Abstract
Immunization of BALB/c mice with an expression genomic library of Toxoplasma gondii induces a Th1-type immune response, with recognition of several T. gondii proteins (21 to 117 kDa) and long-term protective immunity against a lethal challenge. These results support further investigations to achieve a multicomponent anti-T. gondii DNA vaccine.
Toxoplasma gondii is a ubiquitous protozoan parasite that induces lethal encephalitis in immunosuppressed individuals and severe congenital birth defects (8, 22, 33). Several efforts have been concentrated on developing an anti-T. gondii vaccine, the feasibility of which is supported by the long-term immunity induced by the primary infection (7). Furthermore, during chronic infection parasite cysts are well controlled by the host immune response, mainly due to CD8+ effector lymphocytes (4) and T. gondii antigen-elicited production of gamma interferon (IFN-γ) (9). In addition, the immunization of mice with parasite antigens induces a partial protective Th1-type immunity (3, 35). Hence, a vaccine eliciting a Th1 immune response against T. gondii will be a promising tool to confer protective immunity. Presently the most effective vaccine developed is a live attenuated one that requires boosters to sustain resistance (5). Recently, a single injection of T. gondii mutants lacking an enzyme of the de novo pyrimidine biosynthesis pathway has been shown to induce long-term protective immunity to toxoplasmosis (15), opening a new path to vaccine development.
The ability of a DNA vaccine to elicit a Th1 immune response (24) makes it an attractive immunization approach to use against T. gondii. In fact, partial protective immunity was achieved by cloning SAG1/P30, ROP2, GRA1, GRA7, and GRA4 into plasmid vectors (1, 10, 13, 21, 26, 34). In addition, immunization with a DNA cocktail containing plasmids encoding SAG1/P30 and the amino acid 196 to 561 terminal sequence of ROP2 genes elicited a long-lasting protective Th1 immunity (14), supporting further investigations to achieve a multigene anti-T. gondii DNA vaccine.
Taking in account that the majority of the antigens of a pathogen are encoded by its DNA, an expression library of pathogen DNA could be used to immunize a host without the risk of infection. In fact, the immunization of mice with expression libraries resulted in protection after challenge with Leishmania (23, 27), Plasmodium (31), and Mycoplasma (25). Thus, the purpose of the present study was to describe the cloning of a genomic library of T. gondii tachyzoites and to analyze the efficacy of vaccination with this library to promote protective immunity.
Genomic library construction, screening, and purification.
Tachyzoites of the RH strain of T. gondii (28) for chromosomal DNA isolation and subsequent library construction and antigen preparation were produced as described elsewhere (14). For DNA isolation, 5 × 106 tachyzoites growing in vitro were resuspended in 1 ml of lysis buffer (100 mM Tris-HCl [pH 8], 100 mM EDTA [pH 8], 2% sodium dodecyl sulfate, 150 mM NaCl, 200 μg of proteinase K/ml, and 20 μg of RNase/ml) and were incubated for 2 h at 56°C. After phenol-chloroform extraction, the DNA was precipitated with absolute ethanol and then was resuspended in a buffer containing 10 mM Tris and 1 mM EDTA (pH 8). The DNA integrity was evaluated by gel electrophoresis (29), and DNA concentration was estimated with the GeneQuant Apparatus (Pharmacia Biotech). DNA of the eukaryotic expression vector pcDNA3 (Invitrogen) was digested with BamHI (Amersham) and was purified from the gel by using a Master kit (Bio-Rad Laboratories). The genomic DNA was partially digested with Sau3A1 and was size fractionated on an agarose gel to isolate 0.5- to 1.5-kb DNA fragments (1 μg) that were fused to digested vector (2 μg). The ligated plasmids were used to transform competent Escherichia coli XL1-Blue supE44 hshR17recA1 endA1 gyrA46 thi relA1 lac− F′ (proAB+ lacIq lacZΔM15Tn10 [Tetr]) (Stratagene). The stock library was resuspended into 2 ml of Luria-Bertani (LB) medium containing 15% glycerol and was stored at −70°C in separated stocks. A library aliquot was plated onto 2AMP-LB plates, each harboring 2 × 104 clones. After the restriction analysis, the presence of genomic library DNA inserts was demonstrated in 70% of the analyzed plasmids, showing that a large proportion of the plasmids carry T. gondii genetic material. Plasmid DNA for vaccination was prepared by expanding transfected bacteria in LB broth and by using a Qiagen Plasmid Mega kit according to the manufacturer's instructions. Plasmid DNA was dissolved in double-distilled pyrogen-free sterile H2O and was stored at −70°C.
Expression of a variety of T. gondii antigens and induction of Th1-type immune response by genomic library DNA vaccination.
Groups of 6- to 8-week-old male BALB/c mice (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil) were immunized in the tibialis anterior muscles with one, two, or three doses of 100 μg of genomic library DNA (pcDNA3-GL) or pcDNA3 at intervals of 21 days. Controls were injected with saline. In a Western blotting analysis of total native T. gondii antigens after sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 12% polyacrylamide gel (Pharmacia PHAST System), individual sera from mice immunized with pcDNA3-GL reacted with protein bands ranging from 21 to 117 kDa, whereas no T. gondii antigen was recognized by sera of saline-injected or pcDNA3-immunized mice (Fig. 1). Thus, our genomic library contains DNA sequences coding distinct T. gondii protein epitopes that were expressed and that triggered a significant humoral immune response.
FIG. 1.
Several T. gondii antigens are specifically detected by sera of pcDNA3-GL-immunized mice. Shown is Western blotting analysis of native T. gondii antigens in the presence of a pool of sera of mice immunized with total native T. gondii antigens in complete Freund's adjuvant as positive control (lane 1), a pool of sera of saline-injected mice (lane 2), a pool of sera of pcDNA3 plasmid-immunized mice (lane 3), and individual serum of pcDNA3-GL-immunized mice (lanes 4 to 8). The pattern of antigen recognition of the following animals are shown: lane 4, mouse 3 (three doses); lane 5, mouse 6 (two doses); lane 6, mouse 6 (three doses); lane 7, mouse 7 (two doses); lane 8, mouse 7 (three doses). These are representative data of 10 analyzed mice.
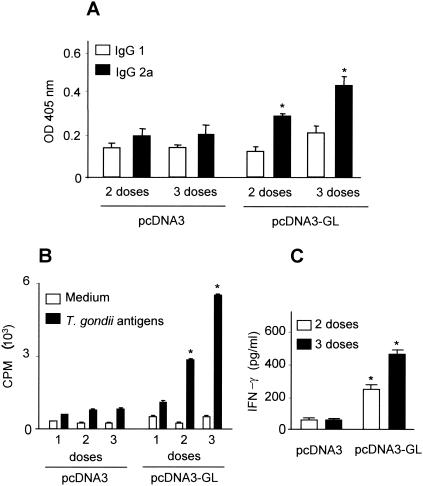
To determine whether a Th1 and/or Th2 response was elicited, antibodies induced by pcDNA3-GL vaccination were initially isotyped by enzyme-linked immunosorbent assay (14). Figure 2A shows that a predominance of immunoglobulin G2a (IgG2a) over IgG1 antibodies against total native T. gondii antigens was observed when BALB/c mice were immunized with two and three doses of pcDNA3-GL, implying that vaccination with the DNA genomic library elicits a humoral Th1 response. Although less intense, IgG2a predominance was also observed in mice immunized with one dose of pcDNA3-GL (data not shown).
FIG. 2.
Specific anti-T. gondii Th-1-type immune response elicited by pcDNA3-GL vaccination. (A) The levels of anti-T. gondii IgG1 (□) and IgG2a (▪) isotype antibodies in sera of mice injected with two or three doses of pcDNA3 plasmid or pcDNA3-GL 15 weeks after the last immunization. Each experimental group was composed of 10 animals. (B) The lymphoproliferative response of splenocytes from mice immunized with one, two, or three doses of pcDNA3 vector or pcDNA3-GL after stimulation with medium alone (□) or total native T. gondii antigens (▪) 17 weeks after the last immunization. (C) IFN-γ production by T. gondii antigen-stimulated splenocytes from mice immunized with two (□) or three (▪) doses of pcDNA3 vector or pcDNA3-GL 17 weeks after the last injection. Each experimental group was composed of five animals. This study was performed three times with similar results. *, P < 0.05 by the Student's t test (STATSYST; Microsoft). OD 405 nm, optical density at 405 nm. CPM, counts per minute.
To determine the ability of pcDNA3-GL to induce a cellular immune response, splenocytes from immunized mice were plated at a density of 5 × 105 cells/well (on a flat-bottomed 96-well plate) in RPMI 1640 medium supplemented with 1% normal mouse serum (Sigma), 2 mM glutamine, 0.05 mM 2-mercaptoethanol, 0.05 mM sodium pyruvate, 0.05 mM minimal essential medium nonessential amino acid solution, and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively), stimulated for 4 days with native T. gondii antigens (10 μg/ml). [3H]thymidine (Amersham) was added at 1 μCi/well during the last 18 h. The cells were harvested onto a glass fiber filter, and the radioactivity (in counts per minute) was measured by liquid scintillation. Figure 2B shows that splenocytes from pcDNA3-GL-vaccinated mice specifically proliferate in a dose-dependent manner when stimulated with native T. gondii antigens, while no significant proliferation was observed in pcDNA3-immunized mice. Splenocytes from immunized and control groups proliferated to comparable levels in response to the mitogen concanavalin A (data not shown). Further, supernatants of splenocytes stimulated with T. gondii antigens were collected after 24 and 72 h and were assayed by ELISA for interleukin-4 (IL-4) and IFN-γ, respectively, as previously described (14). Compared to pcDNA3, vaccination with pcDNA3-GL induced, in a dose-dependent manner, significant levels of IFN-γ (Fig. 2C), whereas IL-4 was undetectable (data not shown). Altogether, these results suggest that the specific cellular immunity was also oriented to a Th1 profile in pcDNA3-GL-vaccinated mice.
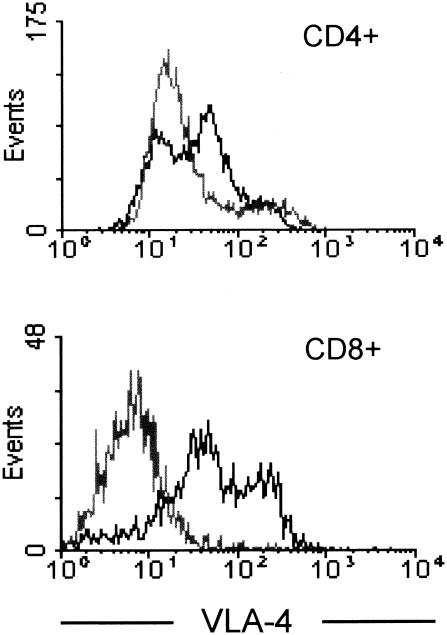
DNA-based immunization elicits both humoral and cellular immune responses, particularly inducing cytotoxic T lymphocytes (CTL) (11), likely via systemic IL-12- and IFN-γ-dependent mechanisms (20, 32). As IFN-γ-producing CD8+ CTL have been shown to have a prominent role in controlling T. gondii infection (16, 17), it is possible that at least part of the detected IFN-γ is produced by activated CD8+ T cells. Although CD4+ T cells have been demonstrated to be crucial for induction of protective immunity for T. gondii (16), a recent study showed that CD4+ T cells are not required for IFN-γ production or CD8+ effector cell activation; however, they are essential for anti-T. gondii long-term memory generation (6). In a first attempt to elucidate whether pcDNA3-GL was able to activate both CD4+ and CD8+ T cells, the expression of very late antigen-4 (VLA-4), a marker of T-cell activation (2, 12), was assessed by flow cytofluorimetry (12) on splenocytes from mice vaccinated three times with pcDNA3-GL 24 weeks after the last injection. Figure 3 shows that CD4+ and, mainly, CD8+ splenocytes isolated from pcDNA3-GL-vaccinated mice (but not from pcDNA3-immunized animals) are responsive to in vitro stimulation with native T. gondii antigens, supporting the idea that our genomic library triggers a long-term T. gondii-specific CD4- and CD8-mediated immune response.
FIG. 3.
Activation of CD4+ and CD8+ splenocytes from pcDNA3-GL-immunized mice by in vitro stimulation with T. gondii antigens. Twenty-four weeks after the last immunization, splenocytes from mice vaccinated with three doses of pcDNA3 (gray line) or pcDNA3-GL (black line) were stimulated in vitro for 4 days with native T. gondii antigens. VLA-4 expression on CD4+ and CD8+ T cells was determined by using flow cytofluorimetry. These are representative data of triplicate cultures of three animals analyzed per group.
Protective immunity induced by pcDNA3-GL immunization.
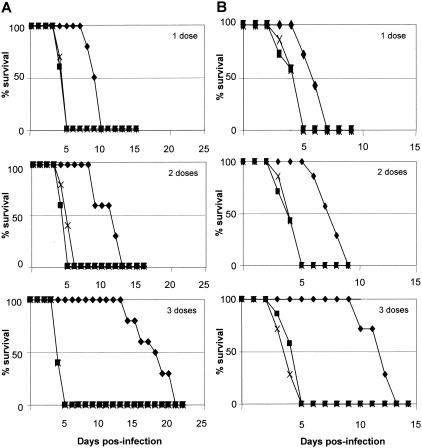
In view of the significant humoral and cellular immunity elicited by pcDNA3-GL immunization, we next tested whether this vaccination protocol could afford protection against infection with the highly virulent T. gondii RH strain. BALB/c mice were immunized with one, two, or three doses of vector or pcDNA3-GL as described above and were challenged with 105 RH strain tachyzoites. Fifteen weeks after the last inoculation, one (3.6 ± 2 days; P < 0.01), two (3.6 ± 0.2 days; P < 0.01), or three (3.4 ± 0.2 days; P < 0.01) doses of vector did not protect mice from lethal challenge any more than saline-inoculated mice were protected (Fig. 4A). Importantly, protective immunity was achieved in animals that received one (8.3 ± 0.3 days; P < 0.01), two (10.1 ± 0.6 days; P < 0.01), or three (16.9 ± 0.9 days; P < 0.01) doses of pcDNA3-GL. Although we were unable to detect significant humoral and cellular immune responses in mice that received one dose of genomic library, these animals also showed protection when lethally challenged. Moreover, significant survival rates were obtained when pcDNA3-GL-immunized mice were challenged 24 weeks after the last immunization (Fig. 4B). This long-term (>6 months) protective immunity was observed in mice that received one (5.1 ± 0.3 days; P < 0.01), two (6.7 ± 0.4 days; P < 0.01), or three (10.7 ± 0.5; days P < 0.01) doses of pcDNA3-GL but not in mice that were immunized with one (3.3 ± 0.4 days), two (3.1 ± 0.3 days), or three (3.4 ± 0.3 days) doses of pcDNA3 or that were injected with saline, demonstrating that although protection decreases slowly over time, significant survival rates were still detected. Nevertheless, one should keep in mind that the readout of our experiments is very stringent where the highly virulent RH T. gondii strain was used, and thus our results showing long-term protective immunity are extremely significant (1, 14).
FIG. 4.
Genomic library vaccination induces long-term protective immunity against T. gondii infection. Survival curves of BALB/c mice injected with pcDNA3-GL (⧫), vector pcDNA3 plasmid (▪), or saline (X) and challenged intraperitoneally with 105 tachyzoite forms of T. gondii strain RH 15 (A) or 24 (B) weeks after the last immunization. Kaplan-Meier plotting was used (STATSYST; Microsoft). Each group was composed of 10 mice. Similar results were obtained in three (A) or two (B) independent experiments.
Studies performed with different animal models showed that antibody response plays a partial protective role against T. gondii infection (18, 30). Further, cellular immunity, mainly due to CD8+ T cell action and IFN-γ production, is required for parasite control (4, 9). Thus, it is likely that the predominantly Th1-driven anti-T. gondii-specific IgG2a antibodies and IFN-γ production elicited by pcDNA3-GL immunization contributes to the elimination of systemic spreading of T. gondii, resulting in increased survival rates. Importantly, considering that CD4+ T cells are required for the generation of long-term memory during T. gondii infection (6), our results showing that CD4+ and CD8+ splenocytes are responsive to stimulation with T. gondii antigens 24 weeks after the last immunization with pcDNA3-GL point to the possibility that both CD4+ and CD8+ T-cell subsets are involved in genomic library-elicited protective immunity.
The possibility of successful vaccine development in toxoplasmosis had been strengthened by recent advances in our understanding of the nature of protective immunity to T. gondii and pathogenesis of diseases (7, 9, 17, 19). Therefore, the results presented here demonstrated that genomic library immunization might be an important approach to achieve a multicomponent vaccine against T. gondii, particularly with respect to generating an efficient long-term protective immunity.
Acknowledgments
We are grateful to Solange de Castro for her help with the statistical analysis and to Danielle R. Martins for helpful technical assistance.
This work was supported by funds from PAPES-III, PDTIS, FAPERJ, and fellowships from CNPq (J.L.V. and L.M.O.P.), FAPERJ (D.R.M.), IOC-Fiocruz (A.F.F.C.), and CAPES (A.P.M.P.M.). In addition, this investigation received support from a grant from ANPCyT (PICT 05-04831), Argentina.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Angus, C. W., D. Klivington-Evan, J. P. Dubey, and J. A. Kovac. 2000. Immunization with a DNA plasmid encoding the SAG1 (P30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J. Infect. Dis. 181:317-324. [DOI] [PubMed] [Google Scholar]
- 2.Beverley, P. C. 1996. Generation of T-cell memory. Curr. Opin. Immunol. 8:327-330. [DOI] [PubMed] [Google Scholar]
- 3.Bourguin, I., T. Chardés, and D. Bout. 1993. Oral immunization with Toxoplasma gondii antigens in association with cholera toxin induces enhanced protective and cell-mediated immunity in C57BL/6 mice. Infect. Immun. 61:2082-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, C. R., and R. Mcleod. 1990. Class I MHC and CD8+ T cells determine cyst number in T. gondii infection. J. Immunol. 145:3438-3441. [PubMed] [Google Scholar]
- 5.Buxton, D. 1993. Toxoplasmosis: the first commercial vaccine. Parasitol. Today 9:335-337. [DOI] [PubMed] [Google Scholar]
- 6.Casciotti, L., K. H. Ely, M. E. William, and I. A. Khan. 2002. CD8+ T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesbron-Delauw, M. F., and A. Capron. 1993. Excreted/secreted antigens of Toxoplasma gondii - their origin and role in the host-parasite interaction. Res. Immunol. 144:7-79. [DOI] [PubMed] [Google Scholar]
- 8.Cook, G. C. 1990. Toxoplasma gondii infection: a potential danger to the unborn fetus and AIDS sufferers. Q. J. Med. 273:3-19. [PubMed] [Google Scholar]
- 9.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desolme, B., M.-N. Mévélec, D. Buzoni-Gatel, and D. Bout. 2000. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii Gra4 gene. Vaccine 18:2512-2521. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 12.dos Santos, P. V. A., E. Roffê, H. C. Santiago, R. A. Torres, A. P. M. P. Marino, C. N. Paiva, A. A. Silva, R. T. Gazzinelli, and J. Lannes-Vieira. 2001. Prevalence of CD8+ αβ T cells in Trypanosoma cruzi-elicited myocarditis is associated with acquisition of CD62LLowLFA-1HighVLA-4High activation phenotype and expression of IFN-γ-inducible adhesion and chemoattractant molecules. Microbes Infect. 3:971-984. [DOI] [PubMed] [Google Scholar]
- 13.Fachado, A., J. L. Gonzalez, C. Hidalgo, D. Torres, T. Vidal, L. Fonseca, E. Alberti, A. M. Montalvo, L. Leal, M. Nazabal, R. Amendoeira, and A. Acosta. 1998. Expression of the major surface antigen of Toxoplasma gondii in the muscle of Balb/c mice. Biotecnologia Aplicada 15:159-161. [Google Scholar]
- 14.Fachado, A., A. Rodriguez, S. O. Angel, D. C. Pinto, I. Vila, A. Acosta, R. M. Amendoeira, and J. Lannes-Vieira. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327-1335. [DOI] [PubMed] [Google Scholar]
- 15.Fox, B. A., and D. J. Bzik. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926-929. [DOI] [PubMed] [Google Scholar]
- 16.Gazinelli, R. T., F. T. Hakin, S. Hieny, G. M. A. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 17.Gazinelli, R. T., S. Hieny, T. A. Wyinn, S. Wolf., and A. Sher. 1993. Interleukin-12 is required for the T-lymphocyte-independent induction to interferon gamma by an intracellular parasite and induces resistance in T cell deficient host. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alfa and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 19.Khan, I. A., T. Matsuura, S. Fonseca, and L. H. Kasper. 1996. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1 −/− mice. J. Immunol. 156:636-643. [PubMed] [Google Scholar]
- 20.Kowalczyk, D. W., and H. C. Ertl. 1999. Immune responses to DNA vaccines. Cell Mol. Life Sci. 55:751-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyva, R., P. Herion, and R. Saavedra. 2001. Genetic immunization with plasmid DNA coding for the ROP2 protein of Toxoplasma gondii. Parasitol. Res. 87:70-79. [DOI] [PubMed] [Google Scholar]
- 22.Luft, B., and J. S. Remington. 1988. AIDS commentary. Toxoplasmic encephalitis. J. Infect. Dis. 157:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Melby, P. C., G. B. Ogden, H. A. Flores, W. Zhao, C. Geldmacher, N. M. Biediger, S. K. Ahuja, J. Uranga, and M. Melendez. 2000. Identification of vaccine candidates for experimental visceral leishmaniasis by immunization with sequential fractions of a cDNA expression library. Infect. Immun. 68:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery, D. L., J. B. Ulmer, J. J. Donnelly, and M. A. Liu. 1997. DNA vaccines. Pharmacol. Ther. 74:195-205. [DOI] [PubMed] [Google Scholar]
- 25.Moore, R. J., C. Lenghaus, S. A. Sheedy, and T. J. Doram. 2002. Improved vector for expression library immunization-application to Mycoplasma hyopneumoniae infection in pig. Vaccine 20:115-120. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen, H. V., S. L. Lauemoller, L. Christiansen, S. Buus, A. Fomsgaard, and E. Petersen. 2000. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect. Immun. 67:6356-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piedrafita, D., D. Xu, D. Hunter, R. A. Harrison, and F. Y. Liew. 1999. Protective immune response induced by vaccination with an expression genomic library of Leishmania major. J. Immunol. 163:1467-1472. [PubMed] [Google Scholar]
- 28.Sabin, A. B. 1941. Toxoplasmic encephalitis in children. JAMA 116:801-807. [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smooker, P. M., Y. Y. Setiady, A. Rainczuk, and T. W. Spithill. 2000. Expression library immunization protects mice against a challenge with virulent rodent malaria. Vaccine 18:2533-2540. [DOI] [PubMed] [Google Scholar]
- 32.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vercammen, M., T. Scorsa, K. Huygen, J. De Braekeleer, R. Diet, D. Jacobs, E. Saman, and H. Verschueren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenner, L., J. Estaquier, F. Darcy, P. Maes, A. Capron, and M. F. Cesbron-Delauw. 1999. Protective immunity in the rat model of congenital toxoplasmosis and the potential of excreted-secreted antigens as vaccine components. Parasite Immunol. 21:261-272. [DOI] [PubMed] [Google Scholar]