Abstract
Transgenic tobacco plants stably expressing recombinant FaeG, which is the major subunit and adhesin of K88ad fimbriae, were obtained. Analysis of sera from immunized mice indicates that in mice, the immunogenicity induced by plant-derived FaeG protein is comparable to that generated with traditional approaches.
Enterotoxigenic Escherichia coli (ETEC) strains are commonly associated with neonatal diarrhea in piglets (6, 11, 12, 13, 19). Among the different ETEC strains, those expressing K88 fimbrial antigen are the most prevalent type (1, 18). Fimbriae (or pili) are the primary pathogenicity factors of this bacterium and are responsible for its adhesion to enterocyte receptors. Analysis of the genetic organization of the K88 gene cluster has revealed that at least eight structural genes are involved in biosynthesis. FaeG (27.6 kDa) is the so-called major fimbrial subunit protein that also carries the adhesive properties of the K88 fimbriae (4, 17, 24, 26).
Vaccines containing purified K88 fimbriae, formalin-inactivated ETEC, or engineered E. coli expressing K88 fimbriae have been used to vaccinate pregnant sows, and passive transfer of lacteal immunity from the vaccinated dams can protect piglets from ETEC infection (5, 10, 15, 21, 25, 27). Although proven effective for the prevention of disease, limiting the widespread use of these vaccines are the fact that bacteria might not be inactivated fully and the high cost of producing and preserving these vaccines.
Recently, the use of plants as bioreactors has become of special interest, as they allow production of recombinant proteins in large quantities at relatively low cost (14, 22, 23, 30). For the development of FaeG-based vaccine against K88 ETEC strains in plants, we constructed pBI8801, a plant binary expression vector containing the K88ad fimbrial major antigen gene (faeG). First, p8801 (31), a parental plasmid which contains faeG without a signal peptide coding region, was digested with BamHI and SacI and the 789-bp faeG fragment was cloned into the digested plant expression vector pBI 121 (Clontech, Palo Alto, Calif.). This led to the creation of pBI8801, a binary vector with a cauliflower mosaic virus (CaMV 35s) promoter and a nopaline synthase terminator. Triparental matings were then performed as described by Ditta et al. (7) to transfer plasmid pBI8801 into Agrobacterium tumefaciens LBA4404.
Thereafter, transgenic tobacco (Nicotiana tabacum) was obtained by a modified leaf-disk cocultivation method using A. tumefaciens harboring pBI8801 (16). PCR analysis was carried out to show the presence of an amplified product of the expected size (789 bp) in the genomic DNAs (8) of tested kanamycin-resistant plants (data not shown). Reverse transcriptase PCR (RT-PCR) was used according to the protocol of a PowerScript RT kit (Clontech) to test whether faeG was transcribed in the transgenic plants; a fragment with expected size was observed for all the tested transformants, whereas no product was observed for nontransgenic plants (Fig. 1A).
FIG. 1.
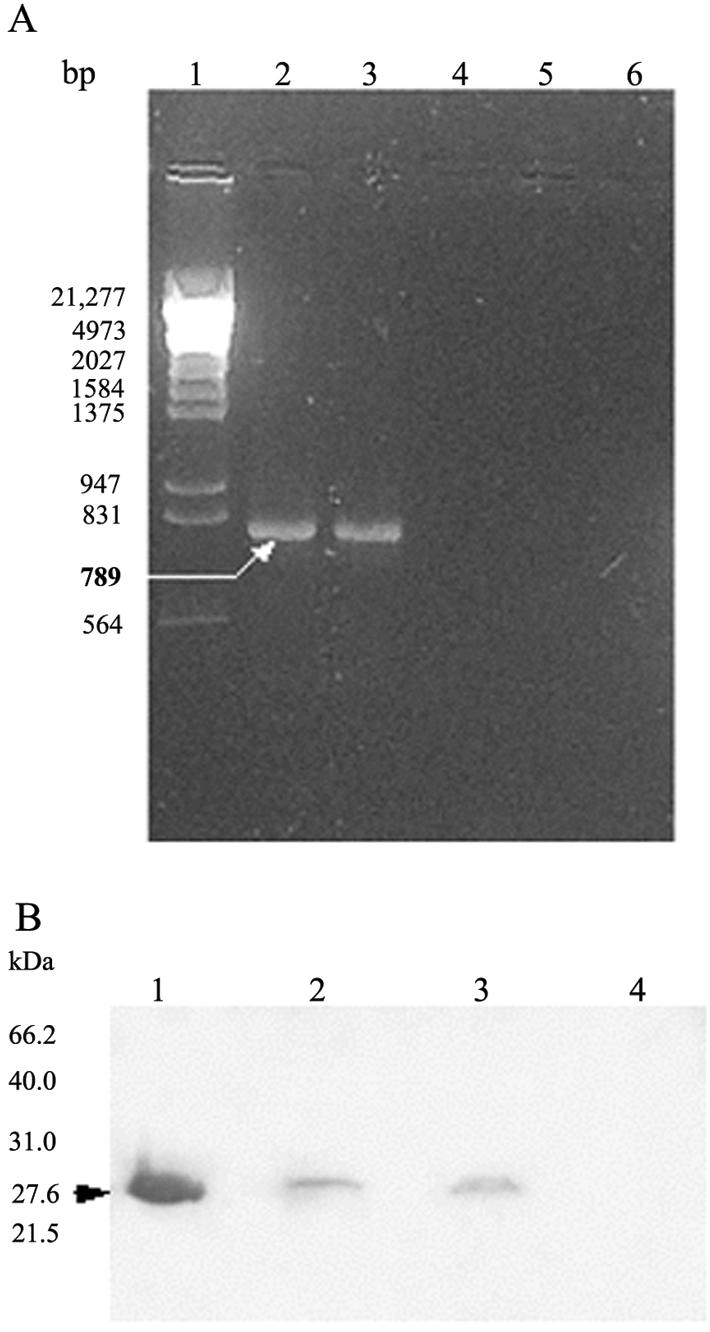
Analysis of the expression of faeG in transgenic plants. (A) Detection (using RT-PCR) of faeG transcription in transgenic plants. RT-PCR was performed (using specific primers that amplify a 789-bp DNA fragment of faeG) with total RNA from leaves of transgenic tobaccos (32). Lane 1, λDNA (digested with HindIII/EcoRI) molecular weight marker; lanes 2 and 3, RNA from transgenic tobacco; lanes 4 and 5, PCR amplification without RT reaction as a control for DNA contamination; lane 6, RNA from nontransgenic tobacco. (B) Immunoblot detection of recombinant FaeG synthesized in transgenic tobacco. TSP from tobacco leaves was fractionated by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis. The recombinant FaeG protein was detected with anti-FaeG antibody as the primary antibody and alkaline phosphatase-conjugated goat anti-rabbit IgG as the secondary antibody. Lane 1, 0.5 μg of purified recombinant FaeG expressed in E. coli BL21(DE3+K88) as a positive control; lanes 2 and 3, 100 μg each of TSP from transgenic tobacco plants; lane 4, 100 μg of TSP from nontransgenic tobacco.
The transgenic plants were further tested for transcriptional activity by examining the expression of faeG at translational level. Total soluble protein (TSP) was obtained from plant leaves following the method described by Arakawa et al. (2). The presence of the recombinant protein in the transformants harboring faeG was investigated by immunoblot analysis using the anti-FaeG serum (1:300) (31) to probe recombinant protein and an alkaline phosphatase-conjugated anti-rabbit immunoglobulin G (IgG) goat antiserum (Promega, Madison, Wis.) (1:5,000) as the second antibody. There were clear blotting bands with a molecular mass of 27.6 kDa in the tested transgenic plants (Fig. 1B), and no cross-reaction with the anti-FaeG serum was observed in nontransgenic plants. Then, Western blot analysis (28) and an enzyme-linked immunosorbent assay (2) were used to quantify the expression level of the recombinant protein in the transgenic plants. Results of these measurements indicated that recombinant FaeG protein constituted approximately 0.15% of the TSP in a highly expressed transgenic tobacco plant.
It has been shown previously that FaeG can be rapidly degraded without the aid of the chaperone molecule (FaeE) in the host ETEC (3). To evaluate recombinant FaeG expression level variations and the stability of expression levels in the different generations of transgenic tobacco plants, the leaves from the first, second, and third generations (T0, T1, and T2, respectively) of two transgenic plant lines were harvested for immunological analysis. Results indicated that the expression levels among the three generations did not differ greatly (Fig. 2). The little variation observed was likely due to a lack of some related protease specific for the digestion of FaeG in plants. Alternatively, within the plant cell there might be some chaperone-like proteins just like FaeE in K88ad ETEC (3) that may serve to stabilize FaeG and prevent its degradation by the protease.
FIG. 2.
Immunoblot analysis of the expression level of recombinant FaeG in T0, T1, and T2 generations of transgenic tobacco plants. A total of 100 μg of TSP was loaded for each lane. Lane 1, a nontransgenic plant; lanes 2 and 3, T0 transgenic plants; lanes 4 and 5, T1 transgenic plants; lanes 6 and 7, T2 transgenic plants.
Since one of the ultimate purposes of this study was to generate recombinant protein with immunogenicity, a group of 24 adult (60- to 90-day-old) female KM mice (purchased from Shanghai Laboratory Animal Center) were immunized intraperitoneally on days 0, 14, 28, and 42 with 0.5-ml transgenic tobacco leaf extracts (containing 7.5 μg of recombinant FaeG per animal per injection) emulsified in an identical volume of complete or incomplete Freund's adjuvant. Mice were bled before each injection. After the last inoculation, the immunized mice were also bled at weekly intervals over a period of 5 weeks. With the same immunizing schedule, each of a group of six KM mice was immunized with nontransgenic plant leaf extracts as a negative control. Moreover, each of a group of six KM mice was immunized with 7.5 μg of purified K88ad fimbriae as a positive control.
By a method described previously (29), K88ad fimbrial antigens were isolated from E. coli strain C83902 (a standard ETEC strain expressing K88ad fimbriae as determined by the China Institute of Veterinary Drug Control). With purified recombinant FaeG expressed in E. coli BL21(DE3+K88) as an antigen (31), an enzyme-linked immunosorbent assay was performed as described by Arakawa et al. (2) to detect specific serum antibodies. Alkaline phosphatase-conjugated anti-mouse goat IgG (Sigma, St. Louis, Mo.) (1:10,000) was used as the second antibody. The reaction was developed by the addition of the substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate and read at 405 nm in a universal microplate reader (Bio-Tek). The antibody titer was defined as the log10 reciprocal of the highest serum dilution that consistently presented (in at least three consecutive independent determinations) readings of optical density at 405 nm of 3 standard deviations over the mean optical density of the sera from six mice immunized with nontransgenic tobacco plants.
As shown in Fig. 3A the recombinant FaeG produced by transgenic plants can elicit a specific antibody response. Specific anti-FaeG antibodies were first detected in the immunized mice 3 weeks after the first antigen injection. The immunized mice developed a serum antibody response that peaked at 104 1 week after the last inoculation and then declined over the next 3 weeks. Mice immunized with the leaf extract from nontransgenic plants showed no immune response. The immune response of mice immunized with transgenic plant extracts was similar to that of mice immunized with purified K88ad fimbriae (Fig. 3A).
FIG. 3.
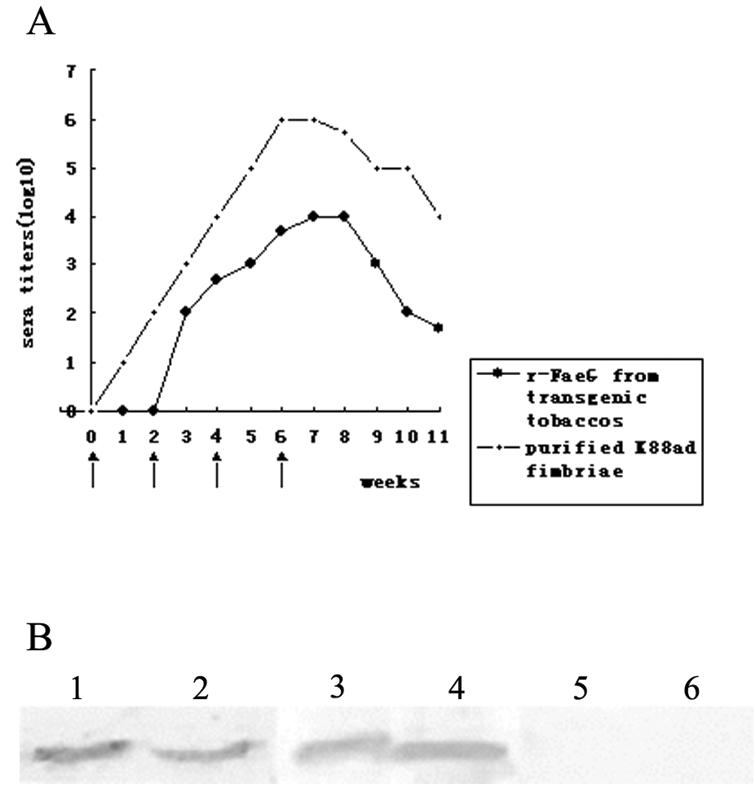
Immunogenicity of recombinant FaeG from transgenic tobacco. (A) Mice were immunized intraperitoneally on days 0, 14, 28, and 42 with either purified K88ad fimbriae or crude extracts from transgenic tobacco leaves. Arrows indicate the inoculating schedule. (B) Anti-FaeG antibodies detected by immunoblot analysis. ETEC K88ad fimbriae and purified recombinant FaeG from E. coli BL21(DE3+K88) were loaded in a well for sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. After the reaction mixtures were incubated with the sera of mice immunized with purified fimbriae, transgenic tobacco leaf extracts, or nontransgenic tobacco leaf extracts and then washed three more times, the reactions were developed by the addition of the substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate. Lanes 1 and 2, serum from a mouse immunized with purified fimbriae as a positive control; lanes 3 and 4, serum from a mouse immunized with transgenic tobacco extracts; lanes 5 and 6, serum from a mouse immunized with nontransgenic tobacco extracts as a negative control. Lanes 1, 3, and 5, 0.5 μg of purified K88ad fimbriae; lanes 2, 4, and 6, 0.5 μg of purified recombinant FaeG from E. coli BL21(DE3+K88).
The specificity of this anti-FaeG response was also confirmed by immunoblot analysis, using the purified K88ad fimbriae and recombinant FaeG from E. coli BL21(DE3+K88) as standard antigens. Sera (diluted 1:50) from mice immunized with transgenic plants expressing recombinant protein specifically recognized proteins with relative mobility identical to that of the protein recognized by the serum raised against bacterium-derived FaeG (Fig. 3B, lanes 1, 2, 3, and 4), whereas no immunological cross-reaction with either purified K88ad fimbriae or recombinant FaeG in serum from the mice immunized with nontransgenic plant leaf extracts was observed (Fig. 3B, lanes 5 and 6).
To test whether the sera from the mice immunized with transgenic plant extract could interact with the K88ad ETEC, a slide agglutination assay was performed as previously described (20). Sera from the mice immunized with transgenic tobacco at a dilution of 1:20 agglutinated strain C83902. In contrast, the use of the sera from the mice immunized with nontransgenic tobacco extract resulted in no agglutination.
The fact that sera from mice immunized with leaf extracts from transgenic plants can cause the agglutination of K88ad ETEC suggests that the same sera can also be effective in mitigating relevant diarrhea symptoms. To this end, ligated ileal loops from rabbits were used to test whether the sera could reduce the adherence of K88ad fimbria-expressing ETEC and inhibit the expression of enterotoxin in this strain in vivo. Rabbit ileal loop studies were performed with 3-kg-body-weight male New Zealand White rabbits as previously described (9). Rabbits were subjected to fasting for 24 h prior to surgery. Laparotomy was performed to externalize the intestine by aseptic technique under anesthesia with intramuscularly administered Sumianxin (purchased from the Quartermaster University of the People's Liberation Army) (0.2 ml/kg). Loops were created in the jejunum by placing ligatures at 6- to 7-cm intervals and separating loops with a 0.5- to 0.6-cm interposing loop. C83902 strains were grown from single colonies in 5 ml of Luria-Bertani medium, and the number of cells was adjusted to approximately 109 CFU/ml, with each aliquot containing 500 μl of C83902. Serum (100 μl) from a mouse immunized intraperitoneally with transgenic tobacco or nontransgenic tobacco was added to ETEC aliquots to neutralize K88ad-expressing ETEC, and the mixture was incubated for 12 h at room temperature. Then, the 0.6-ml mixture was injected into each loop in random fashion, the intestine was internalized, and the incision was closed. After 18 h of incubation, the consecutive ileal loops were excised, the weights of the loops were measured, and then the loops were punctured to permit measurement of the weight of the empty loops for determination of the significance of fluid reduction in the sera from the mice immunized with transgenic plant extracts. The volume of fluid accumulated in ileal loops inoculated with C83902 treated with the sera from the mice immunized with transgenic or nontransgenic plant extracts was measured and expressed as the ratio V/L (volume [V] [in microliters]/loop length [L] [in centimeters]) (Table 1).
TABLE 1.
Parameters of rabbit ileal loop ligation analysisa
| Sampleb | Loop length (cm) | Total weight of ileal loop (g) | Weight of empty ileal loop (g) | Vol of accumulated fluid (ml) | V/L |
|---|---|---|---|---|---|
| A | 6.55 ± 0.07 | 2.803 ± 0.025 | 1.206 ± 0.093 | 1.19 ± 0.01 | 181.70 ± 4.12 |
| B | 6.32 ± 0.47 | 2.107 ± 0.213 | 1.107 ± 0.178 | 0.97 ± 0.12 | 154.48 ± 15.11 |
| C | 6.68 ± 0.58 | 1.834 ± 0.434 | 1.349 ± 0.320 | 0.55 ± 0.29 | 63.61 ± 22.32 |
| D | 6.50 ± 0.28 | 2.175 ± 0.182 | 1.850 ± 0.071 | 0.38 ± 0.11 | 56.31 ± 15.35 |
The ratio of accumulated fluid volume to loop length (V/L [in micrograms per centimeter]) was used to express the serum-neutralized effect on K88ad ETEC in ileal loop ligation as previously described (9). A significant difference in neutralizing K88ad ETEC in the sera from the mice immunized with plant-expressed recombinant FaeG was observed in comparison with the sera from the mice immunized with nontransgenic tobacco extracts.
A, K88ad ETEC not treated by sera; B, K88ad ETEC treated with the sera of mice immunized with nontransgenic plant leaf extract; C, K88ad ETEC treated with the sera of mice immunized with transgenic plant leaf extract; D, physiological saline solution instead of K88ad ETEC.
As shown in Table 1, the mean values of V/L for each group were 63.61 ± 22.32 (mean ± standard error of the mean) for the mice immunized with transgenic tobacco extracts and 154.48 ± 15.11 (mean ± standard error of the mean) for the mice immunized with nontransgenic tobacco extracts. A Student's t test revealed significant fluid reduction in comparisons between the sera from the mice immunized with transgenic tobacco plants extracts and the sera from the mice immunized with nontransgenic tobacco extracts (P < 0.05). Moreover, ileal loops injected with physiological saline solution did not show significant accumulation of fluid (Table 1) or increased volume (data not shown), suggesting that there were no inflammatory responses caused by mechanical disturbances during loop ligation. This result implies that mouse sera stimulated by plant-derived recombinant FaeG can neutralize K88ad fimbria-expressing ETEC in vivo.
The results of this work suggest the possibility of producing a new alternative vaccine for diarrhea of piglets. Compared to traditional vaccines, this alternative vaccine will be less expensive and more convenient to store. To our knowledge, this is the first report to demonstrate that FaeG can be synthesized in plants. This work also provides a model for the use of plants for the production of vaccines against other ETEC with proteinaceous fimbriae or pili.
Acknowledgments
This work was supported by the fund of the National Key Basic Research Developments Program of the Ministry of Science and Technology, People's Republic of China (2001CB109002), the youth fund of the QIMINGXING Project of the Shanghai Committee of Science and Technology (01QC14037), and the youth fund of the Shanghai Academy of Agricultural Sciences (2000-09-01-1).
We sincerely thank Ning Jiang, Sim Soon Liang, Hai Huang, and Shuiping Wang for helpful suggestions and critical reading of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Alexander, T. J. L. 1994. Neonatal diarrhea in pigs, p. 151-170. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, United Kingdom.
- 2.Arakawa, T., J. Yu, D. K. X. Chong, J. Hough, P. C. Engen, and W. H. R. Langridge. 1998. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 16:934-938. [DOI] [PubMed] [Google Scholar]
- 3.Bakker, D., C. E. M. Vader, B. Roosendaal, F. R. Mooi, B. Oudega, and F. K. de Graaf. 1991. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol. Microbiol. 5:875-886. [DOI] [PubMed] [Google Scholar]
- 4.Bakker, D., P. T. J. Willemsen, R. H. Willems, T. T. Huisman, F. R. Mooi, B. Oudega, F. Stegehuis, and F. K. de Graaf. 1992. Identification of minor fimbrial subunits involved in biosynthesis of K88 fimbriae. J. Bacteriol. 174:6350-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barman, N. N., and D. K. Sarma. 1999. Passive immunization of piglets against enterotoxigenic colibacillosis by vaccinating dams with K88ac pili bearing bacterins. Indian J. Exp. Biol. 37:1132-1135. [PubMed] [Google Scholar]
- 6.Blanco, J., E. A. Gonzalez, and M. Blanco. 1989. Prevalence of enterotoxigenic Escherichia coli strains in outbreaks and sporadic cases of diarrhoea in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 8:396-400. [DOI] [PubMed] [Google Scholar]
- 7.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, K., and C. J. Thompson. 1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleckenstein, J. M., L. E. Lindler, E. A. Elsinghorst, and J. B. Dale. 2000. Identification of a gene within a pathogenicity island of enterotoxigenic Escherichia coli H10407 required for maximal secretion of the heat-labile enterotoxin. Infect. Immun. 68:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fürer, E. J., S. J. Cryz, Jr., F. Dorner, J. Nicolet, M. Wanner, and R. Germanier. 1982. Protection against colibacillosis in neonatal piglets by immunization of dams with procholeragenoid. Infect. Immun. 35:887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez, E. A., and J. Blanco. 1985. Comparative study of inhibition of mannose resistant haemaglutination caused by CFA/I, CFA/II, K88 and K99 (+) Escherichia coli strains. FEMS Microbiol. Lett. 29:115-121. [Google Scholar]
- 12.Gonzalez, E. A., and J. Blanco. 1986. Colonization antigens, antibiotic resistance and plasmid content of enterotoxigenic Escherichia coli isolated from piglets with diarrhoea in Galicia (north-western Spain). Vet. Microbiol. 11:271-283. [DOI] [PubMed] [Google Scholar]
- 13.Hacker, J. 1992. Role of fimbriae adhesins in the pathogenesis of Escherichia coli infections. Can. J. Microbiol. 38:720-727. [DOI] [PubMed] [Google Scholar]
- 14.Herbers, K., and U. Sonnewald. 1999. Production of new/modified proteins in transgenic plants. Curr. Opin. Biotechnol. 10:163-168. [DOI] [PubMed] [Google Scholar]
- 15.Hong, M. M., J. L. Zhang, R. Z. Cai, Y. Y. Fan, M. Yu, B. X. Wu, and J. F. Xiao. 1985. Cloning and expression of genetic determinants for K88 antigen. Chin. J. Biotechnol. 1:36-45. [Google Scholar]
- 16.Horsch, R. B., J. E. Fry, N. L. Hoffman, D. Eichholtz, S. G. Rogers, and R. T. Fraley. 1985. A simple and general method for transferring genes into plants. Science 227:1229-1231. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs, A. A. C., B. Roosendaal, J. F. L. Van Breemen, and F. K. de Graaf. 1987. Role of the phenylalanine-150 in the receptor binding domain of the K88 fibrillar subunit. J. Bacteriol. 169:4906-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klemm, P. 1981. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur. J. Biochem. 117:617-627. [DOI] [PubMed] [Google Scholar]
- 19.Levine, M. M. 1987. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic and enteroadherent. J. Infect. Dis. 155:377-389. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Vidal, Y., and A. M. Svennerholm. 1990. Monoclonal antibodies against the different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J. Clin. Microbiol. 28:1906-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx, J. L. 1980. Vaccinating with bacterial pili. Science 209:1103-1106. [DOI] [PubMed] [Google Scholar]
- 22.Mason, H. S., and C. J. Arntzen. 1995. Transgenic plants as vaccine production systems. Trends Biotechnol. 13:388-392. [DOI] [PubMed] [Google Scholar]
- 23.Mason, H. S., H. Warzecha, T. Mor, and C. J. Arntzen. 2002. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 8:324-329. [DOI] [PubMed] [Google Scholar]
- 24.Mooi, F. R., and F. K. de Graaf. 1985. Molecular biology of fimbriae of enterotoxigenic Escherichia coli. Curr. Top. Microbiol. Immunol. 118:119-138. [DOI] [PubMed] [Google Scholar]
- 25.Nagy, B., H. W. Moon, R. E. Isaacson, C. C. To, and C. C. Brinton. 1978. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating pigs with purified pili. Infect. Immun. 21:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oudega, B., M. de Graaf, L. De Boer, D. Bakker, C. E. M. Vader, F. R. Mooi, and F. K. De Graaf. 1989. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol. Microbiol. 3:645-652. [DOI] [PubMed] [Google Scholar]
- 27.Rutter, J. M., and G. W. Jones. 1973. Protection against enteric disease caused by Escherichia coli—a model for vaccination with a virulence determinant? Nature 242:531-532. [DOI] [PubMed] [Google Scholar]
- 28.Vassileva, A., D. A. Chugh, S. Swaminathan, and N. Khanna. 2001. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter. J. Biotechnol. 88:21-35. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez, F., E. A. Gonzalez, J. I. Garabal, S. Valderrama, J. Balanco, and S. B. Baloda. 1996. Development and evaluation of an ELISA to detect Escherichia coli K88 (F4) fimbrial antibody levels. J. Med. Microbiol. 44:453-463. [DOI] [PubMed] [Google Scholar]
- 30.Yu, J., and W. H. R. Langridge. 2001. A plant-based multicomponent vaccine protects mice from enteric diseases. Nat. Biotechnol. 19:548-552. [DOI] [PubMed] [Google Scholar]
- 31.Zhao, Z. J., C. Huang, Y. H. Huang, J. X. Chen, Z. A. Zhou, W. Q. Liang, A. H. Pan, and D. B. Zhang. 2000. Cloning, expression of K88 fimbrial subunit gene and preparation of the antiserum of the recombinant protein. Acta Agric. Shanghai 16:38-41. [Google Scholar]
- 32.Zheng, F. Q., Z. Y. Wang, and J. P. Gao. 1993. Isolation of total RNA from rice endosperm. Plant Physiol. Commun. 29:438-440. [Google Scholar]



