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imberechts, H., H. De Greve, C. Schlicker, H. Bouchet, P. Pohl, G. Charlier, H. Bertschinger, P. Wild, J. Vandekerckhove, J. Van Damme, M. Van Montagu, and P. Lintermans. 1992. Characterization of F107 fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequence of the F107 major fimbrial subunit gene, fedA. Infect. Immun. 60:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jelacic, S., C. L. Wobble, D. R. Boster, M. A. Ciol, S. L. Watkins, P. I. Tarr, and A. E. Stapleton. 2002. ABO and P1 blood group antigen expression and stx genotype and outcome of childhood Escherichia coli O157:H7 infection. J. Infect. Dis. 185:214-219. [DOI] [PubMed] [Google Scholar]
- 14.Karch, H., T. Meyer, H. Russmann, and J. Heesemann. 1992. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect. Immun. 60:3464-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod, D. L., and C. L. Gyles. 1991. Immunization of pigs with a purified Shiga-like toxin II variant toxoid. Vet. Microbiol. 29:309-318. [DOI] [PubMed] [Google Scholar]
- 17.MacLeod, D. L., C. L. Gyles, and B. P. Wilcock. 1991. Reproduction of edema disease of swine with purified Shiga-like toxin II variant. Vet. Pathol. 28:66-73. [DOI] [PubMed] [Google Scholar]
- 18.Marques, L. R. M., J. S. M. Peiris, S. J. Cryz, and A. D. O'Brien. 1987. Escherichia coli strains isolated from pigs with edema disease produce a variant of Shiga-like toxin II. FEMS Microbiol. Lett. 44:33-38. [Google Scholar]
- 19.Matise, I., N. A. Cornick, S. L. Booher, J. E. Samuel, B. T. Bosworth, and H. W. Moon. 2001. Intervention with Shiga toxin (Stx) antibody after infection by Stx-producing Escherichia coli. J. Infect. Dis. 183:347-350. [DOI] [PubMed] [Google Scholar]
- 20.Matise, I., T. Sirinarumitr, B. T. Bosworth, and H. W. Moon. 2000. Vascular ultrastructure and DNA fragmentation in swine infected with Shiga toxin-producing Escherichia coli. Vet. Pathol. 37:318-327. [DOI] [PubMed] [Google Scholar]
- 21.Newburg, D. S., P. Chaturvedi, E. L. Lopez, S. Devoto, A. Fayad, and T. G. Cleary. 1993. Susceptibility to hemolytic-uremic syndrome relates to erythrocyte glycosphingolipid patterns. J. Infect. Dis. 168:476-479. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keush. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 24.Orr, P. H., V. Dong, M. L. Schroeder, and M. R. Ogborn. 1995. P1 blood group antigen expression and epidemic hemolytic uremic syndrome. Pediatr. Nephrol. 9:612-613. [DOI] [PubMed] [Google Scholar]
- 25.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robson, W. L. M., A. K. C. Leung, T. Bowen, R. Brant, and E. Ching. 1994. The P1 blood group and the severity of diarrhea-associated hemolytic uremic syndrome. Clin. Nephrol. 42:288-290. [PubMed] [Google Scholar]
- 28.Samuel, J. E., L. P. Perera, S. Ward, A. D. O'Brien, V. Ginsberg, and H. C. Krivan. 1990. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 58:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarmiento, J. I., T. A. Casey, and H. W. Moon. 1988. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am. J. Vet. Res. 49:1154-1159. [PubMed] [Google Scholar]
- 30.Siegler, R. L., T. J. Physher, V. L. Tesh, and F. B. J. Taylor. 2001. Response to single and divided doses of Shiga toxin-1 in a primate model of hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:1458-1467. [DOI] [PubMed] [Google Scholar]
- 31.Su, C., and L. J. Brandt. 1995. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 123:698-714. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, C. M., D. V. Milford, P. E. Rose, T. C. F. Roy, and B. Rowe. 1990. The expression of blood group P1 in post-enteropathic haemolytic uraemic syndrome. Pediatr. Nephrol. 4:59-61. [DOI] [PubMed] [Google Scholar]
- 33.te Loo, D. M. W. M., A. E. Heuvelink, E. de Boer, J. Nauta, J. van der Walle, C. Schroder, V. W. M. van Hinsbergh, H. Chart, N. C. A. J. van de Kar, and L. P. W. J. van den Heuvel. 2001. Vero cytotoxin binding to polymorphonuclear leukocytes among households with children with hemolytic uremic syndrome. J. Infect. Dis. 184:446-450. [DOI] [PubMed] [Google Scholar]
- 34.te Loo, D. M. W. M., L. A. H. Monnens, T. J. A. M. van der Velden, M. A. Vermeer, F. Preyers, P. N. M. Demacker, L. P. W. J. van den Heuvel, and V. W. M. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95:3396-3402. [PubMed] [Google Scholar]
- 35.te Loo, D. M. W. M., V. W. M. van Hinsbergh, L. P. W. J. van den Heuvel, and L. A. H. Monnens. 2001. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:800-806. [DOI] [PubMed] [Google Scholar]
- 36.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, and A. D. O'Brien. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waddell, T. E., and C. L. Gyles. 1995. Sodium deoxycholate facilitates systemic absorption of verotoxin 2e from pig intestine. Infect. Immun. 63:4953-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]