Abstract
Aims
Preliminary results indicate higher absorption of triclabendazole (TCBZ) administered postprandially. Therefore, the influence of food on the pharmacokinetics of TCBZ and its active sulphoxide (TCBZ-SO) and sulphone (TCBZ-SO2) metabolites was investigated.
Methods
Two single doses (10 mg kg−1) of TCBZ were administered to 20 patients with fascioliasis. Ten patients were first given the drug after a high energy breakfast and then, 48 h later, after an overnight fast. The other 10 patients first received the drug in fasting state and then, 48 h later, after breakfast. A low energy breakfast was served 2 h after drug administration for fasting state.
Results
Compared with the fasting state, an increased AUC and Cmax after food intake (significant, P < 0.0001) was shown from the values of TCBZ, TCBZ-SO and TCBZ-SO2. The mean AUC for TCBZ (fasting: 1.55, fed: 5.72 μmol l−1 h), TCBZ-SO (fasting: 177, fed: 386 μmol l−1 h) and TCBZ-SO2 (fasting: 13.9, fed: 30.5 μmol l−1 h) indicated a large availability increase with food and the strong systemic predominance of the active sulphoxide metabolite over the unchanged drug. (All patients were cured at the end of the trial except one who required a second course of two postprandial doses of triclabendazole (10 mg kg−1 each). Tolerability to the treatment among the patients was good.
Conclusions
The administration of triclabendazole with food is recommended for improved systemic availability in patients with fascioliasis or paragonimiasis.
Keywords: active metabolite, bioavailability, Fasciola hepatica, food, kinetics, patients
Introduction
Triclabendazole (TCBZ), the active ingredient of a registered veterinary preparation, is a benzimidazole anthelmintic indicated in human fascioliasis. In animals, no unchanged drug is detected in plasma after oral dosing. With immature worms of Fasciola hepatica exposed in vitro for 24 h to 1–10 μmol l−1 concentrations of TCBZ or of its sulphoxide metabolite (TCBZ-SO), TCBZ-SO exerted a delayed, but more potent effect on the motility of the parasites than TCBZ [1]. Thus, the activity of the preparation is likely to occur through the active metabolite TCBZ-SO [2].
Triclabendazole is effective in humans presenting with infection by the liver fluke Fasciola hepatica [3, 4] or with the lung fluke Paragonimus sp. [5, 6]. Preliminary pharmacokinetics in European patients indicate that the absorption of TCBZ might be higher in postprandial than in fasting administration conditions [4].
The objective of this trial was to investigate the pharmacokinetics of triclabendazole in patients and to assess the influence of food intake on the kinetics of TCBZ, TCBZ-SO and its sulphone metabolite (TCBZ-SO2).
Methods
Patients
Twenty Peruvian patients (10 male, 10 female), aged from 9 to 62 years (mean ±s.d. = 29±17 year), with a body weight from 21 to 60 kg (mean ±s.d. = 42±12 kg) and presenting a moderate infection with Fasciola hepatica participated in the study. The Ethics Committee of the World Health Organization (WHO) approved the study. Informed consent was obtained from the patients or their legal representatives.
Protocol
The study was performed according to a nonrandomized two-period cross-over design. Patients 1–10 were given the drug in the morning, immediately after a high energy breakfast (day 1) and then, 48 h later, in a fasting state (day 3). Patients 11–20 received the drug first in fasting state (day 1) and then, 48 h later (day 3), after a high energy breakfast. A low energy breakfast was given 2 h after the drug administration in fasting state. The two single oral doses given on days 1 and 3 corresponded to ≈10 mg of TCBZ kg−1 of body weight.
The high energy breakfast, consumed within 30 min before drug intake, consisted of two cups of coffee with coffee cream and sugar, one roll with cheese and one roll with butter and jam (total energy amount, ≈560 kcal). The low energy breakfast (decaffeinated coffee with coffee cream and sugar, two rolls and jam; total energy: about 200 kcal) was served 2 h after the drug had been administered to the fasted patients.
Blood (about 8 ml) was withdrawn into heparinized tubes predose and at 1, 2, 4, 8, 12, 24, 36 and 48 h after the drug administrations. Immediately after collection, blood samples were centrifuged (2200g, approximately) and the plasma stored frozen at about −20° C until analysis.
Analytical method
Concentrations of TCBZ and metabolites in plasma were measured by h.p.l.c. [7]. The limit of quantification was 0.06 μmol l−1 (0.02 μg ml−1) for TCBZ, 0.13 μmol l−1 (0.05 μg ml−1) for TCBZ-SO and 0.05 μmol l−1 (0.02 μg ml−1) for TCBZ-SO2. The method was validated daily. The mean accuracy values (found×100/given) at seven different concentration levels for each compound, were between 97 and 104% and the precision (CV%) was between 4 and 12%.
Pharmacokinetic analysis
The following pharmacokinetic parameters were determined:
AUC(0,48h): Area under the plasma concentration-time curve (μmol l−1 h) calculated by the trapezoidal rule over 48 h.
When the metabolites could still be detected in plasma just before the second dose administration, the AUC after the second administration was corrected to eliminate the contribution of the first administration by using the concentration at 48 h after the first dose and the apparent terminal t1/2.
Cmax: Maximum plasma concentration (μmol l−1). For TCBZ-SO and TCBZ-SO2, the Cmax after the second administration was corrected to eliminate the minor contribution of the first administration by using C48 h, 1st dose and the terminal t1/2.
tmax: Time corresponding to Cmax (h).
t1/2: Elimination half-life (h) determined from the slope of the terminal log-linear segment of the plasma concentration-time curve, calculated only for TCBZ-SO and -SO2. No well defined log-linear decrease of the TCBZ individual concentration-time curves could be observed.
Statistical analyses
The AUC and Cmax of the three measured compounds in fasting and postprandial conditions were subjected to analysis of variance using the log transformed data. The 90% confidence limits for the difference between least squares means on the log-scale, using fasting conditions as the reference standard were antilogged to obtain 90% confidence limits [8]. tmax values were compared by a Wilcoxon matched-pairs signed rank test. The significance level was set at 5%.
Results
Pharmacokinetics
Mean plasma concentration of TCBZ, TCBZ-SO and TCBZ-SO2 are reported in Figure 1.
Figure 1.
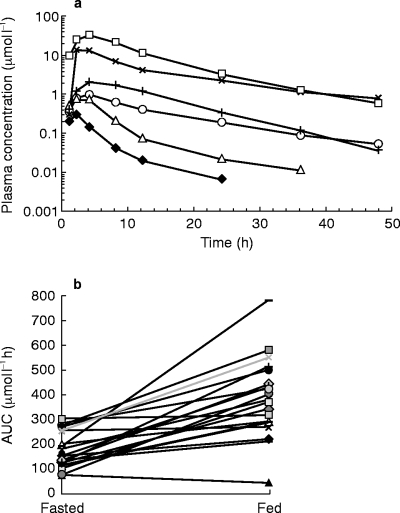
a) Mean (n = 20) concentrations in plasma, log-linear scale, for TCBZ (Fasting: ♦, Fed, ▵), TCBZ-SO (Fasting: ×, Fed: □) and TCBZ-SO2 (Fasting: ○, Fed: +), b) AUC (0,48 h) (μmol l−1) of the sulphoxide metabolite for each patient: Comparison of fasted/fed conditions.
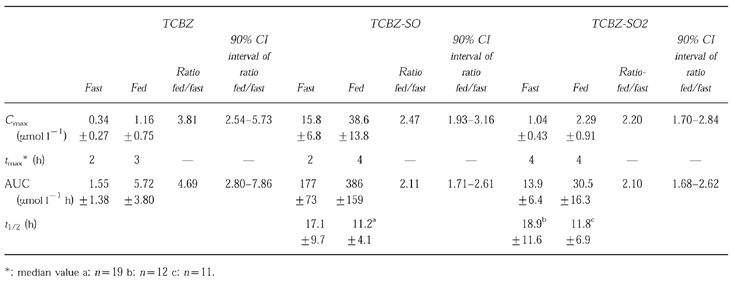
Pharmacokinetic parameters (mean ±s.d.) are reported in Table 1. In comparison with fasting conditions, a highly significant difference (P < 0.0001) in AUC and Cmax of the three compounds was detected after food intake. Whether the patient received the first dose in fasting or fed conditions, no period effects (P = 0.05) was recorded on the three measured compounds either on AUC or Cmax. The ratio (90% confidence interval) for the fed vs fasted conditions (Table 1) was 1.71–2.61 for AUC and 1.96–3.16 for Cmax of TCBZ-SO, the principal active compound. tmax values were moderately, but significantly longer after food for TCBZ. There was no significant difference in tmax for the two metabolites. Analysis of plasma concentration of TCBZ-SO indicated that the mean apparent t1/2±s.d. was 17.1±9.7 h in fasted and 11.2±4.1 h in fed conditions. For TCBZ-SO2 the values were 18.9±11.6 h (fasted) and 11.8±6.9 h (fed).
Table 1.
Triclabendazole and metabolites apparent mean (n = 20) pharmacokinetic parameters ±s.d. in plasma and the fed/fast ratio (geometric mean) of Cmax and AUC with 90% confidence interval for this ratio.
Efficacy
Prior to triclabendazole administration all patients had eggs of Fasciola hepatica in their stools as evidenced by a sedimentation concentration technique. Patients were kept in the hospital for 1 week and followed-up 1 and 3 months after start of therapy. All patients were judged cured at the end of the trial except one (No. 16). Prior to his enrolment he had resisted other fasciolicides. Finally he was cured with a second course of two postprandial doses of triclabendazole (10 mg kg−1 each).
Tolerability
No systemic intolerability was detected but several patients complained of right upper abdominal pain postdrug. Such episodes were relieved by oral spasmolytics.
Discussion
The rapid biotransformation of TCBZ in man and the previous findings in animals [9] provides evidence of presystemic biotransformation including first-pass metabolism of TCBZ. In the present study, the relative ratio of the metabolite levels (AUC and Cmax) vs those of the unchanged drug remained almost constant for the two administration conditions. As a consequence, the metabolism does not seem to be influenced by food.
The increased concentrations of TCBZ and its two metabolites after food intake is most likely due to increased gastro-intestinal absorption. TCBZ is a poorly water soluble compound at 37° C: 0.2 mg l−1 in pH 6.8 phosphate buffer and 32 mg l−1 in HCl, 0.1 mol l−1, pH 1. The partition coefficient (P) between octanol and water (P > 200) indicated that the drug was almost exclusively present in the organic phase. Therefore, greater dissolution of the drug within the gastric and intestinal contents under postprandial conditions is highly probable [10]. In addition, meals including fat are known to prolong gastric drug retention [10–12]. This increased retention favours drug absorption and by consequence the increase of the levels of the three compounds in plasma. This hypothesis is also supported by reports for the related drugs, albendazole and mebendazole, whose bioavailability is increased markedly by administration with a fatty meal [11, 12], as well as for the onchocercacidal drug amocarzine [13].
The terminal elimination half-life calculated from plasma concentrations of TCBZ-SO and TCBZ-SO2 was for most patients longer in the fasting state than after food intake. An explanation might be found in the very low solubility of TCBZ which would produce a slower absorption of the drug resulting in a slower formation of metabolites in the absence of a meal [10]. The slow metabolite formation could interfere with the elimination and produce a longer apparent t1/2 in fasted patients.
TCBZ-SO concentrations were much higher than those of the unchanged drug. Since this metabolite was found to produce in vitro a more potent effect on the motility of the parasites than TCBZ (in animal species where the drug is highly active, unchanged drug was rarely detected in plasma), it is likely that the activity of the drug occurs, largely, through this metabolite [1, 2, 9]. TCBZ-SO could therefore be used as a marker of drug bioavailability. Food results in an enhancement of systemic exposure to TCBZ-SO, and in clinical practice this leads to cure for patients with liver [4] or lung fluke [5, 6].
Tolerability to the treatment among the patients was good. Episodes of right upper abdominal pain recorded by several patients probably originates from the expulsion of dead or dying worm from the hepatobiliary into the intestinal system. Ultrasonographic observations in children from Bolivia and adults from Europe with fascioliasis and who had been treated with postprandial triclabendazole subtantiate this hypothesis (Poltera et al. personal communication).
In conclusion, the plasma concentrations of TCBZ, of its active metabolite TCBZ-SO and of the sulphone metabolite were increased about two fold after food intake. The administration of the drug with food is therefore recommended for improved systemic availability in patients with fascioliasis and paragonimiasis.
References
- 1.Bennett JL, Kohler P. Fasciola hepatica: Action in vitro of triclabendazole on immature and adult stages. Experimental Parasitology. 1987;63:49–57. doi: 10.1016/0014-4894(87)90077-4. [DOI] [PubMed] [Google Scholar]
- 2.Robinson CP. Triclabendazole. Drugs Today. 1985;21:227–233. [Google Scholar]
- 3.Chen MG, Mott KF. Progress in assessment of the morbidity due to Fasciola hepatica infection: A review of the recent literature. Trop Dis Bull. 1990;87:R1–R38. [Google Scholar]
- 4.Loutan L, Bouvier M, Rojanawisut B, et al. Single treatment of invasive Fascioliasis with triclabendazole. Lancet. 1989;8659:383. doi: 10.1016/s0140-6736(89)90557-6. [DOI] [PubMed] [Google Scholar]
- 5.Ripert C, Couprie B, Moyou R, Gaillard F, Appriou M, Tribouley-Duret J. Therapeutic effect of triclabendazole in patients with paragonimiasis in Cameroon: a pilot study. Transac Roy Soc Trop Med Hyg. 1992;86:417. doi: 10.1016/0035-9203(92)90247-a. [DOI] [PubMed] [Google Scholar]
- 6.Calvopiña M, Paredes W, Guderian RH, Poltera AA. Eficacia del triclabendazole en Paragonimiasis pulmonar humana refractaria a la emetina, bithionol y praziquantel. Parasitología al Dia. 1993;17:44–46. [Google Scholar]
- 7.Rouan MC, Le Duigou F, Campestrini J, Lecaillon JB, Godbillon J. Fast liquid chromatography for the determination of drugs in plasma and combination with liquid-solid extraction in a fully automated system. J Chromatogr. 1992;573:59–64. doi: 10.1016/0378-4347(92)80474-5. [DOI] [PubMed] [Google Scholar]
- 8.Bolton S. Pharmaceutical statistics. 2. New York: Marcel Dekker; 1990. chapter 11. [Google Scholar]
- 9.Hennessy DR, Lacey E, Steel JW, Prichard RK. The kinetics of triclabendazole disposition in sheep. J Vet Pharmacol Therap. 1987;10:64–72. doi: 10.1111/j.1365-2885.1987.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Toothaker RD, Welling PG. The effect of food on drug bioavailability. Ann Rev Pharmacol Toxicol. 1980;20:173–199. doi: 10.1146/annurev.pa.20.040180.001133. [DOI] [PubMed] [Google Scholar]
- 11.Lange H, Eggers R, Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34:315–317. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- 12.Welling PG. Influence of food and diet on gastrointestinal drug absorption. A review. J Pharmacokin Biopharm. 1977;5:291–334. doi: 10.1007/BF01061694. [DOI] [PubMed] [Google Scholar]
- 13.Lecaillon JB, Dubois JB, Soula G, Poltera AA. Influence of food on the pharmacokinetics of CGP 6140 after oral administration of a 1200 mg single dose to onchocerciasis patients. Br J Clin Pharmacol. 1990;30:629–633. doi: 10.1111/j.1365-2125.1990.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


