Abstract
Aims
To evaluate a possible positive association between tamoxifen treatment and the risk of developing idiopathic venous thromboembolism (VTE) in women with breast cancer in the absence of clinical risk factors for venous thromboembolism other than breast cancer itself.
Methods
Using information from the large UK-based General Practice Research Database, we identified, within a cohort of more than 10 000 women with breast cancer, all women who developed a first-time diagnosis of deep vein thrombosis or pulmonary embolism of uncertain cause between January 1, 1991 and December 31, 1996. In a case-control analysis, we compared their tamoxifen exposure experience prior to the thromboembolic event with that of a randomly selected group of control women with breast cancer who were matched to cases on age, year of the breast cancer diagnosis and calendar time.
Results
We identified 25 cases of idiopathic VTE and 172 controls, all of whom had breast cancer, but were otherwise free from other risk factors for VTE. Past tamoxifen exposure was not materially associated with an elevated risk of developing VTE, and we therefore combined never and past users as reference group. The relative risk estimate of VTE for current tamoxifen exposure, as compared with never and past use combined, was 7.1 (95% CI 1.5–33), adjusted for body mass index, smoking status and hysterectomy status. High body mass index was an independent predictor of VTE itself.
Conclusions
Our study provides evidence that current use of tamoxifen increases the risk of idiopathic venous thromboembolism.
Keywords: tamoxifen, idiopathic venous thromboembolism
Introduction
The oestrogen receptor antagonist tamoxifen is widely used as adjuvant therapy of breast cancer. Tamoxifen has well documented efficacy in increasing survival time and reducing the risk of recurrent as well as contralateral breast cancer [1, 2]. While tamoxifen is usually relatively well tolerated, it has been associated with a variety of adverse effects related to its actual pharmacological activity [3, 4]. While tamoxifen has antioestrogenic properties, which have been related to symptoms such as hot flushes, vaginal bleeding and pruritus vulvae, it also has partial oestrogen-agonistic effects at particular receptor subtypes. The oestrogenic activity may reduce the risk for ischemic heart disease [5, 6], but may also increase the risk for endometrial cancer [3, 4, 7, 8] and venous thromboembolism (VTE).
Several case-reports of women with breast cancer have been described who developed deep vein thrombosis or pulmonary embolism while being treated with tamoxifen [9–12], and there is growing evidence from randomized clinical trials that the incidence of VTE is indeed higher among tamoxifen exposed women than in the comparison groups, suggesting a possible causal role of tamoxifen [13–19]. However, those women who developed VTE were not necessarily free of cardiovascular risk factors, and they often received additional cancer treatment, such as chemo- or radiotherapy. This led us to conduct a case-control analysis in order to explore further the role of tamoxifen on the risk of developing idiopathic first-time VTE in a large cohort of women with breast cancer in the absence of predisposing risk factors other than breast cancer itself.
Methods
We conducted a case-control study in a base population of women with breast cancer, using the UK-based General Practice Research Database (GPRD). Over four million people in the UK are enrolled with selected general practitioners who use office computers provided by Value Added Medical Products and who have agreed to provide data for research purposes. General practitioners have been trained to record medical information in a standard manner and to supply it anonymously. Information about symptoms, diagnoses, referrals, hospitalizations as well as all drug prescriptions are recorded prospectively and on an ongoing basis by over 400 general practices. The database furthermore contains demographics of the patients as well as information with regard to smoking status, height, weight, hysterectomy status and more. Drug prescriptions are generated by the general practitioner directly with the computer, and it contains the name of the preparation, route of administration, dose, and number of tablets for each prescription. Upon request, anonymized copies of hospital discharge and referral letters are available for review to validate the diagnoses recorded in the computer record. A modification of the Oxford Medical Information System (OXMIS) classification is used to enter medical diagnoses. For the purpose of this study, OXMIS- codes have been mapped onto ICD-codes. The recorded information on drug exposure and on diagnoses in the GPRD has been validated and proved to be of high quality [20, 21], and there is particular experience for studies involving cancer patients [22] as well as subjects with VTE [23] using GPRD-data.
Base population and case selection
The base population consisted of women who had a computer-recorded diagnosis of breast cancer in or after 1980. Within this base population, we identified (by computer-recorded ICD-codes) the potential cases, which were women who were hospitalized for a first-time diagnosis of deep vein thrombosis or pulmonary embolism between January 1, 1991 and December 31, 1996. At the date of their VTE-diagnosis (subsequently referred to as ‘index date’), they had to have been 70 years of age or younger. Potential cases were excluded if they had—according to the computer record—any other malignancies besides breast cancer, a history of VTE or thrombophlebitis, stroke, angina pectoris, myocardial infarction, diabetes mellitus, chronic renal disease, hypertension, hyperlipidemia, intermittent claudication, systemic lupus erythematosus, epilepsy, connective tissue disorders or cystic fibrosis. We furthermore excluded all potential cases who underwent mastectomy, chemotherapy, radiotherapy, trauma (i.e. accident, bone fracture) or major surgery (i.e. abdominal surgery, hip replacement) within 6 months prior to the index date, who had recurrent or metastatic breast cancer, or who were in their terminal phase and died within 6 months after the index date (subjects who died from pulmonary embolism were included). Potential cases had to have been hospitalized and treated with warfarin for the VTE. We sent for hospital discharge letters in order to verify the computer-recorded diagnosis, and for death certificates for women who died from pulmonary embolism. The diagnosis of deep vein thrombosis had to be confirmed by venogram, ultrasound or Doppler test, and pulmonary embolism by ventilation perfusion scan or angiography. We also sent a questionnaire to the general practitioners in order to verify the computer-recorded date of the breast cancer, the index date and the idiopathic aetiology of the first-time VTE by verifying the absence of predisposing events. Computer-based patient profiles of both cases and controls were reviewed without knowledge of tamoxifen exposure.
Controls
For each case we randomly selected up to 10 control women with breast cancer, matched on age (within two years), duration of breast cancer (same year of breast cancer) and calendar year of VTE (same index date). Women were ineligible to be controls if they had—according to the computerized medical record—recurrent or metastatic breast cancer, died within 6 month after the index date, or underwent mastectomy, chemotherapy, radiotherapy, trauma, or major surgery within 6 months prior to the index date.
Exposure to tamoxifen
Tamoxifen exposure history was assessed from the computerized medical record (which gave identical information as the questionnaire filled out by the general practitioner for the cases). The computer recorded information contains the number of tablets per prescription, the dose and the specific instruction of the prescribing doctor on how many tablets to be taken per day. Women were classified as ‘current users’ (if the tablet supply covered the index date), ‘past users’ (last drug intake one or more days prior to the index date), or ‘never users’. The tamoxifen exposure duration among users was categorized into predefined categories of ‘less than 12 months’ and ‘12 months or more’. The tamoxifen dose (10, 20, 40 or 80 mg day−1) was also assessed from the computer record.
Analysis
We conducted a matched analysis by using conditional logistic regression models with the software program SAS, and we obtained relative risk estimates (odds ratios, OR) of developing VTE with regard to current and past use, using never users as reference group. The effect of exposure duration and dose was also assessed with conditional logistic regression models; these subanalyses were done by stratifying current tamoxifen users according to duration and dose, and by comparing these subgroups to never-users. For each case and control, the potential confounders body mass index [BMI] ( < 30, 30+ kg m−2, unknown), smoking status (never, ex, current, unknown), and hysterectomy status (yes, no) were assessed from the computer profiles and evaluated in univariate regression models. Since none of the cases was oophorectomized, used oral steroids or oral contraceptives, and only one case and four controls used oestrogen replacement therapy at the index date, these variables were not included in further analyses.
Results
The base population encompassed 10 011 women with a first-time diagnosis of breast cancer in or after 1980. The case series consisted of 25 confirmed idiopathic cases and 172 controls. Among the cases, 16 had deep vein thrombosis and 9 pulmonary embolism, and for 4 women the pulmonary embolism was fatal. The mean age of cases (57.5 years) and controls (58.2 years) at the index date was closely similar, and the mean duration of breast cancer (the time from the diagnosis of breast cancer up to the index date) was 4.1 years (range 1–12 years) for both cases and controls.
The cases arose from 258 women who developed, at the age of 70 years or younger, a first-time diagnosis of VTE between January 1, 1991 and December 31, 1996. Of these, we excluded 181 potential cases because they had—according to the computer record—metastatic and/or recurrent breast cancer at the index date, or mastectomy, chemotherapy, radiation, surgery or trauma within 6 months prior to the date of the diagnosis. As a result of additional information that we received from the GP, we excluded the remaining 52 cases due to the presence of other risk factors for VTE (n = 25), because the diagnosis of VTE was not confirmed (n = 12), or because we did not get medical records from the GP (n = 15).
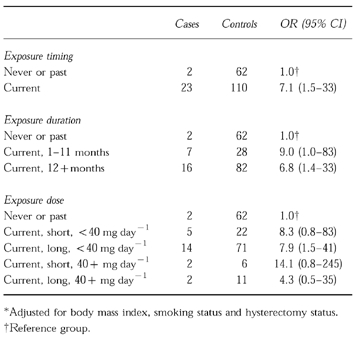
At the index date, 23 of 25 cases (92%) and 110 of 172 controls (64%) were current users of tamoxifen. One case and 16 controls used tamoxifen in the past, and one case and 46 controls were never users of tamoxifen. Since past tamoxifen use was not materially associated with an increased risk of developing VTE, we combined never and past users for further analyses. The relative risk estimate (OR) of developing idiopathic VTE for current tamoxifen use, as compared with never and past use combined, was 7.1 (95% CI 1.5–33) (Table 1), adjusted for body mass index, smoking status and hysterectomy status.
Table 1.
Tamoxifen exposure and relative risk estimates (OR) for venous thromboembolism*.
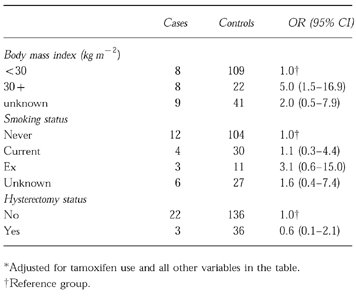
Body mass index was a strong independent risk factor for VTE. Women with a BMI> = 30 kg m−2, as compared with women below 30 kg m−2, were at a significantly increased risk of developing VTE, resulting in a relative risk estimate of 5.0 (95% CI 1.5–16.9), adjusted for tamoxifen exposure, smoking and hysterectomy status. We also assessed the independent effect of smoking (never, current, past, unknown) and hysterectomy (yes/no) status on the risk of developing VTE, but both these parameters were not significantly related to the risk of developing VTE in this group of women (Table 2).
Table 2.
Patient characteristics and relative risk estimates (OR) for venous thromboembolism*.
We also assessed whether the risk of developing VTE was related to the duration of the tamoxifen treatment and the daily tamoxifen dose used. The adjusted relative risk estimates for current use of 1–11 months and current use of 12 months or more were 9.0 (95% CI 1.0–83) and 6.8 (95% CI 1.4–33), respectively, as compared to never and past use combined (Table 1).
The relative risk estimates for current tamoxifen use of less than 40 mg day−1, and of 40 or more mg day−1 (who used the drug for less than a year), as compared with never and past use combined, were 8.3 (95% CI 0.8–83) and 14.1 (95% CI 0.8–245), respectively (Table 1).
Discussion
Our findings in this case-control analysis, based on 25 idiopathic cases and 172 controls from a base population of some 10 000 women with breast cancer, suggest that current use of tamoxifen is associated with an elevated risk of developing idiopathic VTE.
By restricting our analysis to idiopathic cases without prior history of thromboembolism, cardiovascular diseases, cancer (other than breast cancer), metastatic or recurrent cancer or cancer in the terminal phase, surgery, trauma and any treatment for breast cancer within 6 months prior to the index date (i.e. chemotherapy, radiotherapy or mastectomy), and by matching cases and controls on age and duration of breast cancer, we compared women who were closely similar with regard to risk factors for VTE other than tamoxifen exposure itself.
To our knowledge, this is the first formal observational study on tamoxifen-related VTE. A case-series of 18 women with thromboembolism among 441 tamoxifen-treated women [24], and several case-reports of women who developed deep vein thrombosis or pulmonary embolism while being treated with tamoxifen [9–12] have been reported. However, the interpretation of these reports is difficult due to lack of comparison groups and because most of these women had several other risk factors for venous thromboembolism, such as cardiovascular diseases, prior history of VTE, metastases or recent chemotherapy or radiation.
Increasing evidence supporting a causal association between tamoxifen and VTE comes from several large, controlled, randomized clinical trials. In the ‘National Surgical Adjuvant Breast and Bowel Project’ (NSABP), 1.7% of tamoxifen treated women (n = 1422) developed VTE, as compared to only 0.4% in the placebo-treated group (n = 1437) (19). The 10-year experience of 2673 women with breast cancer in the multicentre trial Eastern Cooperative Oncology Group (ECOG) was retrospectively analyzed and reported by Saphner et al. [15]. There was a significant excess of women who experienced VTE among those who received tamoxifen plus chemotherapy, as compared with those who received chemotherapy only or those who were observed only [15]. McDonald et al. also reported a statistically significant increase in the incidence of VTE for current tamoxifen users as compared with non-current users among 1312 women with breast cancer in the randomized Scottish Cancer Trials Breast Group [16]. Furthermore, two smaller trials also suggest that tamoxifen might increase the risk of developing VTE; Tormey et al., as part of the ECOG, randomized 194 women after 5 years of tamoxifen treatment either to continue tamoxifen treatment or to stop tamoxifen (observation only). There were two cases of VTE among the long-term users, and no case in the observation group [17]. In another randomized trial, Pemberton et al. reported that the tamoxifen treated group (n = 89) experienced significantly more VTE events (5.62%) than the untreated group (n = 60) where no VTE event occurred [25].
On the other hand, no association between tamoxifen use and VTE was found by Rutqvist et al. who retrospectively analyzed the experience of 2365 women involved in the Stockholm Breast Cancer Study Group. There was an almost equally distributed number of women with VTE events in the tamoxifen group (40 mg day−1) and in the non-tamoxifen treated group, leading to a RR of 1.06 (95% CI 0.71–1.60) [6].
Oestrogens increase the risk of developing VTE (26–29), and tamoxifen has well-documented oestrogen-agonistic properties. The mechanism of action with regard to changes in coagulation parameters which could potentially explain venous thrombogenic activity of tamoxifen are not completely understood, but tamoxifen tends to decrease antithrombin III and protein C levels in the blood [25, 30–33] which might partially explain venous thrombogenic activity of tamoxifen.
In our study, confounding might have occurred to some degree if cases were much sicker than controls, although only under the assumptions that the clinical condition of patients was both associated with the risk of developing VTE and the likelihood of getting tamoxifen treatment. However, it is a strength of our study that it was restricted to relatively healthy subjects without recurrent cancer or metastases and without known risk factors for VTE. We furthermore matched controls to cases closely on age, duration of breast cancer and calendar time in order to control for potential confounding by these parameters. In addition, information with regard to the clinical condition of cases, which was available to us from the questionnaires that we sent to the GPs, indicated that none of the cases was in poor health (partially or completely bedridden) at the index date (information unavailable for four cases who died from pulmonary embolism). This makes the above mentioned proposition of residual confounding by severity of the disease a rather unlikely explanation for our findings.
A potential bias could have arisen since we did not have exactly the same amount of information for cases and controls; we sent out a questionnaire to the GPs and reviewed hospital discharge letters for 77 potential cases, but not for controls. Based on this additional information, 25 of 77 cases (32.5%) were excluded because they had other risk factors for VTE (such as recent chemotherapy, surgery, metastases) which have not been documented in the computer record. Therefore—at least in theory—up to a third of the 172 controls in the final analysis might also have had additional risk factors for VTE, but we were unaware of that because we did not have detailed information from the GPs for controls. This might have introduced a potential bias, but only under the condition that these potentially ineligible controls had a very low tamoxifen exposure prevalence which drove the exposure prevalence for the entire set of 172 controls down to 64%. Since the exposure prevalence in the 25 cases that we eliminated due to additional risk factors was similarly high (80%) as in the 25 idiopathic cases which we included in the final analysis (92%), it seems unlikely that the tamoxifen exposure prevalence in the potentially ineligible controls differed substantially from the 64% exposure prevalence in the entire set of 172 controls which we included in the final analysis.
A limitation of our study is the fact that we could not control for breast cancer histology and/or oestrogen-receptor status. In theory, these parameters might be associated both with the outcome and the exposure and could therefore confound the results to some extent, although this seems to be an unlikely alternative explanation for the observed finding.
In conclusion, our results suggest that current use of tamoxifen increases the risk of developing VTE in women with breast cancer who are free of other predisposing diseases and known risk factors for VTE.
Acknowledgments
The Boston Collaborative Drug Surveillance Program is supported in part by grants from: Astra AB, Berlex Laboratories, Bayer AG, Glaxo Wellcome Inc., Hoffmann-La Roche, RW Johnson Pharmaceutical Research Institute, Novartis Pharmaceuticals and Pfizer Inc. Christoph Meier is supported in part by a grant from the Swiss National Science Foundation; Freiwillige Akademische Gesellschaft; Basel; and Holderbank Stiftung zur Foerderung der Wissenschaftlichen Fortbildung, Switzerland.
We thank the participating general practitioners for their excellent cooperation and Dr Alan Dean and his team for their generous help. We also wish to thank Professor Alexander M. Walker, Chair, Department of Epidemiology, Harvard School of Public Health, for his helpful comments and suggestions on the manuscript.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. N Engl J Med. 1988;319:1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. Lancet. 1992;339:1–15–71–85. [PubMed] [Google Scholar]
- 3.Catherino WH, Craig Jordan V. A risk-benefit assessment of tamoxifen therapy. Drug Safety. 1993;8:381–397. doi: 10.2165/00002018-199308050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Love RR. Tamoxifen therapy in primary breast cancer: biology, efficacy, and side effects. J Clin Oncol. 1989;7:803–815. doi: 10.1200/JCO.1989.7.6.803. [DOI] [PubMed] [Google Scholar]
- 5.McDonald CC Stewart HJ for the Scottish Breast Cancer Committee. Br Med J. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutqvist LE, Mattsson A. for the Stockholm Breast Cancer Study Group. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. J Natl Cancer Inst. 1993;85:1398–1406. doi: 10.1093/jnci/85.17.1398. [DOI] [PubMed] [Google Scholar]
- 7.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen FE, Benraadt J, Coebergh JWW, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 9.Cutuli BF, Tritsch L, Jung GM, Mantz JM. Embolie pulmonaire mortelle chez une malade en cours de traitement adjuvant par tamoxifene. La Presse Medicale. 1991;20:1948–1949. [PubMed] [Google Scholar]
- 10.Nevasaari K, Heikkinen M, Taskinen PJ. Tamoxifen and thrombosis. Lancet. 1978;i:946–947. doi: 10.1016/s0140-6736(78)91668-9. [DOI] [PubMed] [Google Scholar]
- 11.Hendrick A, Subramanian VP. Tamoxifen and thromboembolism. JAMA. 1980;243:514–515. [PubMed] [Google Scholar]
- 12.Wilson CB, Lambert HE, Scott RD. Subclavian and axillary vein thrombosis following radiotherapy for carcinoma of the breast. Clin Radiol. 1987;38:95–96. doi: 10.1016/s0009-9260(87)80425-7. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Redmond C, Wickerham DL, et al. Doxorubicin-containing regimens for the treatment of stage II breast cancer: The National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 1989;7:572–582. doi: 10.1200/JCO.1989.7.5.572. [DOI] [PubMed] [Google Scholar]
- 15.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 16.McDonald CC, Alexander FE, Whyte BW, Forrest AP Stewart HJ for the Scottish Cancer Trials Breast Group. Br Med J. 1995;311:977–980. doi: 10.1136/bmj.311.7011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. J Natl Cancer Inst. 1996;88:1828–1833. doi: 10.1093/jnci/88.24.1828. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard KI, Paterson AHG, Paul NA, Zee B, Fine S, Pater J for the National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol. 1996;14:2731–2737. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 20.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerized data resource in the United Kingdom. Pharmacoepidemiology and Drug Safety. 1992;1:347–349. [Google Scholar]
- 22.Jick H, Jick S, Derby LE, Vasilakis C, Wald Myers M, Meier CR. Calcium-channel blockers and risk of cancer. Lancet. 1997;349:525–528. doi: 10.1016/S0140-6736(97)80084-0. [DOI] [PubMed] [Google Scholar]
- 23.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet. 1995;346:1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 24.Cutuli B, Petit JC, Fricker JP, Schumacher C, Velten M, Abecassis J. Accidents thromboemboliques chez les patientes menopausees sous traitement adjuvant par tamoxifene. Frequence, facteurs de risque et possibilites de prevention. Bull Cancer. 1995;82:51–56. [PubMed] [Google Scholar]
- 25.Pemberton KD, Melissari E, Kakkar VV. The influence of tamoxifen in vivo on the main natural anticoagulants and fibrinolysis. Blood Coagulation & Fibrinolysis. 1993;4:935–942. [PubMed] [Google Scholar]
- 26.Daly E, Vessey MP, Hawkins MM, Carlson JL, Gough P, Marsh S. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977–980. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 27.Jick H, Derby LE, Myers MW, Vasilakis C, Newton KM. The risk of hospitalization for idiopathic venous thromboembolism among users of postmenopausal estrogens. Lancet. 1996;348:981–983. doi: 10.1016/S0140-6736(96)07114-0. [DOI] [PubMed] [Google Scholar]
- 28.Grodstein F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet. 1996;348:983–987. doi: 10.1016/S0140-6736(96)07308-4. [DOI] [PubMed] [Google Scholar]
- 29.Pérez Gutthann S, Garca Rodrguez LA, Castellsague Pique J, Duque Oliart A. Hormone replacement therapy and risk of venous thromboembolism: population based case-control study. Br Med J. 1997;314:796–800. doi: 10.1136/bmj.314.7083.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enck RE, Rios CN. Tamoxifen treatment of metastatic breast cancer and antithrombin III levels. Cancer. 1984;53:2607–2609. doi: 10.1002/1097-0142(19840615)53:12<2607::aid-cncr2820531206>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Auger MJ, Mackie MJ. Effects of tamoxifen on blood coagulation. Cancer. 1988;61:1316–1319. doi: 10.1002/1097-0142(19880401)61:7<1316::aid-cncr2820610707>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Love RR, Surawicz TS, Williams EC. Antithrombin III level, fibrinogen level, and platelet count changes with adjuvant tamoxifen therapy. Arch Intern Med. 1992;152:317–320. [PubMed] [Google Scholar]
- 33.Mannucci PM, Bettega D, Chantarangkul V, Tripodi A, Sacchini V, Veronesi U. Effect of tamoxifen on measurements of hemostasis in healthy women. Arch Intern Med. 1996;156:1806–1810. [PubMed] [Google Scholar]


