Abstract
Aims
To examine the feasibility of using the human iris in vivo for the assessment of the interaction between tyramine and monoamine oxidase (MAO) inhibitors. To examine the relative roles of the two forms of MAO in terminating the response to sympathomimetic amines in the iris, by comparing the effects of single oral doses of moclobemide, a selective MAO-A inhibitor, and selegiline, a selective MAO-B inhibitor, on mydriatic responses to tyramine.
Methods
Twelve healthy male volunteers participated in three monthly sessions, each associated with ingestion of one capsule (moclobemide 450 mg, selegiline 10 mg, or placebo), according to a double-blind, balanced, cross-over design. Tyramine hydrochloride eye-drops (75 mm, 2×10 μl) were instilled three times in the left conjuctival sac at 40 min intervals. Pupil diameter was monitored with a binocular infrared television pupillometer before and for 4.5 h after ingestion of the capsule. The pupillary response to tyramine was expressed as the area under the pupil diameter×time curve (arbitrary units). A blood sample was taken before and 2 h after ingestion of the capsule, for the assay of platelet MAO-B activity, and plasma 3,4-dihydroxyphenylglycol (DHPG) concentration, an index of MAO-A activity. Platelet MAO activity was assayed radiochemically, using [14C]-phenylethylamine as substrate, and plasma DHPG by high performance liquid chromatography (h.p.l.c.). The results were analysed using analysis of variance with repeated measures, followed by Bonferroni's corrected t-test, using a significance criterion of P < 0.05.
Results
Both moclobemide and selegiline, compared with placebo, caused significant miosis in the right (untreated) eye. The changes in pupil diameter (mm±s.e. mean) from the pretreatment measurement were: placebo −0.09±0.07, moclobemide −0.52±0.09, selegiline −0.26±0.1. The mydriatic response to tyramine was potentiated by moclobemide, compared with the response recorded in the presence of placebo. The responses to tyramine (arbitrary units±s.e. mean) were: placebo 77.08±11.65, moclobemide 140.25±18.9, selegiline 72.75±12.35. Both moclobemide and selegiline significantly reduced platelet MAO activity, compared with placebo. The changes in platelet MAO activity (nmol h−1 mg−1 protein±s.e. mean) from the pretreatment level were: placebo 0.5±0.62, moclobemide −6.7±0.66, selegiline −17.7±0.87. Moclobemide significantly reduced plasma DHPG concentration, compared with placebo. The changes in plasma DHPG concentration (nmol l−1±s.e. mean) from the pretreatment level were: placebo −0.01±0.24, moclobemide −4.98±0.32, selegiline −0.51±0.26.
Conclusions
The potentiation of tyramine-evoked mydriasis by moclobemide is likely to reflect the inhibition of MAO-A activity in the iris, consistent with the activity of this enzyme in sympathetic nerve terminals. The lack of effect of selegiline on tyramine-evoked mydriasis argues against a role of MAO-B in terminating the effects of sympathomimetic amines in the iris. The effects of the two drugs on platelet MAO activity and plasma DHPG concentration are in agreement with previous reports and consistent with the relative selectivity of moclobemide for MAO-A and of selegiline for MAO-B. The miosis caused by the two MAO inhibitors is likely to be due to a central sympatholytic action of the drugs.
Keywords: moclobemide, monoamine oxidase, pupil diameter, selegiline, tyramine
Introduction
Monoamine oxidase (MAO) is a mitochondrial enzyme which occurs in most tissues of the body [1]. The enzyme is responsible for the oxidative deamination of a large range of monoamines, including both physiologically occurring catecholamines and indolamines and exogenously administered synthetic monoamines. The enzyme occurs in two forms (A and B), the A form being responsible, in humans, mainly for the metabolism of noradrenaline and 5-hydroxytryptamine, and the B form for metabolizing phenylethylamine and dopamine. In most tissues the two forms of the enzyme co-occur, however, only the B form has been identified in platelets, and only the A form in placenta [1].It is generally assumed that MAO-A is responsible for the oxidative deamination of noradrenaline in sympathetic nerve endings [2, 3].
Inhibitors of both MAO-A and MAO-B are available. Non-selective inhibitors, which affect both forms of the enzyme, have been available for many years for clinical use as antidepressants [4]. More recently, selective enzyme inhibitors have been developed for clinical use. Moclobemide [5] and brofaromine [6] are selective (and reversible) inhibitors of MAO-A, whereas selegiline (deprenyl) [7] is a selective (irreversible) inhibitor of MAO-B. The selective MAO-A inhibitor moclobemide is available as an antidepressant [8], whereas selegiline is used in the treatment of Parkinson's disease [9].
An important pharmacodynamic effect of MAO inhibitors is their ability to potentiate the effects of the indirectly acting sympathomimetic amine, tyramine [10]. Tyramine produces its pharmacological effect by releasing noradrenaline from sympathetic terminals. When MAO is inhibited, respones to tyramine are enhanced due to the inhibition of the catabolism of both tyramine and noradrenaline. The most widely studied response to tyramine is the pressorresponse evoked by either oral [11] or intravenous [12] administration of the drug. The ability of a MAO inhibitor to potentiate the tyramine-evoked pressor response is regarded as a predictor of the likelihood that the MAO inhibitorwill produce the ‘cheese reaction’ in patients treated with the drug. The cheese reaction consists of a hypertensive crisis evoked by tyramine-containing foodstuffs as a result of the inhibition of the catabolism of tyramine [13]. There is evidence that the two more modern MAO inhibitors, moclobemide [14]and selegiline [15], are less likely to evoke the cheese reaction, and indeed they have reduced ability to potentiate the tyramine-evoked pressor response in volunteer subjects.
Although the tyramine-evoked pressor response test is suitable to assess the interaction of MAO inhibitors and tyramine, it has several drawbacks. Firstly, a test involving supra-normal increases in blood pressure is not without risk, especially if the drug under investigation (i.e. a MAO inhibitor) may produce further increases. Secondly, when orally administered tyramine is used, the first pass metabolism of tyramine in the intestinal wall and in the liver will contaminate the pressor response, thus making inferencesabout the interaction between tyramine and the MAO inhibitor in vascular tissue difficult [12]. Therefore, a test involving the interaction between locally administered tyramine and a MAO inhibitor in a sympathetically innervated tissue would be valuable.
The human iris may provide a suitable, noninvasive, test systemfor studying the interaction between tyramine and MAO inhibitors. The iris receives a dense noradrenergic sympathetic innervation, and it is well documented that the pupil responds with dilatation to a tyramine solution instilled in the conjuctival sac [16–19]. Furthermore, it has been reported that the mydriatric response to tyramine is enhanced in patients receiving chronic treatment with the MAO inhibitors iproniazid, isocarboxazid and clorgyline [20],and benmoxine [21]. However, two previous studies, using single doses of classical irreversible nonselective MAO inhibitors (phenelzine [22], tranylcypromine [23]), failed to demonstrate the potentiation of tyramine-evoked mydriasis in healthy volunteers, although in both studies there was evidence for the potentiation of the tyramine-evoked pressor response.
In the present study, we re-examined the possible interaction between tyramine and MAO inhibitors in the human iris, using an acute dosing protocol with two modern selective MAO inhibitors, moclobemide (MAO-A inhibitor) and selegiline (MAO-B inhibitor). We also measured the concentration of the noradrenaline metabolite 3,4dihydroxyphenylglycol (DHPG) in the plasma, an index of MAO-A activity [24, 25], and MAO activity in blood platelets, an index of MAO-B activity [24, 26]. It has been shown that both plasma DHPG concentration and platelet MAO activity are reduced by single oral doses of moclobemide and selegiline, respectively [24–26].
Some of these results have been presented to the British Pharmacological Society [27].
Methods
Subjects
Twelve healthy male volunteers, with blue or light brown irides, aged 18–30 years (mean±s.d., 24.4±5.6), and weighing 60–89 kg (mean±s.d., 77±9.2), participated in the study. Subjects were all medication-free for at least 1 month prior to the study and remained so throughout the experiment. Two of them were smokers and all were occasional caffeine and/or social alcohol consumers. They were requested to stop smoking and to avoid drinking alcohol for at least 36 h prior to each session. Food and caffeine-containing beverages were not allowed after 22.00 h on the preceding night. The study protocol was approved by the University of Nottingham Medical School Ethics Committee. All volunteers gave their written consent following a verbal explanation of the study, and after reading a detailed information sheet.
Drugs
Moclobemide 450 mg, selegiline 10 mg and placebo were administered orally in matching capsules. All subjects participated in three monthly experimental sessions, each session being associated with one of the drug treatments. An interval of 30 days was interspersed between sessions since it has been shown that at least 14 days are required to attain full recovery of platelet MAO-B activity following inhibition by a single dose of 10 mg selegiline [26]. Subjects were allocated to drug-treatment conditions and experimental sessions according to a double-blind, balanced, cross-over design. Tyramine hydrochloride solution (75 mm, buffered to pH 8.0 with borate, tear-isotonic), for instillation in the conjunctival sac, was prepared in our laboratory. The tyramine hydrochloride solution was instilled into the conjunctival sac of the left eye on three occasions at 40 min intervals, according to an open design (see below, Procedure).
Tests and apparatus
Pupillometry
An infrared binocular television pupillometer (TVP 1015B Applied Science Laboratories, Waltham, MA, USA) was used for the recording of pupil diameter in darkness and in ambient light. In the ambient light condition the degree of the illumination of the room was defined in luminence units (candela [cd] m−2) measured at the level of the eyes; the luminence was 32 cd m−2. Pupil diameter was recorded in each eye at 10 min intervals, on 25 occasions in each session (see Figure 1). On each occasion, three 15 s recordings were taken at 5 s intervals, and the mean of the three recordings was calculated as the mean pupil diameter for that occasion. The recordings took place in a sound-attenuated room and the subjects fixed their gaze on a dim red spot of light positioned 2.5 m in front of them, in order to avoid accommodative constriction of the pupils during these measurements. Pupillary measures were digitized and stored on a floppy disk for off-line analysis.
Figure 1.

Design of experimental session. The horizontal line represents running time (min) measured from the end of an initial 10 min rest period (see Procedure). Arrows above the time- base correspond to recordings of pupil diameter (long arrows: in darkness; short arrows: under constant illumination of 32 cd m−2). At each time-point, as indicated by the arrows, three 15 s recordings of pupil diameter were taken at 5 s intervals (see text for details). Recordings in darkness at 10, 20 and 30 min, and under illumination at 100, 110 and 120 min, served for the calculation of the presystemic-treatment and pre-eyedrop baselines, respectively. Timings of the administration of systemic (‘capsule’) and local (‘Tyr’) treatments, and of blood sampling is indicated by arrows below the time-base.
Platelet MAO-B activity
Blood samples for determination of platelet MAO-B activity were taken at the times indicated in Figure 1. Venous blood (5ml) was collected in chilled evacuated tubes containing 0.1 ml of Na2-EDTA-solution. Platelets were isolated [24] and stored at −70° C until analysis. MAO-B activity was assayed radiochemically using [14C]-phenylethylamine as the substrate [24]. Enzyme activity was expressed as nmol of deaminated metabolites formed h−1 mg−1 protein. Protein content was measured with the method of Peterson [28] with bovine serum albumin as standard. The intra and interassay coefficients of variation were 2.7% and 6.0% (25.6 nmol h−1 mg−1). The MAO-B activity in human isolated platelets was found to be stable for at least 2 years when stored at −70° C [25].
Plasma DHPG
Another 5 ml of blood was collected for plasma DHPG determination into chilled tubes containing Na2-EDTA, and promptly chilled and centrifuged at 0–4° C. The plasma was separated and stored at −70° C until analysis. The concentration of DHPG was determined using high performance liquid chromatography with coulometric electrochemical detection [24, 30]. The intra and interassay coefficients of variation were 2.6% and 8.9%, respectively, at a concentration of 1.97 nmol l−1 and 1.8% and 4.7% at a concentration of 6.2 nmol l−1. The DHPG concentration in plasma was found to be stable for at least 10 months when stored at −70° C (unpublished).
Procedure
The design and time course of the experimental session is shown in Figure 1. At the beginning of each session, after a 10 min rest period, the subject adapted to darkness for 10 min and underwent three recordings, at 10 min intervals, of his pupil diameter in darkness. This was followed by taking a blood sample from the subject and the ingestion of the drug capsules by the subject. A time-interval of 70 min was interposed between the taking of the blood sample and the next pupil diameter measurement (see Figure 1), minimizing the potential effect of the venepuncture on pupil diameter (i.e. psychosensory pupil dilatation evoked by pain, apprehension, etc.). Furthermore, as the design of each session was the same, the cross-over nature of the experiment controlled for any possible effects related to the venepuncture. Recordings of pupil diameter in the right (untreated) eye and taking of a blood sample were repeated 120 min after ingestion of the capsule.
Each eye was used separately for the assessment of the effects of the drugs: the right eye served to evaluate the effects of the systemically taken drugs on pupil diameter, and the left eye served to assess the effect of locally instilled tyramine and its interaction with systemic treatments, on pupil diameter. The effects of the systemically taken drugs on pupil diameter were assessed by comparing the pre- and post-treatment recordings of the diameter of the pupil of the right (treated) eye, in darkness.
The diameter of the left (tyramine-treated) eye was recorded prior to the instillation of the eye-drops in order to obtain a baseline against which the response to tyramine could be measured. These pre-tyramine recordings were carried out in standard ambient illumination (32 cd m−2), 65, 75 and 85 min after ingestion of the capsule. Tyramine hydrochloride eye-drops (75 mm, 2×10 μl) were instilled into the subject's left conjunctival sac three times at 40 min intervals (i.e. 90, 130 and 170 min after ingestion of the capsule). Pupil diameter of the treated eye was recorded in ambient light (32 cd m−2) at 10 min intervals for 265 min following drug ingestion. The mydriatic response to tyramine was recorded in light in order to minimize the possible curtailment of the response by a ‘ceiling effect’ resulting from pupillary dilatation in the dark [31].
The timing of the blood sampling was based on reports in the literature that single doses of both moclobemide [24, 25] and selegiline [26] produce maximum inhibition of MAO-A and MAO-B, respectively, 2 h after dosing. The timing of the instillation of tyramine hydrochloride eye-drops was based on pilot studies in our laboratory showing that the peak response to tyramine is obtained 40 min after instillation [19]. Since the mydriatic response to tyramine fades quickly after 40 min, the instillation of the eye-drops was repeated three times at 40 min intervals. This procedure ensured that prolonged mydriatic responses to tyramine were obtained, enabling the use of the area under the response×time curve as a measure of the response.
Data reduction and analysis
The response to tyramine was expressed as the area under the pupil diameter×time curve obtained between 60 and 270 min following drug ingestion (see Figure 3). The responses (in arbitrary units) were analysed by one-way ANOVA with repeated measures, followed by Bonferroni-corrected t-tests. A significance criterion of P < 0.05 was adopted. Identical analyses were used to compare the effects of the drug treatments on resting pupil diameter measured in the right (untreated) eye in darkness, on platelet MAO activity and plasma DHPG concentration.
Figure 3.

Mydriatic response to tyramine. a) Pupil diameter (mm) at different time points following the instillation of tyramine eye-drops. The horizontal axis represents time elapsed since the start of the session. Points correspond to mean (n = 12) values at different time points obtained in the three treatment conditions (circles: placebo; triangles: moclobemide; squares: selegiline). Horizontal broken lines correspond to pre-eyedrop baselines in the placebo (top), selegiline (middle) and moclobemide (bottom) treatment conditions. b) Size of mydriatic response to tyramine in the three treatment conditions (area-under-the-curve, mean±s.e. mean, n = 12). Conventions as in Figure 1.
Results
Pupillary measures
Resting pupil diameter
Any change in pupil diameter in response to the systemic treatments was assessed against a baseline obtained in darkness. This pre-treatment baseline was calculated by taking the mean of three pupil diameter measurements at 10, 20 and 30 min after the start of the session, during a 30-min dark adaptation period (see Figure 1). Pre-treatment resting pupil diameters (mm; mean±s.e. mean), associated with the three treatment conditions, were as follows: placebo condition: 7.41±0.18; moclobemide condition: 7.55±0.19; selegiline condition: 7.51±0.18. ANOVA revealed no significant differences between the pretreatment values in the three conditions. Figure 2 shows the changes from pre-treatment in resting pupil diameter (mm) of the right (untreated) eye which was measured in darkness before and 2 h after treatment. All three treatments reduced pupil diameter. ANOVA showed a significant main effect of treatment (F = 11.24, d.f. = 2, 22), and comparisons of individual active treatments with placebo showed that moclobemide caused a significantly greater miosis than placebo. Although selegiline also tended to reduce pupil diameter to a greater extent than placebo (see Figure 2), the difference between the effects of selegiline and placebo did not reach statistical significance. The differences [mean (95 CI)] between placebo and the active treatments were: placebo vs moclobemide 0.44 (0.32, 0.56); placebo vs selegiline 0.17 (−0.08, 0.43).
Figure 2.
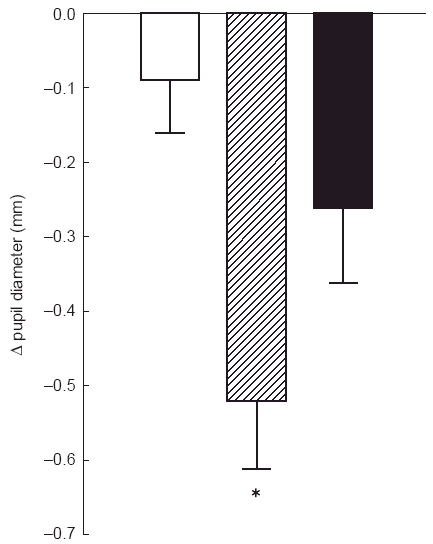
Change in resting pupil diameter from pretreatment, measured in darkness. Columns correspond to mean±s.e. mean (n = 12) obtained in the three treatment conditions (open column: placebo, hatched column: moclobemide, closed column: selegiline). Asterisk denotes significant difference from placebo (P < 0.05).
Response to tyramine
Any change in pupil diameter in response to locally instilled tyramine was assessed against a baseline obtained in constant illumination (32 cd m−2). This ‘pre-eyedrop’ baseline was calculated for each systemic treatment condition by taking the mean of three pupil diameter measurements at 100, 110 and 120 min after the start of the session. The pre-eyedrop baselines (mm, mean±s.e. mean) were: placebo condition 5.24±0.11; moclobemide condition 4.56±0.15; selegiline condition: 5.09±0.17. ANOVA showed a significant main effect of treatment (F = 8.07, d.f. = 2,22), and comparisons of individual active treatments with placebo showed that the baseline was significantly lower in the moclobemide condition than in the placebo condition. There was no significant difference between the selegiline and placebo conditions. The differences [mean (95 CI)] between placebo and the active treatments were: placebo vs moclobemide 0.67 (0.28, 1.06); placebo vs selegiline 0.15 (−0.23, 0.53).
Figure 3a shows the time course of the change in the diameter (mm) of the left pupil in the presence of the three treatments, following the instillation of tyramine hydrochloride eye-drops, measured between 60 and 270 min following ingestion of the drug capsule. Figure 3b shows the size of the mydriatic response to tyramine expressed as the area under the pupil diameter×time curve (arbitrary units). The response to tyramine was increased (by ≈82%) in the presence of moclobemide, whereas it was slightly reduced (by ≈6%) in the presence of selegiline, compared with the response recorded after the administration of placebo. ANOVA showed a significant main effect of drug treatment (F = 29.72, d.f. = 2,22), and comparisons of individual active treatments with placebo indicated that the response to tyramine was significantly larger following treatment with moclobemide than following treatment with placebo. The size of the response in the presence of selegiline did not differ significantly from that obtained in the presence of placebo. The differences [mean (95 CI)] between placebo and the active treatments were: placebo vs moclobemide 63.2 (40.0, 86.3); placebo vs selegiline 4.3 (−4.7, 13.4). In order to address the possibility that the differences in the sizes of the mydriatic responses to tyramine under the three treatment conditions were secondary to differences in the pre-eyedrop baselines, an ANCOVA of the area-under-the-curve data, with pre-eyedrop baseline as the covariate, was carried out. This analysis did not reveal a significant effect of the regression (F < 1), and the significant effect of treatment on area-under-the-curve was not removed (F = 17.2, d.f. = 2,22, P < 0.001).
Biochemical indices
Platelet MAO activity
Pre-treatment platelet MAO activities (nmol h−1mg−1 protein; mean±s.e. mean), associated with the three treatment conditions, were as follows: placebo condition: 21.1±0.76; moclobemide condition: 23.3±0.88; selegiline condition: 20.6±0.89. Figure 4a shows the change from pretreatment in platelet MAO activity (nmol h−1mg−1 protein), measured 2 h after drug ingestion. Both moclobemide and selegiline reduced platelet MAO activity, whereas placebo had little effect. The reduction after the administration of selegiline was approximately three times greater than that observed after the administration of moclobemide. ANOVA showed a significant main effect of drug treatment (F = 58.7, d.f. = 2,22), and comparisons of individual active treatment with placebo indicated that both active drugs significantly reduced MAO activity compared to placebo. The differences [mean (95 CI)] between placebo and the active treatments were: placebo vs moclobemide 6.58 (3.14, 10.03); placebo vs selegiline 18.53 (14.78, 22.39).
Figure 4.

Biochemical indices of MAO activity. a) Change in platelet MAO activity (nmol h−1 mg−1 protein) from pretreatment. Conventions as in Figure 1. b) Change in plasma DHPG concentration (nmol l−1) from pretreatment. Conventions as in Figure 1.
Plasma DHPG concentration
Pre-treatment plasma DHPG concentrations (nmol l−1), associated with the three treatment conditions, were as follows: placebo condition 5.97±0.42; moclobemide condition: 5.97±0.30; selegiline condition: 5.57±0.34. Figure 4b shows the change from pretreatment in plasma DHPG concentration (nmol l−1) measured two hours after drug taking. Moclobemide reduced DHPG concentration, whereas placebo and selegiline had little effect. ANOVA showed a significant main effect of drug treatment (F = 115.7, d.f. = 2,22), and comparisons of individual active treatments with placebo indicated that moclobemide significantly reduced plasma DHPG concentration. The differences [mean (95 CI)] between placebo and the active treatments were: placebo vs moclobemide 4.58 (3.67, 5.48), placebo vs selegiline 0.29 (−0.21, 0.79).
Discussion
The biochemical indices of MAO activity were affected in the predictable way in the present study. Moclobemide (450 mg) caused ≈83% reduction in plasma DHPG concentration, whereas selegiline (10 mg) was without effect. On the other hand, platelet MAO activity was reduced preferentially (by ≈86%) by selegiline, although moclobemide also caused a small (≈29%) reduction. These findings are in good agreement with previous reports, and confirm the relative selectivity of moclobemide for MAO-A and of selegiline for MAO-B [24–26]. The relatively small inhibition of platelet MAO-B by moclobemide has been observed previously and is attributed to MAO-B inhibition by some metabolites of the drug [24]. Although selegiline (10 mg) was completely selective for MAO-B in the present experiment, in agreement with previous reports [24], it has been shown that higher (e.g. 30 mg) single doses of the drug have some MAO-A inhibitory activity [11].
Tyramine hydrochloride solution, instilled in the conjunctival sac, evoked consistent mydriatic responses in the present experiment, as shown in numerous previous studies [16–19]. It should be noted that in a number of previous studies a more prolonged time course (later onset of peak, longer duration) of the response to tyramine [17, 21] was observed than in the present experiment. The prolonged time course of the response in those earlier studies is likely to be due to the vehicle used in the commercially available tyramine hydrochloride preparation (Mydrial, Winzer Pharmaceuticals) applied [17, 21].
Both moclobemide and selegiline reduced resting pupil size. The reduction in pupil size was ascertained in darkness, in the untreated eye, prior to the development of the mydriatic response to tyramine in the fellow eye. Thus, it can be excluded that the miosis was due to a consensual adjustment by the light reflex resulting from mydriasis in the fellow eye [32]. There is little information about the effects of MAO inhibitors on resting pupil diameter in man. It has been reported that a single dose of moclobemide (200 mg) was without any effect on pupil diameter until about 8 h after dosing [33], and that the selective MAO inhibitor befloxatone (5, 10 and 20 mg) [34] had no effect on pupil diameter for up to 8.5 h. The miosis observed in response to the MAO inhibitors in the present experiment was an unexpected finding, since on the basis of MAO inhibition in the iris the predicted effect would have been mydriasis, due to the inhibition of the catabolism of endogenous noradrenaline in the sympathetic terminals. Indeed, it has been reported that the local administration of the MAO inhibitor tranylcypromine causes mydriasis in the eye of rabbits [35]. A possible explanation for the miosis could be that, like the well-documented hypotensive effect of MAO inhibitors [36], it is due to central sympathetic inhibition. In this respect, the MAO inhibitors act similarly to clonidine, a mixed α2-adrenoceptor/imidazoline receptor agonist which has potent central sympatholytic effects, including miosis, in man [37]. It is of interest that it has been shown recently that there is a close molecular and pharmacological association between imidazoline receptors and MAO enzymes [38, 39].
As sedation due to a variety of causes (e.g. physiological somnolence, narcolepsy, anaesthesia) may be accompanied by miosis [40], the possibility arises that the miosis evoked by the MAO inhibitors may have been secondary to sedation. Observation of the behaviour of the subjects in the laboratory indicated that moclobemide may have caused some sedation, but no such effects were observed in association with selegiline. It should be noted, however, that selegiline also tended to reduce resting pupil diameter (see Figure 2). Furthermore, there is evidence that sedation and miosis do not always occur together, and thus the mechanisms underlying the two phenomena can be dissociated. Thus, it has been reported that in some experimental animals (e.g. mice) the clonidine induced sedation is accompanied by mydriasis rather than miosis [41]. Furthermore, diazepam has distinct sedative effects in humans without affecting resting pupil size [42].
The mydriatric response to tyramine was potentiated by moclobemide, but not by selegiline. It should be noted that apparent potentiation of the response could be due to the lowering of the baseline [31], and indeed moclobemide had a miotic effect. However, it is unlikely that the observed potentiation is entirely due to such a ‘baseline effect’, since the response to tyramine in the presence of moclobemide was associated with a larger absolute pupil diameter than that recorded in the presence of placebo (see Figure 3). Furthermore, there was no significant effect of the baseline on the size of the response to tyramine. The potentiation of the mydriatic response to tyramine by moclobemide is likely to reflect the inhibition of MAO in sympathetic nerve endings in the iris, leading to the impaired catabolism of both tyramine and endogenous noradrenaline, which in turn would lead to the enhancement of the effect of tyramine. As moclobemide is a selective MAO-A inhibitor, our observation confirms the role of MAO-A in terminating the effects of tyramine and noradrenaline in sympathetic nerve terminals. It should be noted, however, that moclobemide may also have some MAO-B inhibitory activity, via some of its metabolites [24], as indicated by its ability to inhibit platelet MAO-B. The lack of effect of selegiline, which in the dosage used had a selective inhibitory effect on MAO-B, seems to argue against the involvement of MAO-B in the catabolism of tyramine and noradrenaline in the iris. It should be noted, however, that it has been reported that selegiline can antagonize pharmacological responses to tyramine [43], either by blocking its uptake into nerve terminals [44], or by blocking postjunctional α1-adrenoceptors [45], or via both mechanisms. Therefore, it is possible that selegiline may have caused some increase in the size of the mydriatic response to tyramine due to the inhibition of MAO-B, however, this was masked by a reduction in the size of the response brought about by the blockade of tyramine uptake and/or of postjunctional α1-adrenoceptors.
Our results show that the pupil provides a suitable noninvasive test system for assessment of the interaction between tyramine and acute single doses of MAO inhibitors. It should be noted however, that caution is needed with the use of mydriatic drugs, such as tyramine, since they may provoke an attack of closed angle glaucoma in susceptible individuals. Therefore, this technique is not recommended for use in elderly subjects. Furthermore, care should be taken to use subjects with light coloured irides since iris pigmentation can reduce the response to mydriatric drugs [46].
In conclusion, our results show that the potentiation of tyramine-evoked mydriasis by moclobemide is consistent with a role of MAO-A in terminating the pharmacological effects of tyramine in sympathetically innervated tissues. The lack of potentiation of tyramine-evoked mydriasis by selegiline seems to argue against a similar role of MAO-B, although some involvement of this enzyme cannot be entirely excluded. The reduction in resting pupil size caused by moclobemide and, to a smaller extent, by selegiline may be interpreted in terms of a postulated central sympatholytic effect of the drugs.
Acknowledgments
P.B. was supported by a scholarship from the State Scholarship Foundation of Greece (I.K.Y.).
References
- 1.Berry MD, Juorio AV, Paterson IA. The functional role of monoamine oxidases A and B in the mammalian central nervous system. Progr Neurobiol. 1994;42:375–391. doi: 10.1016/0301-0082(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DL, Sunderland T, Garrick NA, Aulakh CS, Cohen RM. Selective amine oxidase inhibitors: basic to clinical studies. In: Dahl SG, Gram LF, Paul SM, Potter WZ, editors. Clinical Pharmacology in Psychiatry. Berlin: Springer-Verlag; 1987. pp. 135–146. [Google Scholar]
- 3.Broadley KJ. Autonomic pharmacology. London: Taylor & Francis; 1996. [Google Scholar]
- 4.Szabadi E, Bradshaw CM. Affective disorders: 1. Antidepressants. In. In: King DJ, editor. Seminars in Clinical Psychopharmacology. London: Royal College of Psychiatrists/Gaskall; 1995. pp. 138–192. [Google Scholar]
- 5.Haefely W, Burkard WP, Cesura A, et al. Pharmacology of moclobemide. Clin Neuropharmacol. 1993;16(Suppl 2):S8–S18. [PubMed] [Google Scholar]
- 6.Volz H-P, Gleiter CH, Waldmeier PC, Struck M, Möller H-J. Brofaromine—a review of its pharmacological properties and therapeutic use. J Neural Transmission. 1996;103:217–245. doi: 10.1007/BF01292628. [DOI] [PubMed] [Google Scholar]
- 7.Knoll J. The pharmacology of (-) deprenyl. J Neural Transmission. 1986;22S:75–89. [PubMed] [Google Scholar]
- 8.Amrein R, Hetzel W, Stabl M, Schmid-Bungk W. RIMA—a new concept in the treatment of depression with moclobemide. Int Clin Psychopharmacol. 1993;7:123–132. doi: 10.1097/00004850-199300730-00001. [DOI] [PubMed] [Google Scholar]
- 9.Elizan TS. (-) Deprenyl combined with L-DOPA in the treatment of Parkinson's disease. In: Szelenyi I, editor. Inhibitors of monoamine oxidase B. Basel: Birkhäuser Verlag; 1993. pp. 277–288. [Google Scholar]
- 10.Rand MJ, Trinker FR. The mechanism of the augmentation of responses to indirectly acting sympathomimetic amines by monoamine oxidase inhibitors. Br J Pharmacol Chemother. 1968;33:287–303. doi: 10.1111/j.1476-5381.1968.tb00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad A, Glover V, Goodwin BL, Sandler M, Signy M, Smith SE. Enhanced pressor sensitivity to oral tyramine challenge following high dose selegiline treatment. Psychopharmacol. 1988;95:540–543. doi: 10.1007/BF00172970. [DOI] [PubMed] [Google Scholar]
- 12.Pickar D, Cohen RM, Jimerson DC, Murphy DL. Tyramine infusions and selective monoamine oxidase inhibitor treatment. I. Changes in pressor sensitivity. Psychopharmacol. 1981;74:4–7. doi: 10.1007/BF00431747. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell B, Marley E, Price J, Taylor D. Hypertensive interactions between monoamine oxidase inhibitors and foodstuffs. Br J Psychiatry. 1967;113:349–365. doi: 10.1192/bjp.113.497.349. [DOI] [PubMed] [Google Scholar]
- 14.Audebert C, Blin O, Monjanel-Mouterde S, et al. Influence of food on the tyramine pressor effect during chronic moclobemide treatment of healthy volunteers. Eur J Clin Pharmacol. 1992;43:507–512. doi: 10.1007/BF02285092. [DOI] [PubMed] [Google Scholar]
- 15.Elsworth JD, Glover V, Reynolds GP, et al. Deprenyl administration in man: a selective monoamine oxidase B inhibitor without the ‘cheese effect’. Psychopharmacol. 1978;57:33–38. doi: 10.1007/BF00426954. [DOI] [PubMed] [Google Scholar]
- 16.Szabadi E, Besson J, Bradshaw CM. Pupil responsiveness to tyramine in depressed patients treated with amitriptyline. Br J Clin Pharmacol. 1975;2:362–363. doi: 10.1111/j.1365-2125.1975.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peet M, Yates RA, Shields AG. Dose–response relationship for mydriasis produced by topical ocular tyramine in man. Br J Clin Pharmacol. 1980;9:96–98. doi: 10.1111/j.1365-2125.1980.tb04804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr FA, Szabadi E. Comparison of the effects of chronic administration of ciclazindol and desipramine on pupillary responses to tyramine, methoxamine and pilocarpine in healthy volunteers. Br J Clin Pharmacol. 1985;19:639–647. doi: 10.1111/j.1365-2125.1985.tb02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitsios P, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of clonidine on tyramine- and methoxamine-evoked mydriasis in man. Br J Clin Pharmacol. 1996;41:269–275. doi: 10.1046/j.1365-2125.1996.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevan-Jones B, Lind NA. Interactions of monoamine oxidase inhibition and sympathomimetic amines on the human iris. Br J Pharmacol. 1971;41((2)):428P–429P. [PMC free article] [PubMed] [Google Scholar]
- 21.Palm D, Fengler H-J, Güllner H-G, et al. Quantitation of irreversible inhibition of monoamine oxidase in man. Eur J Clin Pharmacol. 1971;3:82–92. [Google Scholar]
- 22.Trembath PW, Turner P. Effect of a single oral dose of phenelzine on the tyramine pressor and ocular responses in man. J Pharm Pharmacol. 1979;31:59–60. doi: 10.1111/j.2042-7158.1979.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 23.Peet M, Yates RA, Carroll JA, Middlemiss DN. The interaction of tyramine with a single dose of tranylcypromine in healthy volunteers. Br J Clin Pharmacol. 1981;11:212–213. doi: 10.1111/j.1365-2125.1981.tb01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koulu M, Scheinin M, Kaarttinen A, et al. Inhibition of monoamine oxidase by moclobemide: effects on monoamine metabolism and secretion of anterior pituitary hormones and cortisol in healthy volunteers. Br J Clin Pharmacol. 1989;27:243–255. doi: 10.1111/j.1365-2125.1989.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holford NHG, Guentert TW, Dingemanse J, Banken L. Monoamine oxidase-A: pharmacodynamics in humans of moclobemide, a reversible and selective inhibitor. Br J Clin Pharmacol. 1994;37:433–439. doi: 10.1111/j.1365-2125.1994.tb05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinonen EH, Anttila MI, Lammintausta RAS. Pharmacokinetic aspects of l-deprenyl (selegiline) and its metabolites. Clin Pharmacol Ther. 1994;56:742–749. doi: 10.1038/clpt.1994.204. [DOI] [PubMed] [Google Scholar]
- 27.Bitsios P, Langley RW, Tavernor S, Szabadi E, Bradshaw CM. Comparison of the effects of moclobemide and selegiline on tyramine-evoked mydriasis in man. Br J Clin Pharmacol. 1997;43:551–552P. doi: 10.1046/j.1365-2125.1998.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 29.Heinonen EH, Anttila MI, Nyman LM, Pyykkö KA, Vuorinen JA, Lammintausta RAS. Inhibition of platelet monoamine oxidase type B by selegiline. J Clin Pharmacol. 1997;37:597–601. doi: 10.1002/j.1552-4604.1997.tb04341.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheinin M, Karhuvaara S, Ojala-Karlsson P, Kallio A, Koulu M. Plasma 3,4- dihydroxyphenylglycol (DHPG) and 3-methoxy-4-hydroxyphenylglycol (MHPG) are insensitive indicators of α2-adrenoceptor mediated regulation of norepinephrine release in healthy human volunteers. Life Sci. 1991;49:75–84. doi: 10.1016/0024-3205(91)90581-u. [DOI] [PubMed] [Google Scholar]
- 31.Longmore J, Theofilopoulos N, Szabadi E, Bradshaw CM. Modification of the pupillary light reflex by miotic and mydriatic drugs: applicability of model of functional interaction. Br J Clin Pharmacol. 1987;23:610–611P. [Google Scholar]
- 32.Theofilopoulos N, Longmore J, Kerr FA, Szabadi E, Bradshaw CM. Consensual pupillary responses to mydriatic and miotic drugs. Br J Clin Pharmacol. 1988;26:697–702. doi: 10.1111/j.1365-2125.1988.tb05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grünberger Von J, Linzmayer L, Cepko H, Saletu B. Lichtevozierte dynamische Pupillometrie zur Differenzierung psychotroper Substanzen. Drug Res. 1987;37:357–360. [PubMed] [Google Scholar]
- 34.Warot D, Berlin I, Patat A, Durrieu G, Zieleniuk I, Puech AJ. Effects of befloxatone, a reversible selective monoamine oxidase-A inhibitor, on psychomotor function and memory in healthy subjects. J Clin Pharmacol. 1996;36:942–950. doi: 10.1002/j.1552-4604.1996.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoyng FJ, van Alphen GWHM. Behaviour of IOP and pupil size after topical tranylcypromine in the rabbit eye. Documenta Ophthalmologica. 1981;51:225–234. doi: 10.1007/BF00143886. [DOI] [PubMed] [Google Scholar]
- 36.McDaniel KD. Clinical pharmacology of monoamine oxidase inhibitors. Clin Neuropharmacol. 1986;9:207–234. doi: 10.1097/00002826-198606000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Szabadi E, Bradshaw CM. Autonomic pharmacology of α2-adrenoceptors. J Psychopharmacol. 1996;10(Suppl 3):6–18. [Google Scholar]
- 38.Parini A, Moudanos CG, Pizzinat N, Lanier SM. The elusive family of imidazoline binding sites. Trends Pharmacol Sci. 1996;17:13–16. doi: 10.1016/0165-6147(96)81564-1. [DOI] [PubMed] [Google Scholar]
- 39.Olmos G, Alemany R, García-Sevilla JA. Pharmacological and molecular discrimination of brain I2-imidazoline receptor subtypes. Naunyn-Schmiedeberg's Arch Pharmacol. 1996;354:709–716. doi: 10.1007/BF00166896. [DOI] [PubMed] [Google Scholar]
- 40.Loewenfeld IE. Pupillary pharmacology. In: Loewenfeld IE, editor. The pupil. Iowa City: Iowa State University Press; 1993. pp. 683–827. [Google Scholar]
- 41.Heal DJ, Prow MR, Buckett WR. Clonidine produces mydriasis in conscious mice by activating central α2-adrenoceptors. Eur J Pharmacol. 1989;170:11–18. doi: 10.1016/0014-2999(89)90127-1. [DOI] [PubMed] [Google Scholar]
- 42.Bitsios P, Szabadi E, Bradshaw CM. The sensitivity of the fear-inhibited light reflex to diazepam. Psychopharmacol. 1998;135:93–98. doi: 10.1007/s002130050489. [DOI] [PubMed] [Google Scholar]
- 43.Knoll J, Vizi ES, Somogyi G. Phenylisopropylmethylpropynylamine (E-250), a monoamine oxidase inhibitor antagonising the effects of tyramine. Arzneimittelforschung. 1968;18:109–112. [Google Scholar]
- 44.Knoll J, Magyar K. Some puzzling effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol. 1972;5:393–408. [PubMed] [Google Scholar]
- 45.Nedergaard OA, Möller J. Inhibition by (-) -deprenyl of agonist-evoked contractions in rabbit aorta. Pharmacol Toxicol. 1994;75:377–383. doi: 10.1111/j.1600-0773.1994.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 46.Seidehamel RJ, Tye A, Patil PN. An analysis of ephedrine mydriasis in relationship to iris pigmentation in the guinea-pig eye in vitro. J Pharmacol Exp Ther. 1970;171:205–213. [PubMed] [Google Scholar]


