Abstract
Aims
The pharmacokinetics of intramuscular artemether and its major plasma metabolite–dihydroartemisinin, were investigated in patients with severe manifestations of falciparum malaria.
Methods
Six severe falciparum malaria patients with acute renal failure (ARF) and 11 without ARF were recruited into the study. They were treated with intramuscular artemether at a loading dose of 160 mg, followed by daily doses of 80 mg for another 6 days (total dose 640 mg).
Results
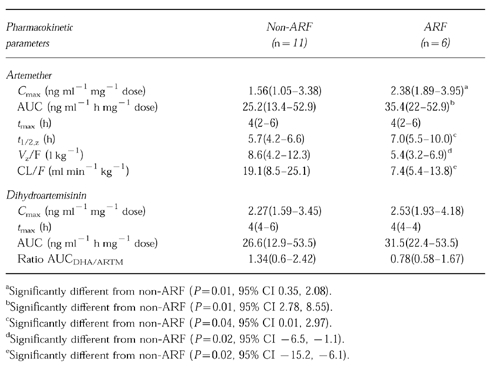
Patients with and without ARF showed a good initial response to treatment; the parasite and fever clearance times were 66(30–164) and 76(36–140) h [median(range)], respectively. None had reappearance of parasitaemia in their peripheral blood smear within 7 days of initiation of treatment. In comatose patients, the time to recovery of consciousness was 51.6(22–144) h. Artemether was detected in plasma as early as 1 h after a 160 mg dose, and declined to undetectable levels within 24 h in most cases. Patients with ARF had significantly higher Cmax [2.38(1.89–3.95) vs1.56(1.05–3.38) ng ml−1 mg−1 dose], AUC [35.4(22–52.9) vs 25.2(13.4–52.9) ng ml−1 h mg−1 dose], and lower Vz/F [5.45(3.2–6.9) vs8.6(4.2–12.3) l kg−1] and CL/F [7.4(5.4–13.8) vs19.1(8.5–25.1) ml min−1 kg−1] when compared with those without ARF. In addition, t1/2,z was significantly longer in ARF patients [7.0(5.5–10.0) vs5.7(4.2–6.6) h]. The pharmacokinetics of dihydroartemisinin in the two groups of patients were comparable.
Conclusions
ARF significantly modified the pharmacokinetics of intramuscular artemether. The changes could be attributed to either improved absorption/ bioavailability, a reduction of systemic clearance, or a change in plasma protein binding of the drug.
Keywords: pharmacokinetics, artemether, dihydroartemisinin, acute renal failure, severe malaria
Introduction
Resistance of Plasmodium falciparum towards the currently used antimalarials is rapidly increasing [1]. Artemether is a derivative of artemisinin (qinghaosu), which has been used extensively and successfully in the treatment of patients with multidrug resistant falciparum malaria, both in severe and uncomplicated cases [2–12]. Its onset of action is rapid but with the existing regimens, it is associated with a high recrudescence rate particularly in severe malaria [2, 5]. Severe side-effects with artemether have never been reported despite extensive usage of this drug in China, Myanmar, Vietnam and Thailand [2–12]. Due to its high clinical efficacy and low toxicity, there is potential usage of the drug in severe falciparum malaria in areas with multidrug resistance. Reliability of absorption of intramuscular artemether in patients with severe malaria has, however, been a major concern. This is particularly important in patients with acute renal failure, one of the common causes of death in severe malaria [13]. The aim of the present study was to investigate the pharmacokinetics of intramuscular artemether and its active plasma metabolite—dihydroartemisinin in falciparum malaria patients with severe manifestations.
Methods
Study site
The study was carried out during 1992–1994, at the Prapok-klao Hospital, Chantaburi Province, southeast Thailand, an area where multidrug resistant falciparum malaria exists.
Patients
Seventeen Thai male patients (aged 16–53 years, weight 45–61 kg) with severe manifestations of malaria [13] (six cases with acute renal failure, and 11 cases without acute renal failure) were recruited into the study. Acute renal failure (ARF) was defined as urine output less than 400 ml day−1 or less than 30 ml h−1 after appropriate fluid and electrolyte resuscitation, and when serum creatinine levels exceeded 3 mg dl−1. All patients denied having received antimalarial treatment within the 24 h prior to admission. Patients with concurrent diseases other than complications of malaria were excluded. Written informed consent for participation to the study was obtained from patients’ relatives. The study was approved by the Ethics Committee of the Ministry of Public Health, Bangkok, Thailand.
Treatment
Patients were treated with intramuscular artemether (Kunming Pharmaceuticals, People's Republic of China: 80 mg ml−1 per ampoule in ground nut oil) at a loading dose of 160 mg, followed by 80 mg, daily for another six doses (total dose of 640 mg). The injection site was in the area of upper outer quadrant of the buttock (single injection for each dose).
Parasite counts were performed every 6 h until negative for at least 24 h, then once daily until day 28. Parasites in thick and thin peripheral blood smears were identified by Giemsa stain, and parasite counts were reported per 1000 red blood cells or per 200 white blood cells. Haemodialysis was performed in patients with ARF after the completion of the kinetic study.
Blood for pharmacokinetics
Blood (5 ml) was taken from an indwelling cannula at hours 0, 1, 2, 3, 4, 6, 8, 10, 12, 16, 20 and 24, then by venepuncture prior to the doses on days 2, 3, 4, 5, 6 and 7 after the initial dose of 160 mg artemether. Plasma samples were separated immediately by centrifugation (2000 g) within 5 min and stored at −70° C until analysis.
Drug analysis
Concentrations of artemether and its major plasma metabolite—dihydroartemisinin in plasma were measured by high performance liquid chromatography with electrochemical detection (HPLC-EC), according to the method of Karbwang et al. [14]. The procedures involved the extraction of both compounds and the internal standard, artemisinin with a mixture of dichloromethane: tert-methyl-butyl-ether (1:1, v/v). Chromatographic separation consisted of the mobile phase (acetonitrile: water containing 0.1 m acetic acid pH 5.0 = 20:80%) running through the μBondapak CN column. The average recoveries of artemether and dihydroartemisinin at concentrations of 10, 80, 240 and 260 ng ml−1 were 82–93%. Coefficients of variation for both compounds were below 15% at a concentration of 10 ng ml−1, and below 10% at concentrations of 80, 240 and 640 ng ml−1. The limits of quantification for artemether and dihydroartemisinin were 5 and 3 ng ml−1, respectively. Artemether and dihydroartemisinin were found to be stable for at least 3 years when stored at −70° C, without successive freezing and thawing.
Pharmacokinetic analysis
The plasma concentration-time profiles of artemether and dihydroartemisinin were analysed by model-independent methods [15]. The time at which the maximum concentration occurred (tmax), and the maximum concentration (Cmax) were obtained directly from the plasma concentration-time data. The terminal phase elimination half-life (t1/2,z) was calculated from log-linear regression of at least four plasma concentrations. The area under the curve from zero to the last observed time AUC(0,t) was calculated by log-trapezoidal rule for ascending and descending data points. The area under the curve extrapolated from the last data point to infinity was estimated by dividing the regressed concentration at the last time point by the estimated elimination rate constant (λz). The extrapolations in all cases contributed less than 10%. The apparent total body clearance and apparent volume of distribution associated with the terminal phase were calculated as CL/F = dose/AUC and Vz/F = [CL/F]/λz, respectively.
Statistical analysis
Comparison of pharmacokinetic parameters of artemether and dihydroartemisinin between severe malaria patients with ARF and non-ARF was performed by a Mann-Whitney U test at a statistical significance level of P = 0.05.
Results
Patients in the two groups were comparable in age, body weight, admission parasitaemia, haematocrit, white blood cell count and liver function tests. Geometric mean (range) values of admission parasitaemia in ARF and non-ARF patients were 53 380 (15,550–175,050) vs 73 300 (31,200–143,330) per μl, respectively. Serum creatinine [4.8(3.7–12.8) vs 1.7(0.8–3.0) mg%] and BUN [89(56–186) vs20(10–38) mg%] concentrations were significantly higher in ARF patients.
Patients with ARF and non-ARF showed a good initial response to artemether; parasite and fever clearance times were 66(30–164) and 76(36–140) h, respectively [median(range)]. None of the patients had reappearance of parasitaemia in their peripheral blood smear within 7 days of the initiation of treatment. The time to recovery of consciousness in comatosed patients (14 cases) was 51.6(22–144) h.
Artemether was detected in plasma within 1 h of an intramuscular initial dose of 160 mg artemether (Figure 1). The drug concentrations declined to under the limit of quantification within 24 h in nine patients (one ARF, eight non-ARF). At 24 h, concentrations were still detectable in four each, of the patients with ARF and without ARF. Neither artemether nor dihydroartemisinin were detected in plasma prior to each dose on the following days. Cmax of artemether was reached at a median time (range) of 4(2–4) h in both groups of patients. Patients with ARF had significantly higher Cmax[2.38(1.89–3.95) vs1.56(1.05–3.38) ng ml−1 mg−1 dose], AUC [35.4(22–52.9) vs25.2(13.4–52.9) ng ml−1 h mg−1 dose], and lower Vz/F [5.45(3.2–6.9) vs8.6(4.2–12.3) l kg−1] and CL/F [7.4(5.4–13.8) vs19.1 (8.5–25.1) ml min−1 kg−1] when compared with those without ARF. Furthermore, t1/2,z was significantly longer in the first group [7.0(5.5–10) vs5.7(4.2–6.6) h]. The metabolite–dihydroartemisinin, reached peak concentrations at a median time of 4 h. Its pharmacokinetics were comparable between the two groups of patients with severe manifestations (Table 1).
Figure 1.

Plasma concentrations of artemether (ARTM) and dihydroartemisinin (DHA) in severe malaria patients with a) ARF (n = 6) and b) non-ARF (n = 11), following a single intramuscular dose of 160 mg ARTM.
Table 1.
Pharmacokinetics of artemether and dihydroartemisinin following an intramuscular dose of 160 mg in severe malaria patients with or without ARF (presented as median and range).
Discussion
Artemether is a very potent antimalarial against multidrug resistant falciparum malaria. However, the superiority of its clinical efficacy over the standard treatment (quinine) for severe falciparum malaria remains controversial [4–11]. It is anticipated that absorption of intramuscular artemether might not be sufficient in severely ill patients due to limited blood flow at the site of injection. The results of the present study have demonstrated the presence of artemether and its active metabolite–dihydroartemisinin in plasma as early as 1 h after intramuscular injection. The pharmacokinetics of intramuscular artemether in severe malaria were markedly influenced by ARF. Cmax and AUC of artemether were significantly increased. In addition, the systemic clearance and apparent volume of distribution were significantly reduced, and the terminal phase elimination half-life was prolonged. The systemic availability of artemether following intramuscular injection in severe malaria patients appears to be altered in association with ARF. The median AUC of artemether observed in healthy subjects in a previous study was 18.5 ng ml−1 h mg−1 dose [16], whereas the values in severe malaria patients with and without ARF were 35.4 and 25.2 ng ml−1 h mg−1 dose, respectively. Increased absorption/bioavailability of the drug during severe malaria and ARF phase was the likely explanation for the kinetic changes. A reduction of the apparent volume of distribution or systemic clearance might also, in part, contribute to the overall changes. Contraction of volume of distribution in severe malaria patients with ARF could be due to increased binding of the drug to plasma proteins during the acute phase of renal failure. Modulation of systemic clearance of artemether could be a consequence of inhibition of hepatic or extrahepatic drug metabolizing enzymes, or a reduction in liver blood flow.
In severe malaria patients, artemether was metabolised rapidly to dihydroartemisinin. The rate and extent of dihydroartemisinin formation appears similar in ARF and non-ARF patients. As systemic exposure of dihydroartemisinin was comparable, the lower metabolic ratio (AUCDHA/ARTM) seen in severe malaria patients compared with healthy subjects [16] was mainly a result of increased systemic exposure of artemether in severely ill patients.
Our pharmacokinetic data suggest that the absorption of intramuscular artemether in severe malaria is adequate. The concentrations of artemether/dihydroartemisinin achieved after intramuscular administration might have been sufficient as supported by the clearance of parasitaemia and suppression of the symptoms during the acute phase of the infection. However, if curative response (i.e. no reappearance of parasitaemia) is also of concern, modification of dosage regimens is required. If the killing effect of artemether is concentration-dependent (i.e. blood schizontocidal activity increases as the concentration increases), then an increase in the dose may be necessary. It was seen in a study with oral artemether that when the loading dose was increased to 300 mg instead of 200 mg, the cure rate was improved [2, 3]. On the other hand, if the killing effect of artemether does not increase with concentration, sustained exposure of parasites to artemether may be required. In this case, more frequent dosing is necessary as artemether/dihydroartemisinin have rather short half-lives. Increasing the duration of treatment may be another alternative to achieve sufficient and sustainable concentrations of active moieties for the sequestered parasites which would be expected to be released into the circulation. It was shown in a recent study that the cure rate of severe falciparum malaria was improved markedly when the duration of treatment was extended from 5 to 7 days [5]. The present study confirms the adequacy of absorption of intramuscular artemether in severe malaria, but further dose-optimisation is required.
Acknowledgments
The study was supported by UNDP/World Bank Special Programme on Research in Tropical Diseases. J. K. is supported by National Science and Technology Development Agency of Thailand. K. N. is supported by The Thailand Research Fund.
References
- 1.Wernsdorfer WH. Epidemiology of drug resistance in malaria. Acta Tropica. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 2.Bunnag D, Karbwang J, Harinasuta T. Artemether in the treatment of multiple drug resistant falciparum malaria. Southeast Asian J Trop Med Public Health. 1992;23:762–767. [PubMed] [Google Scholar]
- 3.Karbwang J, Na-Bangchang K, Thanavibul A, Bunnag D, Chongsupphajaisiddhi T, Harinasuta T. Comparison of oral artemether and mefloquine in acute uncomplicated falciparum malaria. Lancet. 1992;340:1245–1248. doi: 10.1016/0140-6736(92)92947-e. [DOI] [PubMed] [Google Scholar]
- 4.Karbwang J, Sukontason K, Rimchala W, et al. Preliminary report: a comparative clinical trial of artemether and quinine in severe falciparum malaria. Southeast Asian J Trop Med Public Health. 1992;23:768–772. [PubMed] [Google Scholar]
- 5.Karbwang J, Na-Bangchang K, Wattanakoon Y, Thanavibul A, Harinasuta T. Artemether 5 versus 7 day regimen for severe falciparum malaria. Southeast Asian J Trop Med Public Health. 1994;25:702–706. [PubMed] [Google Scholar]
- 6.Karbwang J, Tin T, Rimchala W, et al. Comparison of artemether and quinine in the treatment of severe falciparum malaria in south-east Thailand. Trans R Soc Trop Med Hyg. 1995;89:668–671. doi: 10.1016/0035-9203(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 7.Taylor TE, Wills BA, Kazembe P, et al. Rapid comma resolution with artemether in Malawian children with cerebral malaria. Lancet. 1992;93:661–662. doi: 10.1016/0140-6736(93)90423-e. [DOI] [PubMed] [Google Scholar]
- 8.Hensbroek MBV, Onyiorah E, Jaffar S, et al. A trial of artemether or quinine in children with cerebral malaria. New Engl J Med. 1996;335:69–75. doi: 10.1056/NEJM199607113350201. [DOI] [PubMed] [Google Scholar]
- 9.Myint PT, Shwe T. A controlled clinical trial of artemether (qinghaosu derivative) versus quinine in complicated and severe falciparum malaria. Trans R Soc Trop Med Hyg. 1987;81:559–561. doi: 10.1016/0035-9203(87)90406-8. [DOI] [PubMed] [Google Scholar]
- 10.Myint PT, Shwe T, Lin S. Clinical study of the treatment of cerebral malaria with artemether (qinghaosu derivative) Trans R Soc Trop Med Hyg. 1989;83:72. doi: 10.1016/0035-9203(89)90711-6. [DOI] [PubMed] [Google Scholar]
- 11.Hein TT, Day NPJ, Phu NN, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. New Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 12.China Cooperative Research Group on Qinghaosu and Its Derivatives as Antimalarials. Clinical studies on the treatment of malaria with qinghaosu and its derivatives. J Trad Chin Med. 1982;2:17–24. [PubMed] [Google Scholar]
- 13.World Health Organization. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 2. 1990. [PubMed]
- 14.Karbwang J, Na-Bangchang K, Molunto P, Banmairuroi V, Congpuong K. Determination of artemether and its major metabolite, dihydroartemisinin in plasma using high performance liquid chromatography with electrochemical detection. J Chromatogr. 1997;690:259–265. doi: 10.1016/s0378-4347(96)00422-7. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi M. In: Biopharmaceutics and clinical pharmacokinetics. 4. Lea, Febiger, editors. U.K: 1991. pp. 14–23. [Google Scholar]
- 16.Karbwang J, Na-Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol. 1997;52:307–310. doi: 10.1007/s002280050295. [DOI] [PubMed] [Google Scholar]


