Abstract
We tested the potential of insulin-like growth factor I (IGF-I) to induce functional recovery in an animal model of cerebellar ataxia because this motor impairment is accompanied in humans and rodents by distinct changes in several components of the IGF-I trophic system. Rats rendered ataxic by deafferentation of the cerebellar cortex with 3-acetylpyridine recovered motor function after IGF-I was administered, as determined by behavioral and electrophysiological tests. When treated with IGF-I, inferior olive neurons, the targets of the neurotoxin, were rescued to various degrees (from 92 to 27% of surviving neurons), depending on the time that treatment with IGF-I was initiated. Furthermore, full recovery was obtained regardless of the route by which the trophic factor was administered (intraventricular or subcutaneous) even in rats with severe neuronal loss. These results suggest that human ataxia could be treated with IGF-I by a simple procedure.
Motor pathways in the cerebellum and brainstem express several components of the insulin-like growth factor I (IGF-I) trophic system and are modulated by IGF-I in a paracrine/autocrine fashion (1, 2). Available evidence indicates that this trophic peptide is involved not only in coordination of the development of the cerebellum but also in maintenance of its adult function (3). Moreover, recent observations indicate that neurodegeneration of cerebellar pathways is related to changes in the IGF-I system in humans and in rodents (4–6). A common feature in these cases of neurodegeneration is a depletion of IGF-I levels in both serum and brain tissue. In addition, changes in endogenous IGF-I input by diverse experimental approaches modulate the response of the cerebellum to deafferentation (7). These data strongly suggest that neuronal death in the cerebellum, and possibly in other areas also (8), is related to deficits of IGF-I neurotrophic input.
Although the use of neurotrophic factors for treatment of neurodegenerative illnesses and even brain aging is currently widely advocated (9), strong experimental evidence in favor of their use in humans is at best scarce and mainly based on in vitro observations and preliminary success in animal models (10). Hence, detailed studies in experimental models of human neurodegenerative diseases are very much needed. By using a well-characterized rat model of human cerebellar ataxia (11), we have done a detailed study of the role of the IGF-I trophic system in the pathogenesis of this disease. Because our observations indicated that inappropriate IGF-I input to the cerebellum could underlie the inability of these animals to recover motor coordination, we administered recombinant IGF-I to them. Prolonged infusion of IGF-I to ataxic rats resulted in full recovery of motor coordination, whereas vehicle-treated animals showed no recovery at all.
MATERIALS AND METHODS
Experimental Design.
We used rats injected with 3-acetylpyridine (3AP), a neurotoxin that selectively destroys inferior olive neurons in the brainstem (6), as an animal model of human olivo-cerebellar degeneration resulting in cerebellar ataxia. We determined the potential therapeutic effects of IGF-I on this model of ataxia by assessing diverse parameters of olivo-cerebellar function. These included behavioral, biochemical, electrophysiological, and morphological tests.
Rat Models of Ataxia.
Induction of ataxia in adult rats was achieved either by intraperitoneal injection of 3AP (50–65 mg/kg of body weight) (11) or by bilateral electrocoagulation of the inferior olive [applied current of 10 μA for 1 sec, with a monopolar electrode; stereotaxic coordinates (12) were 10.5 mm ventral, −3.8 mm from lambda, and ±0.6 mm lateral]. Because both procedures destroy the inferior olive, the cerebellar cortex losses its climbing fiber input and as a result the rats become permanently ataxic. Thus, after 3AP injection rats showed clear motor deficits including staggering of their hind limbs and gait disturbances. In addition, the animals showed a 10-fold reduction in the time spent in the rota-rod as compared with intact animals (see Fig. 1). Ten animals were used for each experimental group.
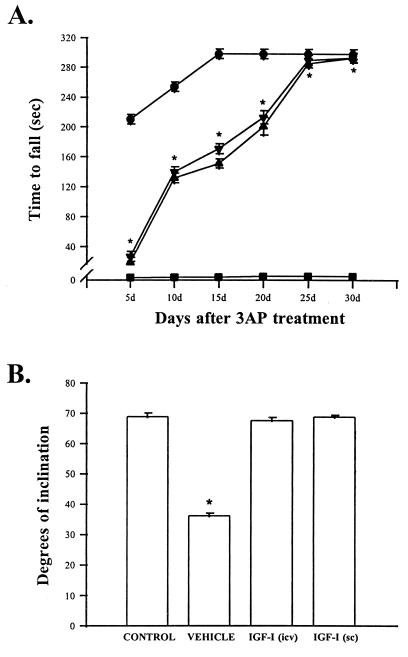
Figure 1.
Motor coordination in ataxic rats is normalized by IGF-I treatment. (A) Motor performance of 3AP-injected animals in the rota-rod test. Although untreated 3AP rats (vehicle group) were unable to stay in the rotating rod for longer than 2–4 sec, 3AP rats treated with IGF-I s.c. or i.c.v. showed a gradual recovery of their motoric abilities and reached normal performance (300 sec in the rotating rod) after 1 month of constant infusion. Statistical significance was determined by one-way analysis of variance followed by Student’s t test in all experiments. ∗, P < 0.001 vs. vehicle-injected rats for both IGF-I-treated groups. •, Controls; ▪, 3AP + vehicle; ▾, 3AP + IGF-I (s.c.); ▴, 3AP + IGF-I (i.c.v.). Error bars in the vehicle group are smaller than the symbols. (B) Motor performance in the inclined plane task (13). The average score for the inclined plane task was 69.9 ± 1.3° (including all three orientations) in both control and IGF-I-treated 3AP animals. However, vehicle-treated 3AP rats exhibited a marked decrease in scores in the inclined plane. ∗, P < 0.001 vs. all other groups. Results shown in these and subsequent figures are mean ± SEM. Number of animals per group was 10. The two vehicle groups used (receiving 3AP plus either s.c. or i.c.v. osmotic minipumps filled with vehicle, n = 10 for each group) gave identical results and are pooled in a single group for clarity.
Evaluation of Motor Coordination.
Motor coordination of ataxic animals was determined by two different tests. In the first one, animals were placed in a rota-rod apparatus (Ugo Basile, Italy) with increasing acceleration. The apparatus consisted of a horizontal motor-driven rotating rod in which the animals were placed perpendicular to the long axis of the rod, with the head directed against the direction of rotation so that the rat has to progress forward to avoid falling. The trial was stopped when the animal fell down or after a maximum of 5 min. The time spent in the rotating rod was recorded for each animal and trial. Animals received a pretraining session to familiarize them with the procedure before being rendered ataxic. Thereafter, a total of six consecutive trials were done for every animal in each session. Only the results from the sixth trial session for each animal were used for statistical comparison (see Fig. 1A). Animals were tested every 5 days in the rota-rod after injection of 3AP. In a second type of test, we used the inclined plane paradigm that measures the animal’s ability to maintain its body position for 5 sec on an inclined plane covered with a corrugated rubber mat (13). The angle of the surface is changed from 10° to 90° at 5° intervals until the animal is unable to remain in position. Animals were placed in three positions: head up, right side up, and left side up. The average score for these three positions was used for all data analysis. Animals were tested at day 30 of the study (last time point in Fig. 1).
IGF-I Administration.
IGF-I was administered to the rats either simultaneously with 3AP (50 mg/kg, i.p.) or 5 days after the injection of the neurotoxin. In both cases, animals showed clear symptoms of ataxia (see Results) well before any beneficial effect of the trophic factor became apparent. Prolonged IGF-I treatment was carried out with osmotic minipumps (Alzet 2002; infusion rate, 0.5 μl/h for 14 days). Pumps were filled with IGF-I by following the manufacturer’s instructions. Minipumps were replaced with new ones after 2 weeks to maintain constant IGF-I infusion for an additional 2-week period. Attached cannulas were implanted stereotaxically in the lateral cerebral ventricle [coordinates (12): −0.8 mm anteroposterior, 4 mm ventral, and 1.5 mm lateral; IGF-I at 100 μg/ml). For subcutaneous delivery, pumps (filled with IGF-I at 1 mg/ml) were placed in the back of the animal in the scapula. Doses of IGF-I were chosen as described in published protocols (7). Control animals received pumps containing the saline vehicle. After 4 weeks of treatment with IGF-I, animals showed a minor, but not significant, increase in blood glucose levels: 79.5–82.1 mg/dl in controls and 84.6–87.6 mg/dl in IGF-I-treated 3AP animals (n = 10 per group).
Electrophysiology.
Parasagittal slices (350 μm thick) from the cerebellar vermis were obtained from the different groups of rats by using a vibroslicer. Slices were incubated for 1 h in oxygenated buffer solution containing 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM dextrose. Recordings were made in a submerged chamber with the slices perfused with oxygenated buffer solution (flow, 2–3 ml/min) at 30°C. Extracellular recordings were made with a glass micropipette filled with 1 M KCl or buffer solution. A bipolar stimulating electrode (FHC, Bowdoinham, ME) was placed on the white matter below the Purkinje cell layer. A recording electrode was moved along the Purkinje cell layer until spontaneous activity of a cell was detected. We unequivocally identified the Purkinje cell layer as the source of the detected current because moving the recording electrode along the molecular layer of the cerebellum resulted in an inversion of the response polarity (14).
Immunocytochemistry.
Animals (n = 5 per group) were deeply anesthetized with pentobarbital (50 mg/kg) and perfused transcardially with 0.9% NaCl, followed by 400 ml of fixative (4% paraformaldehyde/0.1 M sodium phosphate, pH 7.4; PB). Brains were removed, postfixed 3 h in the same fixative at room temperature, and cryoprotected by immersion in 30% sucrose solution in PB at 4°C. Serial 30-μm-thick coronal frozen sections were cut in a cryostat and processed for immunocytochemistry. The sections were soaked in PB containing 10% methanol and 0.3% H2O2 for 30 min at room temperature to block endogenous peroxidase and then rinsed in PB. To visualize calbindin, sections were incubated overnight at 4°C with a monoclonal antibody (anti-calbindin, Sigma) diluted 1:1,000 in PB containing 0.1% bovine albumin, 3% nonimmune goat serum, and 0.2% Triton X-100. After several washes in PB, sections were incubated with a goat anti-mouse IgG horseradish peroxidase conjugate (Bio-Rad) diluted 1:100. The peroxidase activity was visualized by incubating the sections in PB containing 0.03% 3,3′-diaminobenzidine tetrahydrochloride (Sigma) and 0.03% H2O2 for 10 min. For quantitative analysis, sections were observed through a ×10 objective in a Leitz microscope. The entire inferior olivary complex of each animal was evaluated (between 60 and 70 consecutive sections per animal). Calbindin-positive cells within a given section were counted, and the number of cells per section was expressed as the mean of all the sections obtained for each animal.
Biochemical Markers of Cerebellar Function.
Calbindin and glutamate receptor subunit 1 levels in cerebellar extracts from the different groups of animals were measured by Western blot analysis as described elsewhere in detail (15) and expressed as percent of control levels. γ-Aminobutyric acid (GABA) and glutamate levels were assessed by HPLC as described (2). IGF-I in cerebellum and serum was determined with an specific radioimmunoassay (16).
RESULTS
Cerebellar Ataxia and IGF-I.
Motor discoordination in 3AP-injected rats was evaluated with the rota-rod test. One week after 3AP injection, animals stayed in the rotating rod almost 10 times less (20.1 ± 13.3 sec, mean ± SEM) than control rats (189 ± 16 sec; F = 2.3; P < 0.0001), indicating that their motor coordination was severely impaired. The rats remained ataxic for the duration of the study: 6 weeks after 3AP injection they showed an identical inability to stay in the rod (<20 sec).
Previous studies indicated a marked decrease in cerebellar IGF-I levels in ataxic animals (6). IGF-I acts as a trophic factor for Purkinje cells both in vivo and in vitro (2, 17). Hence, we evaluated several phenotypic markers of these cerebellar neurons to determine whether lower IGF-I levels due to cerebellar deafferentation would result in deleterious changes in Purkinje cells. We found that calbindin, GABA, and glutamate receptor subunit 1 levels were significantly decreased 2 weeks after injection of 3AP (Table 1). In accordance with previous observations, we found that glutamate and IGF-I levels in the cerebellum were also significantly depleted (Tables 1 and 2 and refs. 6 and 11).
Table 1.
Indicators of cerebellar function in 3AP ataxic rats
| Calbindin
|
Glur1
|
GABA
|
Glutamate
|
|||||
|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 2 weeks | 4 weeks | 2 weeks | 4 weeks | 2 weeks | 4 weeks | |
| Control | 100 ± 8 | 100 ± 6 | 100 ± 5 | 100 ± 6 | 5.8 ± 0.6 | 5.7 ± 0.7 | 19.9 ± 0.9 | 19.6 ± 0.7 |
| 3AP | 37.6 ± 12* | 101 ± 10 | 42.6 ± 9.2* | 99.3 ± 8.3 | 3.8 ± 0.3* | 5.4 ± 0.5 | 11.4 ± 1.5* | 18.6 ± 2 |
Levels (% of control for calbindin and Glur1 and ng/mg protein for GABA and glutamate) of the various markers showed that animals injected with 3AP have a generalized depressed cerebellar function 2 weeks after injection of the neurotoxin. Although 3AP rats showed no signs of behavioral recovery throughout the time of the study (see Fig. 1), the levels of the various cerebellar indicators return to normal after 4 weeks of the injection of the neurotoxin. This time course of recovery parallels that reported for normalization of the spontaneous activity of Purkinje cells, which is attributed to plastic rearrangements of cerebellar circuitries (18). Calbindin and glutamate receptor subunit 1 (GluR1) are specific markers for Purkinje cells in the cerebellum (19), and GABA and glutamate are used as the major phenotypic markers of cerebellar neurons, including Purkinje cells (GABAergic), granule cells (glutamatergic), and the cerebellar interneurons (GABAergic). ∗, P < 0.01 vs. control, as determined by Student’s t test.
Table 2.
Levels of IGF-I in rodent models of cerebellar ataxia
| Treatment | Cerebellum IGF-I pg/mg protein
|
Serum IGF-I ng/ml serum
|
||
|---|---|---|---|---|
| 2 weeks | 4 weeks | 2 weeks | 4 weeks | |
| Control | 670.3 ± 10.2 | 672.5 ± 10 | 142.2 ± 1.6 | 140 ± 2.8 |
| 3AP | 440.8 ± 10.2** | 500.6 ± 10.2** | 112.6 ± 5.1*** | 131.6 ± 3.4 |
| Control EC | 502.9 ± 19.8 | ND | 164.8 ± 4.9 | ND |
| EC | 270.2 ± 10.8** | ND | 129.6 ± 8.2** | ND |
| 3AP + IGF-I (i.c.v.) | ND | 3038.2 ± 166.1* | ND | 446.9 ± 33.7* |
| 3AP + IGF-I (s.c.) | ND | 1320 ± 150.8*** | ND | 452.5 ± 38.6* |
Peripheral (serum) and local (cerebellum) IGF-I levels changed in parallel after induction of ataxia. During early phases of the ataxic process (first 2 weeks), IGF-I levels in both compartments are depressed, but after a month of the injection of 3AP IGF-I levels tend to recover. Ataxic animals showed changes in IGF-I levels regardless of the method employed to elicit ataxia (neurotoxin or electrolytic lesion). As expected, infusion of IGF-I by either s.c. or i.c.v. osmotic minipumps resulted in significantly increased IGF-I levels both in cerebellum and in serum. These results suggest that decreased serum IGF-I may be related to olivo-cerebellar dysfunction because in human olivo-ponto-cerebellar atrophy similar decreases are found (5). ∗, P < 0.001; ∗∗, P < 0.01; ∗∗∗, P < 0.05 vs. respective controls (Student’s t test). ND, not determined; EC, electrocoagulation.
Because recent findings show that serum IGF-I levels varied in parallel with cerebellar IGF-I levels and that peripheral IGF-I can be selectively taken up from the blood stream into the brain parenchyma (20), we also evaluated serum IGF-I levels in these animals and found a significant decrease in 3AP-injected rats (Table 2). This reinforces the use of 3AP-injected rats as a model of human cerebellar ataxia because in this disease a decrease in both serum IGF-I levels and cerebellar calbindin is found (5, 21).
To rule out a possible unspecific toxic action of 3AP on the liver, the main source of circulating IGF-I (22), we also evaluated the effect of an alternative way of lesioning the inferior olive and found that a similar decrease in IGF-I levels both in serum and cerebellum was present after ablation of this brainstem nucleus by electrocoagulation (Table 2).
Although injection of 3AP at the doses used renders the animals permanently ataxic, plastic rearrangements in the cerebellar cortex circuitry of these animals take place within 1–2 months of the 3AP injection (18). These poorly characterized events are modulated by endogenous IGF-I (7) and eventually lead to recovery of the spontaneous electrical activity of the Purkinje cell, even though these neurons are permanently deprived of their climbing fiber input (18). To determine whether there is a relationship between cerebellar plasticity and IGF-I levels, we measured levels of this trophic peptide and cerebellar cell markers 4 weeks after 3AP injection. Functional indicators of cerebellar function showed a striking recovery correlating with an increase in serum IGF-I levels (Tables 1 and 2, 4 weeks). Thus, recovery of Purkinje cell function, as determined by electrophysiological (18) and biochemical (Table 1) parameters, is paralleled by recovery of serum IGF-I levels (Table 2). Interestingly, IGF-I levels in cerebellum lagged behind serum levels and did not return to normal until 6 weeks after injection of 3AP (data not shown). Moreover, even though in this model of ataxia the animals recovered Purkinje cell function (18), they never recovered normal motor coordination.
Recovery from Ataxia by Administration of IGF-I.
From these observations, we postulated that a lack of recovery of motor coordination in these animals was due to the fact that normalization of cerebellar IGF-I levels occurs too late to rescue any inferior olivary neurons. These neurons die within hours after injection of the neurotoxin (23). We reasoned that if we provided IGF-I before olivary neurons die, we might be able to rescue these neurons. Thus, a second group of rats was injected with 3AP, implanted simultaneously with osmotic minipumps containing either IGF-I or vehicle, and connected to an intracerebroventricular (i.c.v.) cannula. Continued evaluation of the motor coordination in these rats showed that ataxic animals receiving IGF-I recovered gradually throughout time. Although for the first 5 days of treatment 3AP-injected animals were still ataxic, by day 10 they started to recover and gained full motor coordination after 4 weeks (Fig. 1). A continuous supply of IGF-I for more than 3 weeks was necessary because interruption of the treatment at day 15 resulted in blockade of further recovery (data not shown). Vehicle-injected 3AP animals remained ataxic throughout the duration of the study.
Motor ability of IGF-I-treated ataxic animals was further evaluated by using an additional test of motor performance. As shown in Fig. 1B, ataxic animals receiving IGF-I and control animals stayed on a platform that was tilted to a similar degree of inclination and that was significantly steeper than the degree of inclination resisted by vehicle-treated ataxic rats (F = 3.5; P < 0.001). Hence, we concluded that full recovery of motor coordination may be achieved when IGF-I levels are therapeutically increased at the same time that olivary neurons are degenerating.
Previous findings in human cerebellar ataxia and observations in several animal models support the idea of a biologically significant cross-talk between the peripheral (endocrine) and central (paracrine/autocrine) IGF-I trophic system (5, 20). By assuming the hypothesis that serum IGF-I levels are directly influencing brain IGF-I levels, we administered IGF-I by a peripheral route with the hope of finding an easy way to treat human ataxia. Indeed, the recovery elicited in 3AP rats by subcutaneous (s.c.) administration of IGF-I was comparable to that obtained after central delivery of the peptide (Fig. 1). Thus, regardless of the route used for administration of IGF-I to the rats, both cerebellar and serum IGF-I levels were greatly increased (Table 2). Although the increase in serum IGF-I levels after i.c.v. administration may be accounted for by the transport from the cerebrospinal fluid to the circulation, increased cerebellar levels of IGF-I after s.c. administration of the peptide may be explained by specific uptake of IGF-I by the brain (20). Hence, we conclude that peripherally administered IGF-I is able to reach the brain parenchyma in sufficient amounts to induce functional recovery. Alternatively, elevated blood IGF-I levels may regulate the brain IGF-I system by as yet undetermined mechanisms that eventually result in increased brain IGF-I. At any rate, these findings open the possibility of treating human ataxia with subcutaneous administration of IGF-I.
Because pilot studies in ataxic rats indicated that the ability to recover motor coordination depended on the number of surviving inferior olive neurons and this, in turn, depended on the dose of 3AP used, we hypothesized that the recovery elicited by IGF-I was due to its ability to rescue substantial numbers of climbing fiber axons by both promoting increased neuronal survival and inducing axon sprouting (24). Thus, we determined the integrity of the olivocerebellar pathway of IGF-I-treated ataxic rats by both electrophysiological and morphological analyses. Evaluation of the climbing fiber input to the cerebellar cortex of these rats indicated that 3AP-injected animals treated with IGF-I showed a normal pattern of activation of the cerebellar cortex by climbing fiber afferents but, in vehicle-treated 3AP animals, this input was absent (Fig. 2). Similarly, analysis of the number of neurons in IGF-I-treated animals showed a survival of 92% of calbindin-positive cells in the inferior olive nucleus, the source of climbing fiber afferents to the cerebellar cortex, but in vehicle-treated 3AP animals, less than 20% of these neurons remained alive (F = 2.6; P < 0.001; Fig. 3).
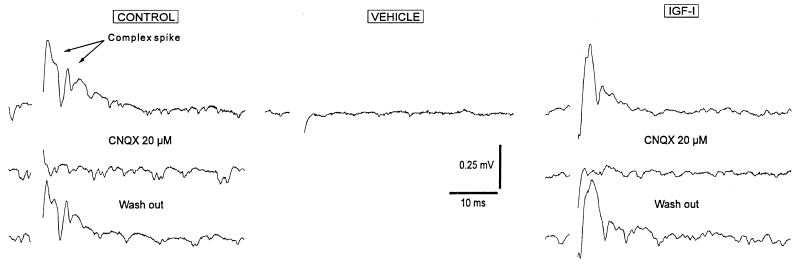
Figure 2.
Treatment of ataxic rats with IGF-I results in maintenance of the climbing fiber input to Purkinje cells. Electrophysiological recordings were made from cerebellar slices of control rats (Control traces), rats injected with 3AP and treated with IGF-I (IGF-I traces), and 3AP rats receiving a constant infusion of saline (Vehicle traces). Typical all-or-none climbing fibers responses (complex spikes) were readily obtained in both controls (91 of 100 trials, n = 7) and IGF-I-injected ataxic rats (82 of 100, n = 7) using a moderate stimulation intensity (range of 10–40 nA for all experimental groups). Synaptic responses were completely abolished by using the selective non-N-methyl-d-aspartate glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM, Alexis, Laufelfingen, Switzerland) and recovered after wash out. When the same experimental procedure was used in the vehicle group, synaptic responses were very rarely obtained (28 of 100, n = 7) even under stronger stimulation intensity (up to 70 nA). The latter agrees with previous results in rats deafferented by either 3AP or electrocoagulation of the inferior olive where a loss of the climbing fibers response is found (18, 25). Results shown for the group treated with IGF-I correspond to a representative 3AP animal receiving IGF-I s.c.
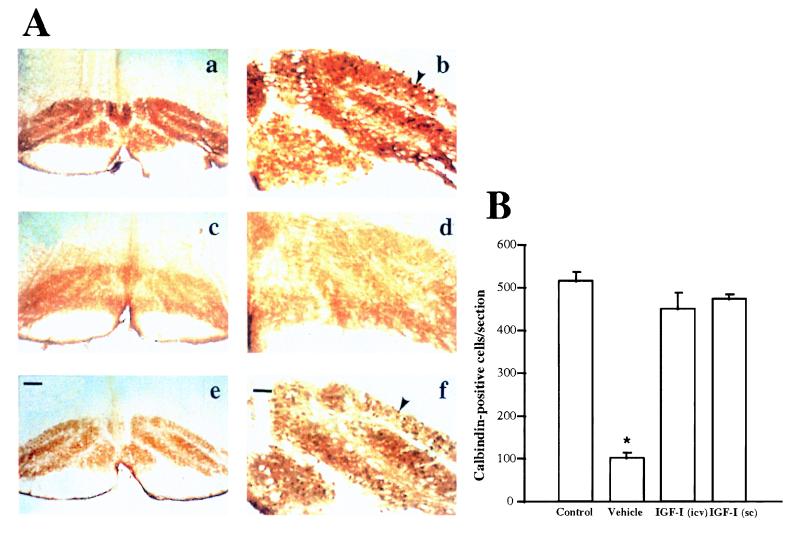
Figure 3.
IGF-I treatment of ataxic rats resulted in greatly increased survival of inferior olive neurons. (A) Representative photomicrographs of inferior olive neurons stained with calbindin, a specific marker of these neurons (26) from control (a and b), vehicle-treated 3AP rats (c and d), and IGF-I-treated 3AP rats (e and f). Note that vehicle-treated 3AP rats have almost no surviving neurons but those treated with IGF-I have almost as much as intact animals. Cresyl violet staining confirmed neurodegeneration and gliosis of the inferior olive nuclei after 3AP injection, as reported (6, 11). (a, c, and e) General overview of the inferior olive nuclei. (Bar = 250 μm.) (b, d, and f) Higher magnification view of inferior olive neurons. (Bar = 50 μm.) Arrowheads show calbindin-positive neuronal bodies. (B) Quantification of neurons in each group showed that IGF-I-treated ataxic rats have significantly greater number of inferior olive neurons compared with vehicle-treated 3AP animals and almost as many as control animals. ∗, P < 0.001 vs. all other groups. Data are the number of calbindin-positive cells per section (mean ± SEM).
We also evaluated the efficacy of delayed IGF-I treatment of ataxic animals to determine whether recovery of function is possible even after severe neuronal loss. Osmotic minipumps filled with IGF-I (1 mg/ml) were implanted (s.c.) in rats that had been injected with 3AP 5 days earlier. Delayed treatment with IGF-I also resulted in full behavioral recovery as determined by the rota-rod test: controls, 299 ± 0.5 sec; vehicle-treated ataxic rats, 2.7 ± 0.5 sec; IGF-I-treated ataxic rats, 292 ± 0.7 sec (day 30 of treatment, P < 0.001 vs. vehicle-treated ataxic rats, Student’s t test). Importantly, the number of neurons rescued by delayed IGF-I treatment represented only 27 ± 8% of the total inferior olive population. These results strengthen the therapeutical potential of IGF-I because even after severe neuronal depletion it still promotes functional recovery.
DISCUSSION
Our results show that full behavioral recovery can be achieved after peripheral administration of IGF-I in a rat model of human olivo-cerebellar degeneration. Complete recovery occurred in parallel with normalization of olivo-cerebellar function. It is noteworthy that neuronal death is usually a protacted process spanning many years in humans (27). Further, the present results show that IGF-I treatment restored full motor function even in ataxic animals with severe neuronal depletion. Hence, a potential therapeutic use of IGF-I in at least a selected subset of human neurodegenerative diseases affecting cerebellar function is supported by these findings.
IGF-I is just one of the many neurotrophic substances known. Previous characterization of its trophic activities in the cerebellar cortex during development and in the adult (2, 3, 15, 17) allowed us to hypothesize its involvement in the pathogenesis of cerebellar degeneration leading to ataxia (5). Several other models of cerebellar ataxia are currently used in addition to the 3AP model used in this study. These include different types of mouse neurological mutants showing various degrees of neuronal loss and affecting different subsets of cerebellar and cerebellar-related pathways (11). Cerebellar degeneration in humans also includes a variety of different conditions and syndromes of diverse pathogenesis such as Friedreich ataxia, spontaneous cerebellar cortical degeneration, or Wernicke–Korsakoff syndrome. Most of these diseases show a very wide range of age of onset and duration and frequently develop over the course of years. Although the pathogenesis of these types of syndromes is most likely multifactorial, it ultimately results in insults to specific populations of neurons within motor pathways (28). Thus, any reparative agent, such as IGF-I may help injured neurons to recover.
Although the mechanisms underlying the trophic effects of IGF-I on injured inferior olive neurons are unknown, Purkinje cells express IGF-I during their entire lifespan, with prominent expression at times coincident with the establishment of the olivo-cerebellar pathway (2, 29). Thus, IGF-I may be a target-derived trophic factor for inferior olive neurons not only during development but also during adulthood. At any rate, the ability of IGF-I to rescue inferior olive neurons is remarkable in view of the fact that IGF-I is also a survival factor for developing Purkinje neurons, the postsynaptic targets of inferior olive neurons (15), and for immature cerebellar granule cells (30). Because an IGF-I trophic circuitry linking the inferior olive and cerebellar cortex and encompassing the entire cerebellar motor pathway has been described (1, 17), the present observation may help explain the biological significance of this trophic circuitry: a single neurotrophic factor may contribute to the maintenance of an entire functional circuitry. Although further work is needed to ascertain this possibility, these data suggest that IGF-I promotes the survival of interconnected populations of neurons within cerebellar circuitries.
It is worth noting that although the number of patients with the different types of cerebellar ataxia fortunately do not reach the devastating proportion of other neurological illnesses such as Alzheimer or Parkinson’s disease, the complete lack of even paliative treatments for this ailment makes it of significant societal interest. IGF-I is currently being tested as a therapeutical aid in diseases such as Laron dwarfism, diabetic neuropathy, and amyotrophic lateral sclerosis (8, 31, 32). Thus, its possible use in the treatment of human cerebellar ataxia appears of great promise.
Acknowledgments
We thank Genentech for their gift of recombinant IGF-I. We also thank Dr. L.M. Garcia-Segura (Instituto Cajal) for help and advise and Drs. Castro-Alamancos (Brown University), Rodriguez-Tébar and Nieto-Sampedro (Instituto Cajal) for their criticism of previous versions of this report. This work was supported by a grant from DGCYT. Additional support by Fundacion Salud 2000 and by the Comunidad de Madrid is also acknowledged. A.M.F. is a Comunidad Autonoma de Madrid fellow.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: IGF-I, insulin-like growth factor I; 3AP, 3-acetylpyridine; i.c.v., intracerebroventricular; GABA, γ-aminobutyric acid.
References
- 1.Bondy C A. J Neurosci. 1991;11:3442–3455. doi: 10.1523/JNEUROSCI.11-11-03442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres-Aleman I, Pons P, Arevalo M A. J Neurosci Res. 1994;38:117–126. doi: 10.1002/jnr.490390202. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Alamancos C A, Torres-Aleman I. Proc Nat Acad Sci USA. 1994;91:10203–10207. doi: 10.1073/pnas.91.21.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vig P J S, Desaiah D, Joshi P, Subramony S H, Fratkin J D, Currier R D. J Neurol Sci. 1994;124:38–44. doi: 10.1016/0022-510x(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 5.Torres-Aleman I, Barrios V, Lledó A, Berciano J. Ann Neurol. 1996;39:335–342. doi: 10.1002/ana.410390310. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Aleman I, Pons S, Garcia-Segura L M. Brain Res. 1991;564:348–351. doi: 10.1016/0006-8993(91)91476-h. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez A M, Garcia-Estrada J, Garcia-Segura L M, Torres-Aleman I. Neuroscience. 1997;76:117–122. doi: 10.1016/s0306-4522(96)00395-8. [DOI] [PubMed] [Google Scholar]
- 8.Ishii D. Brain Res Rev. 1995;20:47–67. doi: 10.1016/0165-0173(94)00005-a. [DOI] [PubMed] [Google Scholar]
- 9.Hefti F. J Neurobiol. 1994;25:1418–1435. doi: 10.1002/neu.480251109. [DOI] [PubMed] [Google Scholar]
- 10.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde Y A. Nature (London) 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 11.Butterworth R F. In: Neuromethods: Animal Models of Neurological Disease. Boulton A, Baker G, Butterworth R, editors. I. Clifton, NJ: Humana; 1992. pp. 275–294. [Google Scholar]
- 12.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- 13.Behrmann D L, Bresnahan J C, Beattie M S. Exp Neurol. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 14.Eccles J C, Llinás R, Sasaki K, Voorhoeve P E. J Physiol (London) 1996;182:297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Aleman I, Pons S, Santos-Benito F F. Eur J Neurosci. 1992;4:864–869. doi: 10.1111/j.1460-9568.1992.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 16.Pons S, Torres-Aleman I. Endocrinology. 1992;131:2271–2278. doi: 10.1210/endo.131.5.1385099. [DOI] [PubMed] [Google Scholar]
- 17.Nieto-Bona M P, Busiguina S, Torres-Aleman I. J Neurosci Res. 1995;42:371–376. doi: 10.1002/jnr.490420311. [DOI] [PubMed] [Google Scholar]
- 18.Rossi F, Strata P. Prog Neurobiol. 1995;47:341–369. [PubMed] [Google Scholar]
- 19.Celio M R. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt R R, Bondy C A. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa K, Mizusawa H, Fujita T, Ohkoshi N, Doi M, Komatsuzaki Y, Iwamoto H, Ogata T, Shoji S. J Neurol Sci. 1995;129:179–185. doi: 10.1016/0022-510x(94)00279-w. [DOI] [PubMed] [Google Scholar]
- 22.Daughaday W H, Rotwein P. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Segura L M, Perrelet A. Brain Res. 1982;236:253–260. doi: 10.1016/0006-8993(82)90712-0. [DOI] [PubMed] [Google Scholar]
- 24.Guthrie K M, Nguyen T, Gall C M. J Comp Neurol. 1995;352:147–160. doi: 10.1002/cne.903520111. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti F, Montarolo P G, Rabacchi S. Exp Brain Res. 1984;55:368–371. doi: 10.1007/BF00237287. [DOI] [PubMed] [Google Scholar]
- 26.Saotti A L. J Anat. 1995;187:649–659. [PMC free article] [PubMed] [Google Scholar]
- 27.Rewcastle N B. In: Textbook of Neuropathology. 2nd Ed. Davis R L, Robertson D M, editors. Baltimore: Williams & Wilkins; 1991. pp. 904–961. [Google Scholar]
- 28.Oppenheimer D R, Esiri M M. In: Greenfield’s Neuropathology. 5th Ed. Adams J H, Duchen L W, editors. London: Edward Arnold; 1992. pp. 988–1045. [Google Scholar]
- 29.Sherrard R M, Richardson N A, Sara V R. Dev Brain Res. 1997;98:102–113. doi: 10.1016/s0165-3806(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 30.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 31.Laron Z, Anin S, Klipper-Aurbach Y, Klinger B. Lancet. 1992;339:1258–1261. doi: 10.1016/0140-6736(92)91594-x. [DOI] [PubMed] [Google Scholar]
- 32.Festoff, B. W., Yang, S. X., Vaught, J., Bryan, C. & Ma, J. Y. (1995) J. Neurol. Sci. 129, Suppl. 1, 114–121. [DOI] [PubMed]