Abstract
Citrobacter rodentium, an attaching-effacing bacterial pathogen, establishes an acute infection of the murine colonic epithelium and induces a mild colitis in immunocompetent mice. This study describes the role of T-cell subsets and B lymphocytes in immunity to C. rodentium. C57Bl/6 mice orally infected with C. rodentium resolved infection within 3 to 4 weeks. Conversely, systemic and colonic tissues of RAG1−/− mice orally infected with C. rodentium contained high and sustained pathogen loads, and in the colon this resulted in a severe colitis. C57Bl/6 mice depleted of CD4+ T cells, but not CD8+ T cells, were highly susceptible to infection and also developed severe colitis. Mice depleted of CD4+ T cells also had diminished immunoglobulin G (IgG) and IgA antibody responses to two C. rodentium virulence-associated determinants, i.e., EspA and intimin, despite having a massively increased pathogen burden. Mice with an intact T-cell compartment, but lacking B cells (μMT mice), were highly susceptible to C. rodentium infection. Systemic immunity, but not mucosal immunity, could be restored by adoptive transfer of convalescent immune sera to infected μMT mice. Adoptive transfer of immune B cells, but not naïve B cells, provided highly variable immunity to recipient μMT mice. The results suggest that B-cell-mediated immune responses are central to resolution of a C. rodentium infection but that the mechanism through which this occurs requires further investigation. These data are relevant to understanding immunity to enteric attaching and effacing bacterial pathogens of humans.
Many enteric bacterial pathogens (e.g., Listeria monocytogenes, Salmonella enterica serovar Typhi, and Shigella flexneri) establish infection of systemic and mucosal tissues by actively invading the host through the gastrointestinal epithelia. In doing so, they are subjected to a barrage of relatively well-characterized innate and acquired host-immune effectors (9, 10, 23). Other enteric pathogens, like the diarrheal agents enterotoxigenic Escherichia coli (ETEC), enteropathogenic E. coli (EPEC), and enterohemorrhagic E. coli (EHEC), colonize the lumenal side of the gastrointestinal epithelium. To combat bacterial colonization at this site, the host presumably requires a different set of immune defense mechanisms in order to achieve pathogen eradication. The immunological events associated with eradication of pathogens that employ attaching and effacing (A/E) lesion formation have been partly addressed (8, 13, 14, 28, 31, 32), yet much more remains to be learned, particularly on the role of B- and T-cell interactions and their role in immunity.
Human-specific EPEC and EHEC strains are members of a family of noninvasive enteric bacterial pathogens that share conserved virulence mechanisms. A characteristic of members of this family of pathogens is their ability to actively subvert intestinal epithelial cell function to produce a histopathological feature known as the A/E lesion. E. coli capable of forming A/E lesions have been recovered from diseased cattle (6), rabbits (15), pigs (1), and dogs and cats (5). In mice, the natural mouse pathogen Citrobacter rodentium colonizes large-intestinal enterocytes via A/E lesion formation and causes acute colitis (3). Two bacterium-expressed, surface-associated proteins, intimin and EspA, are, among others, essential for A/E lesion formation (33).
Infection of mice with C. rodentium has been used as a surrogate model for studying host responses to pathogens dependent upon A/E lesion formation for colonization of the host (14, 28, 32). Importantly, C. rodentium possesses both established and putative virulence determinants common to human-specific strains of EPEC and EHEC, including a large pathogenicity island (7) and an immunomodulating toxin that is distinct from Shiga-like toxins (17). Furthermore, the A/E lesion induced by C. rodentium is ultrastructurally indistinguishable from those formed by EHEC and EPEC in animals and humans (26). In naturally or experimentally infected mice, C. rodentium infection is associated with colonic crypt hyperplasia, goblet cell depletion, and mucosal erosion (2, 16). Many of these pathological features also occur in other genetic or immunologically driven mouse models of colitis. In most of these models, CD4+ T cells are critical mediators of the pathological changes that occur in the colonic mucosa (29).
The aim of this study was to elucidate the role of T-cell subsets and B cells in the development of immunity to C. rodentium and in the colitis that occurs during infection. The results suggested that CD4+ T cells and B cells are essential for resolution of a primary C. rodentium infection.
MATERIALS AND METHODS
Mice.
Female or male 6- to 8-week-old C57Bl/6J mice were purchased from Harlan Olac (Bichester, United Kingdom) or Bantin & Kingman (Hull, United Kingdom). μMT mice (backcrossed to C57Bl/6 background at least 10 times) were originally purchased from Jackson Laboratories and were maintained by homozygous matings under contract at B&K Universal (Hull, United Kingdom). RAG1−/− mice were originally purchased from Jackson Laboratories and were bred under barrier containment facilities by homozygous mating. All mice used in these studies came from colonies which were specific pathogen free. Sentinel animals were screened for common murine pathogens every 2 months. All animals were housed in individually HEPA-filtered cages with sterile bedding and free access to sterilized food and water. The absence of B cells in μMT mice and B and T cells in RAG1−/− mice was verified by flow cytometry using fluorochrome-conjugated antibodies to CD3 and B220 (Becton Dickinson, Cowley, United Kingdom). All mice were between 6 and 12 weeks old when first infected with C. rodentium. All animal procedures were approved under the United Kingdom Animal (Scientific) Procedures Act.
Bacterial strains and oral infection of mice.
The C. rodentium strain DBS100 used in this study was originally supplied by D. Schauer and has been described previously (27). Bacterial inoculums were prepared by culturing bacteria overnight at 37°C in 100 ml of L broth containing nalidixic acid (100 μg/ml). Cultures were harvested by centrifugation and resuspended in a 1/10 volume of phosphate-buffered saline (PBS). Mice were orally inoculated, without anesthetic, using a gavage needle with 200 μl of the bacterial suspension. The viable count of the inocula was determined by retrospective plating on L agar containing nalidixic acid (100 μg/ml).
Antibodies and in vivo T-cell depletion.
The anti-CD4 and anti-CD8 monoclonal antibodies (MAbs) used in T-cell depletion experiments were derived from the clones GK1.5 and YTS169, respectively. In depletion experiments, C57B/6 mice were administered 100 μl of ascites fluid (diluted in 500 μl of PBS) containing either GK1.5 or YTS169 intraperitoneally 3 days prior to oral infection with between 1 × 109 and 5 × 109 CFU of C. rodentium. Mice were administered 100 μl of ascites fluid again on days 4, 11s and 18. The efficiency of depletion in infected mice (n = 3 per time point) was monitored by weekly flow-cytometric analysis of single-cell suspensions of spleens and mesenteric lymph nodes (MLNs) using the noncompeting anti-CD4 MAb RM4-4 (Pharmingen, Oxford, United Kingdom) and the anti-CD8 MAb 53-6.7 (Becton Dickinson, United Kingdom). At all time points monitored, the efficiency of CD4 or CD8 depletion was greater than 92%.
Recombinant proteins.
EspA was cloned from EPEC strain E2348/69, expressed as a His-tagged fusion protein in E. coli, and purified by nickel affinity chromatography as previously described (18). Similarly, the C-terminal 280 amino acids of intimin (Int280β) from a 0114:H2 EPEC strain (expresses β-intimin) were purified by nickel affinity chromatography as previously described (28).
Measurement of pathogen burden.
At selected time points postinfection, mice were killed by cardiac exsanguination under terminal anesthesia or by cervical dislocation. The terminal 6 cm of colon was removed, and the colon was weighed after removal of fecal pellets. Spleens, livers, and the remaining colon were removed and homogenized mechanically using a Seward 80 stomacher (Seward Medical, London, England). The number of viable bacteria in organ homogenates was determined by viable count on L agar containing naladixic acid.
Analysis of humoral immune responses.
At selected times postimmunization, 0.2 ml of blood was collected from the tail vein. The serum was collected and stored at −20°C until analyzed. For analysis of antigen-specific antibody responses, wells of microtiter plates (Maxisorb, Nunc, Denmark) were coated overnight at 4°C with 100 μl of a bicarbonate solution (pH 9.6) containing recombinant EspA (1.5 μg/ml) or Int280β (1 μg/m). After washing with PBS-Tween 20, wells were blocked by addition of 1.5% (wt/vol) bovine serum albumin (BSA) in PBS for 1 h. Plates were then washed twice with PBS-Tween 20 before sera from individual mice were added and serially diluted in PBS-Tween 20 containing 0.2% (wt/vol) BSA and incubated for 2 h at 37°C. For the determination of immunoglobulin A (IgA) or IgG antibody titers, wells were washed with PBS-Tween 20 before addition of 100 μl of either an IgG- or IgA-specific horseradish peroxidase (HRP) conjugate (Dako, Buckinghamshire, United Kingdom) diluted 1/1,000 in PBS-Tween 20 containing 0.2% (wt/vol) BSA for 2 h at 37°C. Finally, after washing with PBS-Tween 20, bound antibody was detected by addition of o-phenylenediamine substrate (Sigma) and the A490 was measured. Titers were determined arbitrarily as the reciprocal of the serum dilution corresponding to an optical density of 0.3. The minimum detectable titer was 100.
Determination of total and EspA-specific IgA in fecal samples.
Fecal pellets were collected from mice infected with C. rodentium 28 days previously, weighed, and then added (100 mg/ml) to sterile PBS containing 0.1% sodium azide. The pellets were homogenized by continuous shaking for 10 min on a vortex. Particulate debris was removed by centrifugation for 5 min at 15,000 × g, and supernatants were collected and stored frozen at −70°C. Total IgA levels in fecal extracts were determined by enzyme-linked immunosorbent assay. Briefly, levels of total IgA were measured by coating 96-well enzyme-linked immunosorbent assay plates with 2 μg of goat anti-mouse α-chain-specific antibodies (Sigma, Poole, United Kingdom)/ml. The plates were then blocked with 1.5% BSA in PBS for 1 h at 37°C. Dilutions of stool supernatants were added to the wells, incubated for 2 h at 37°C, and washed, and then a 1/1,000 dilution of an IgA-specific HRP-conjugated (Dako) antibody was added to each well. Bound antibody was detected by addition of o-phenylenediamine substrate (Sigma), and the A490 was measured. Total IgA in each sample was determined by comparison with a standard curve generated using mouse myeloma IgA (ICN). Titers of EspA-specific IgA were determined using the IgA-specific HRP-conjugated (Dako) antibody as described above. The EspA-specific IgA titers were determined arbitrarily as the reciprocal of the sample dilution corresponding to an optical density of 0.3. The EspA-specific IgA titers thus obtained were divided by the total amount of IgA in the sample to correct for mouse-to-mouse variation and also for the amount of stool collected.
Histology.
In some experiments the terminal 0.5 cm of colon was removed into 10% (vol/vol) buffered formalin for subsequent histological analysis. Transverse, paraffin-embedded sections were prepared and stained with hematoxylin and eosin. The biopsies were assessed by a pathologist blinded to the experimental groups from which the animals came.
Transfer of immune sera.
Convalescent-phase sera used in transfer experiments were derived by cardiac exsanguination of mice that had been orally challenged twice with 109 CFU of C. rodentium 1 month apart. Sera were collected 14 days after the last challenge infection. Normal mouse sera used in transfer experiments were obtained by cardiac exsanguination of naïve C57Bl/6 mice. To establish infection in μMT mice, groups of six animals were orally infected with 109 CFU of C. rodentium. Infected μMT mice received 0.5-ml intraperitoneal injections of PBS or sera (normal mouse sera or convalescent-phase sera) on days 10, 14, 17, and 21 postinfection, and the pathogen burden in spleen, liver, and distal colon was determined on day 24.
Adoptive transfer of B cells.
Single-cell suspensions were prepared from spleen and MLNs harvested from naïve C57Bl/6 mice and in parallel, from C57Bl/6 mice that had been infected with C. rodentium 5 weeks previously. A FACSVantage SE flow cytometer was used to sort CD19+ lymphocytes. Purity and viability was 97 to 98%. Sorted B cells (5 × 106) from naive or convalescent mice were then injected intravenously into separate groups of naive μMT mice, which were orally infected 24 h later with 109 CFU of C. rodentium. Control μMT mice received PBS instead of purified B cells. Mice were killed 5 weeks postinfection, and the pathogen burden was measured in various tissues.
Statistics.
The unpaired Student t test was used to compare normally distributed values from groups of mice. The nonparametric Mann-Whitney test was used to compare non-normally distributed values.
RESULTS
RAG1−/− mice are highly susceptible to C. rodentium infection.
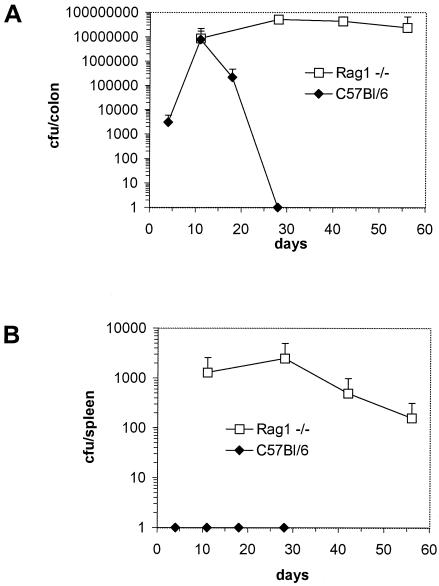
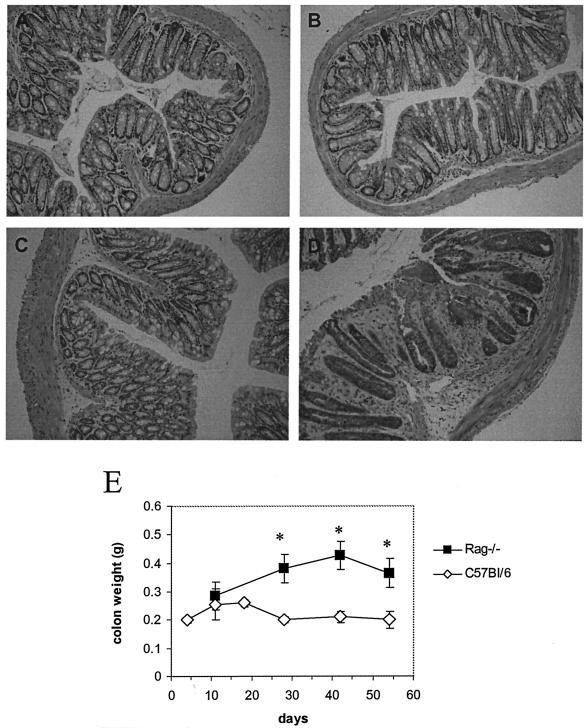
The contribution of T and B cells to the development of immunity and mucosal injury during infection with C. rodentium, or indeed any other A/E bacterial pathogen, has not been systematically described. To address this, RAG1−/− mice and control C57Bl/6 mice were orally infected with 2 × 109 CFU of C. rodentium. Mucosal and systemic tissues were collected from infected mice at different times postinfection, and the size of the pathogen burden was determined by viable count. RAG1−/− mice had significantly greater pathogen burdens in colonic and systemic tissue (spleen) than C57Bl/6 animals (Fig. 1A and B). Unlike C57Bl/6 mice, which eradicated infection within 4 weeks, RAG1−/− mice were chronically infected at least till day 56. In addition to the increased pathogen burden, approximately 25% of RAG1−/− mice also developed diarrhea during the course of infection. Although mortality among RAG1−/− mice did not exceed 5%, experiments were not continued beyond 8 weeks, since 10 to 15% of animals had prolapsed rectums. Pathological changes in the colonic mucosa of infected RAG1−/− mice were grossly and histologically more severe than in infected C57Bl/6 mice (Fig. 2A to D). The submucosa and lamina propria of RAG1−/− mice infected 28 days previously contained a patchy focal polymorphonuclear infiltrate with coincident crypt hyperplasia. In contrast, on day 28 postinfection there was little evidence of inflammation in the mucosa of C57Bl/6 mice. The distal colons of infected RAG1−/− mice were visibly thickened and weighed significantly more than colons of infected C57Bl/6 mice 4 weeks after infection and thereafter (Fig. 2E).
FIG. 1.
Viable counts of C. rodentium recovered from colons and spleens of orally infected C57Bl/6 and RAG1−/− mice. The data depict the mean number (± standard deviation) of C. rodentium CFU recovered from colons (A) and spleens (B) of RAG1−/− and C57Bl/6 mice (n = five to six per time point) orally infected with 2 × 109 CFU of C. rodentium. C. rodentium could not be recovered from C57Bl/6 mice beyond 28 days postinfection. The data shown are from one of two experiments performed which gave identical results.
FIG. 2.
Histology and weights of colons from C57Bl/6 and RAG1−/− mice infected 28 days previously. The panels (hematoxylin and eosin-stained sections; original magnification, ×200) show representative stained colonic sections from uninfected C57Bl/6 mice (A), infected C57Bl/6 mice (B), uninfected RAG1−/− mice (C), and infected RAG1−/− mice (D). Colonic crypts in infected RAG1−/− mice were elongated and irregular. The lamina propria and submucosa also contained a focal polymorphonuclear infiltrate. (E) The distal colon of infected RAG1−/− mice weighed significantly more than the colons of infected C57Bl/6 mice at different times during infection (asterisk, P < 0.05).
CD4+-T-cell-depleted mice are highly susceptible to C. rodentium infection.
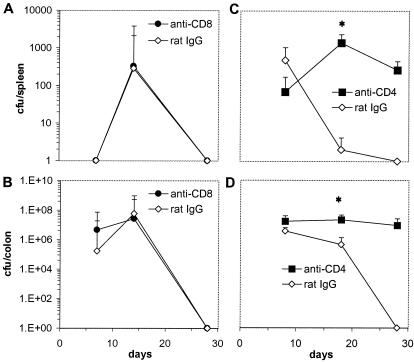
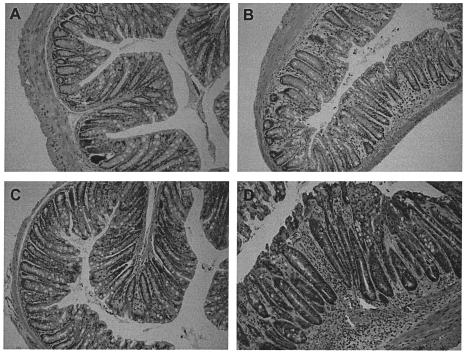
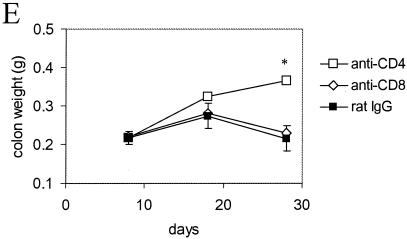
To help discriminate between the relative contributions of B and T cells in immunity to C. rodentium, C57Bl/6 mice were depleted of CD4+ or CD8+ T cells using MAbs GK1.5 (rat anti-mouse CD4) or YTS169 (rat anti-mouse CD8) just prior to and during a primary infection with 109 CFU of C. rodentium. Control mice received an equivalent intraperitoneal dose of purified rat IgG. The affect of T-cell depletion on immunity was assessed by bacterial culture of splenic and colonic homogenates following oral C. rodentium challenge. Mice depleted of CD8+ T cells were as competent as control mice in their ability to eradicate C. rodentium from mucosal and systemic tissue (Fig. 3A and B). In contrast, mice depleted of CD4+ T cells had dramatically higher pathogen burdens in mucosal and systemic tissue on days 18 and 28 postinfection (Fig. 3C and D). Although no CD4+-T-cell-depleted mice died during infection, the experiments were not continued beyond 28 days, since some mice had prolapsed rectums. Histological analysis of colonic tissue from infected mice depleted of CD4+ T cells showed edema in the submucosa and a mixed cellular infiltrate in the lamina propria and submucosa (Fig. 4). There was also substantial crypt hyperplasia and disruption of crypt organization. Macroscopically, the colons from CD4-depleted mice were grossly thickened and weighed significantly more than colons from either CD8-depleted or control mice (Fig. 4E). Collectively, these results imply an important role for CD4+ T cells, but not CD8+ T cells, in immunity to C. rodentium.
FIG. 3.
Viable counts of C. rodentium recovered from colons and spleens of mice depleted of CD4+ or CD8+ T cells. The data depict the mean number of C. rodentium CFU recovered from spleens (A) and colons (B) of C57Bl/6 mice (n = five per time point) infected orally with 109 CFU of C. rodentium and treated with the CD8+-T-cell-depleting MAb YTS169 or the control antibody, rat IgG. Results from a parallel experiment show the number of C. rodentium CFU recovered from the spleens (C) and colons (D) of C57Bl/6 mice treated with the CD4+-T-cell-depleting MAb GK1.5 or the control antibody, rat IgG. There were significantly more C. rodentium bacteria recovered from the spleen and colons of CD4+-T-cell-depleted mice on day 18 postinfection than from rat IgG-treated mice (asterisk, P < 0.05). On day 28 postinfection, no viable C. rodentium bacteria could be recovered from C57Bl/6 mice treated with rat IgG. The data shown are from one of two experiments performed which gave similar results.
FIG. 4.
Histology and weights of colons from infected mice depleted of CD4+ T cells. The panels (hematoxylin and eosin-stained sections; original magnification, ×200) show representative stained colonic sections from uninfected C57Bl/6 mice treated with rat IgG (A) or CD4-depleting MAb GK1.5 (C) for the previous 4 weeks. Sections from C. rodentium infected mice treated with rat IgG (B) or CD4-depleting MAb GK1.5 (D) for the previous 4 weeks are shown in comparison. There were focal inflammatory cell infiltrates, crypt hyperplasia, disruption of the crypt architecture, and evidence of vasculitis in the submucosa of infected CD4+-T-cell-depleted mice. (E) On day 28 postinfection, the distal colon of infected CD4+-T-cell-depleted mice also weighed significantly more (asterisk, P < 0.05) than the colons of infected CD8+-T-cell-depleted mice or rat IgG-treated control mice.
Mice depleted of CD4+ T cells mount weaker humoral immune responses to EspA.
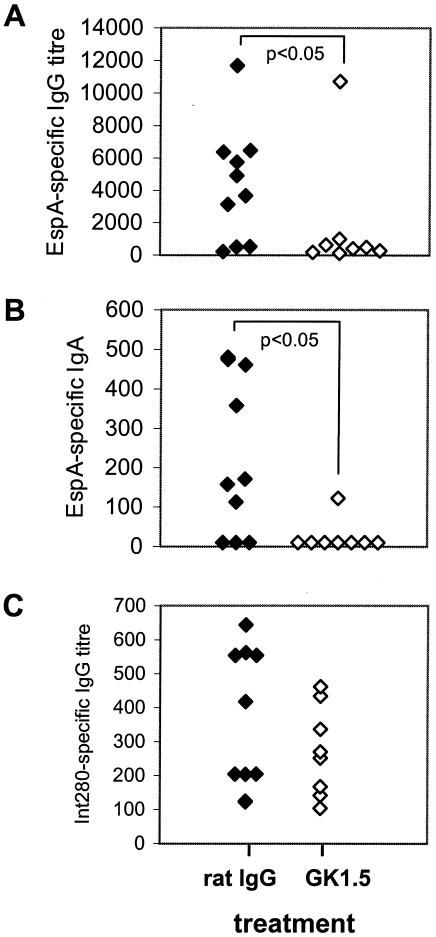
The previous data demonstrated a critical role for CD4+ T cells in immunity to C. rodentium. It remained unclear, however, whether this T-cell subset contributed to immunity via the secretion of cytokines, which activated antibacterial mechanisms in host cells, and/or via provision of “help” to B cells in the lymphoid follicles for anti-C. rodentium antibody production. Therefore, serum IgG and IgA antibody responses were measured against the C. rodentium surface-associated proteins Int280β and EspA 28 days postinfection to determine if the depletion of CD4+ T cells affected pathogen-specific antibody responses. Both antigens are the targets of antibody responses following C. rodentium infection of mice and are essential for the formation of A/E lesions (12). Mice depleted of CD4+ T cells had dramatically diminished IgA and IgG antibody responses to EspA compared to mice administered the control antibody (Fig. 5A and B). This observation was against the background of a substantially greater pathogen burden in mice depleted of CD4+ T cells. Mice infected with C. rodentium also mounted EspA-specific IgA responses that were detected in supernatants of fecal homogenates. However, fecal homogenate EspA-specific IgA titers were low, highly variable, and not significantly different between infected mice depleted of CD4+ T cells and those treated with the control antibody (data not shown). Serum IgG responses to Int280β were more variable than those observed against EspA and of a lower titer. Although there was a trend towards diminished IgG (Fig. 5C) antibody responses to intimin in CD4+-T-cell-depleted mice, these were not significantly lower than in control mice. Serum IgA titers to Int280β were absent or very low in both groups of mice and were not compared. Collectively, these data suggest that mice depleted of CD4+ T cells, despite having a substantially greater load of C. rodentium in host tissues, have an impaired ability to mount serum EspA-specific, and to a lesser extent intimin-specific, antibody responses. These data suggested that impaired B-cell responses to C. rodentium antigens might form a basis for the susceptibility of CD4+-T-cell-depleted mice. Therefore, the role of B cells in immunity to C. rodentium was examined.
FIG. 5.
Serum IgG and IgA responses to EspA and intimin in CD4+-T-cell-depleted and control mice. Shown are individual IgG (A) and IgA (B) antibody responses to EspA in serum for mice depleted of CD4+ T cells (administered GK1.5 MAb) or control mice (administered rat IgG). (C) Individual IgG serum antibody responses to the C-terminal 280-99 of intimin in the same mice are shown. IgG and IgA antibody responses to EspA were significantly lesser for mice administered GK1.5 than for control mice that received rat IgG (P < 0.05).
Role of B cells in immunity to C. rodentium.
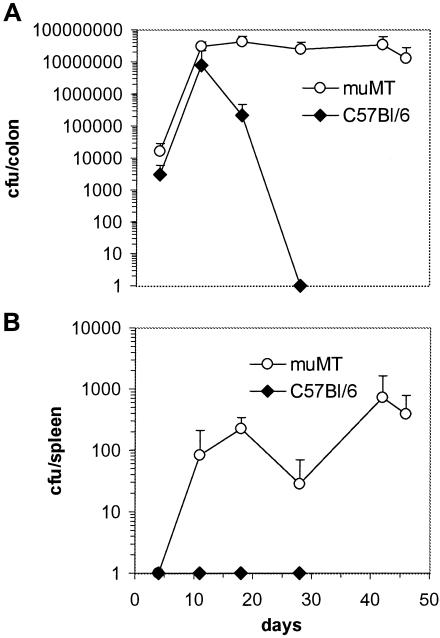
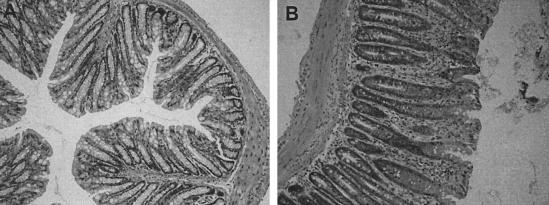
To determine if B cells contribute to immunity to C. rodentium, μMT mice (B cell deficient) and control C57Bl/6 mice were orally infected with 3 × 109 CFU of C. rodentium. Infected μMT mice had significantly higher pathogen burdens in mucosal and systemic tissues than C57Bl/6 controls on days 8 and 18 (Fig. 6). C57Bl/6 mice cleared C. rodentium from host tissues by day 28. In contrast, mucosal and systemic tissues of μMT mice remained infected at least until day 46. Although no μMT mice died during infection, the experiments in μMT mice were not continued beyond day 46, since ∼10% of mice had prolapsed rectums. Histological analysis of colonic tissue from infected μMT mice revealed focal, occasionally transmural cellular infiltrates plus crypt hyperplasia and disruption (Fig. 7).
FIG. 6.
Viable counts of C. rodentium recovered from colons and spleens of orally infected μMT and C57Bl/6 control mice. Mice were orally infected with 3 × 109 CFU of C. rodentium. The data depict the mean number (± standard deviation) of C. rodentium recovered from colons (A) and spleens (B) of muMT and C57Bl/6 mice. There were significantly more C. rodentium bacteria recovered from the colons of muMT mice on day 18 postinfection than with control C57Bl/6 mice (asterisk, P < 0.05). C. rodentium could not be recovered from C57Bl/6 mice beyond day 28. The data shown are from one of two experiments performed which gave similar results.
FIG. 7.
Histological changes in the colonic mucosa of muMT mice 28 days postinfection. Mice were orally infected with 3 × 109 CFU of C. rodentium, and 28 days postinfection, sections from the distal colon were examined histologically. The panels show colonic sections from uninfected (A) and infected (B) muMT mice (hematoxylin and eosin-stained sections; original magnification, ×200). There was an increase in lamina propria inflammatory cells and extensive crypt hyperplasia in infected muMT mice.
Aside from antibody production, B cells have a probable role in the maintenance of memory T-cell responses to pathogens (22). The impaired resistance of μMT mice to C. rodentium infection did not appear to be due to broadly deficient T-cell responses, however, since flow cytometry revealed as many CD4+ and CD8+ T cells with an activated phenotype (CD45RBlow) in the spleens and MLNs of infected μMT mice as in infected C57Bl/6 animals (data not shown). Collectively, the substantially increased susceptibility of B-cell-deficient mice to C. rodentium infection suggested a critical role for antibody in immunity to this agent.
Adoptive transfer of convalescent mouse sera only partially restores immunity to μMT mice infected with C. rodentium.
To assess if sera containing C. rodentium-specific antibody could complement the defective host response in μMT mice, pooled sera from mice that had resolved two challenge infections with C. rodentium were transferred into μMT mice that had been infected 10 days previously with 109 CFU of C. rodentium. In parallel, infected μMT mice received either PBS or normal mouse sera. Infected μMT mice received sera on days 10, 14, 17, and 21 postinfection, and the pathogen burden was determined on day 24. The adoptive transfer of immune mouse sera significantly reduced the number of C. rodentium that were recovered from systemic tissue (spleens) of infected μMT mice (Table 1). However, in colonic tissue, the affect of transferred immune sera on pathogen burden was not significant (Table 1).
TABLE 1.
Mean number of C. rodentium CFU (log 10) recovered from tissues of infected μMT mice treated with PBS, normal mouse sera, or convalescent-phase seraa
| Organ | Bacterial count with treatment
|
||
|---|---|---|---|
| PBS | NMSb | Convalescent sera | |
| Colon | 6.3 ± 0.4 | 6.8 ± 0.5 | 6.9 ± 0.9 |
| Spleen | 2.8 ± 0.8 | 2.4 ± 0.3 | 0.5 ± 0.7* |
| Liver | 2.7 ± 0.1 | 3.1 ± 0.1 | 0.9 ± 0.9* |
Mice (n = 6) were orally infected with 109 CFU of C. rodentium and received sera on days 10, 14, 17, and 21 postinfection. The number of bacteria in each organ was determined on day 24. Asterisk, P < 0.05 (student t test) compared to results for mice treated with PBS or NMS.
Normal mouse sera.
Adoptive transfer of convalescent B cells to μMT mice confers partial resistance to C. rodentium infection.
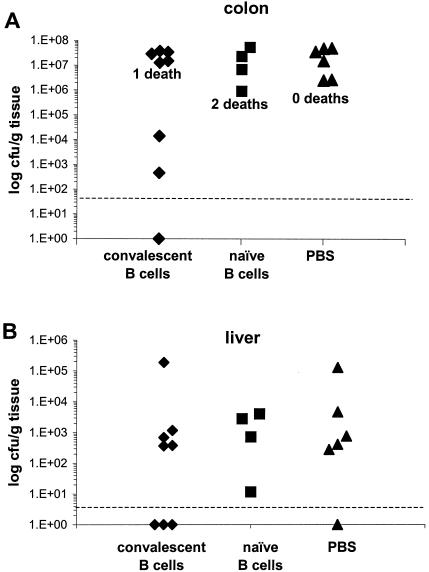
The previous experiment failed to show a significant effect of immune sera on the pathogen burden in the colons of μMT mice. To examine whether purified B cells could reconstitute mucosal immunity to C. rodentium in μMT mice, we adoptively transferred into μMT mice 5 × 106 B cells from naïve C57Bl/6 mice or 5 × 106 B cells from C57Bl/6 mice that had previously resolved a C. rodentium infection. Control μMT mice received PBS instead of purified B cells. One day later all mice were orally infected with C. rodentium. In the following weeks, there were three deaths among infected mice, two in the group that received naïve B cells (days 25 and 31) and one in the group that received convalescent B cells (day 15). All remaining mice were killed on day 35. Measurement of the pathogen burden in the colons and livers of mice revealed that three of eight surviving mice that received convalescent B cells had substantially lower colon counts than mice that received PBS or naive B cells (Fig. 8A). C. rodentium was also absent from the livers (Fig. 8B) and spleens (not shown) of these same three mice. The reduced pathogen burden in these three animals was accompanied by significantly reduced pathology in the colon compared to the rest of the mice in the group (mean colon weight, 0.21 ± 0.03 g versus 0.5 ± 0.06 g). Surprisingly, on day 35, flow cytometry failed to detect CD19+ B cells in the spleens or MLNs of any μMT mouse that received B cells prior to infection.
FIG. 8.
Viable counts of C. rodentium recovered from colons and livers of μMT mice that were recipients of B cells from either naïve or convalescent mice. Mice were intravenously administered 5 × 106 B cells and then 24 h later challenged with 109 CFU of C. rodentium. The data depict the number of C. rodentium recovered from the colons (A) and livers (B) of individual mice 35 days postinfection.
DISCUSSION
The mechanisms through which the host eradicates bacterial infections that are confined to the lumenal side of the gut epithelium are relatively poorly understood. In part, this has been due to the absence of robust, manipulable animal models of infection for enteric bacterial pathogens, such as Vibrio cholerae and pathogenic E. coli. To help overcome this, infection of mice by C. rodentium has been used as a surrogate model of infections caused by A/E EPEC. Members of our group and others have used this model to test vaccine candidates (12), characterize virulence determinants (24, 25), and identify components of the immune response that contribute to protection and infection-associated mucosal pathology (13, 14, 28, 32). This study dissects the relative contributions of T and B cells in the eradication of C. rodentium from the murine colonic mucosa. A critical role for B cells in immunity to primary infection represents a key finding of this study.
RAG1−/− mice are unable to perform V(D)J recombination of Ig and T-cell receptor genes and therefore lack mature T and B cells. In this study, RAG1−/− mice were highly susceptible to mucosal and systemic infection with C. rodentium, and this was associated with more-severe clinical signs, e.g., diarrhea. These results imply that acquired immune responses are essential for pathogen clearance and that innate immune responses in themselves are insufficient. The heightened susceptibility of RAG1−/− mice was also associated with greater colonic pathology as revealed by histology at day 28 and measurement of colon weight. Vallance et al. have previously described the susceptibility of RAG1−/− mice to mucosal C. rodentium infection (32). These authors observed significantly greater mortality in RAG1−/− mice (40 to 70% in first 3 weeks) than was observed in the present study (∼5% in 8 weeks) and found that on day 21, mucosal inflammation was less pronounced in RAG1−/− animals than in C57Bl/6 control mice. The contrasting pathology and mortality results in our two studies might be best ascribed to the timing of the sampling for histology, i.e., day 21 versus day 28, and to differences in the ages of the mice at infection; adult mice were used in this study, while 3- to 4-week-old mice were used in the study by Vallance et al. That infection of young mice results in higher mortality rates has been documented previously (2).
Mice systemically depleted of CD4+ T cells, but not CD8+ T cells, failed to eradicate C. rodentium from mucosal or systemic tissues, and the pathogen burden was as much as 107-fold greater than in control mice 4 weeks after infection. The susceptibility of CD4+-T-cell depleted mice, like that of RAG1−/− mice, was highlighted by the presence of significant numbers of viable C. rodentium bacteria in spleens, implying impaired host resistance in both mucosal and systemic compartments. Although we only determined the number of viable C. rodentium bacteria in spleens, it seems probable that other bacteria resident in the colon could also be translocated across the inflamed epithelium to internal tissues, thereby exacerbating inflammation. The enormous contrast in infection outcome between animals depleted of CD4+ or CD8+ T cells was surprising and suggested that the basis for susceptibility to infection in CD4+-T-cell-depleted animals was linked to a property uniquely associated with CD4+ T cells. This led to the hypothesis that inadequate CD4+-T-cell help for conventional (B2) B-cell responses to C. rodentium antigens formed the basis for the susceptibility of CD4+-T-cell-depleted mice. Subsequently, systemic antibody responses to an immunogenic and essential C. rodentium virulence determinant, EspA, were found to be significantly reduced in CD4+-depleted animals, despite their having a massively greater pathogen burden. Similarly, there was a trend, despite their enormously greater antigen exposure, for CD4+-T-cell-depleted mice to mount lower-level antibody responses to intimin than control mice, although this was not statistically different. The importance of immune responses to intimin is suggested from a previous study whereby vaccination of mice with the C-terminal portion of intimin afforded partial protection against C. rodentium infection (12). More generally, evidence supporting a role for antibody in immunity to A/E pathogens stems from the observation that disease severity in human subjects experiencing a secondary EPEC infection is inversely correlated with the level of prechallenge serum IgG specific for the lipopolysaccharide on the challenge strain (8). Furthermore, antibodies derived from serum and colostrum are functionally relevant and can limit adherence of EHEC or EPEC to cultured epithelial cells in vitro (11, 21).
The role of B cells in immunity to C. rodentium infection was tested using μMT mice. These animals are deficient in IgG+ and IgM+ B cells but have a small number of IgA+ B1 cells (20). μMT mice were extremely susceptible to C. rodentium colonization, a phenotype discernible as early as 1 week postinfection. The ability of adoptively transferred immune sera to restore systemic immunity, although not mucosal immunity, to infected μMT mice implies that B-cell-derived antibodies are the most significant effectors missing in systemic tissue of these animals. In this setting, transferred specific antibody might facilitate systemic immunity through opsonization of bacteria for complement-mediated lysis or phagocytosis by reticuloendothelial phagocytes. The reasons why adoptively transferred convalescent-phase sera did not significantly modulate mucosal infection in μMT mice are not clear. Conceivably this might be due to qualitative (e.g., antibody class and avidity) and quantitative (absolute amount) differences between the sera transferred and that which is produced in the tissues of infected immunocompetent mice. In further attempts at reconstituting immunity in μMT mice, we performed adoptive B-cell transfer experiments. The adoptive transfer of B cells from convalescent mice, but not naive mice, partially restored immunity to 3 of 8 μMT mice. The mechanism through which adoptive transfer achieved this is not immediately obvious, however, since CD19+ B cells were absent from the lymphoid tissues of μMT recipients at day 35 and we could not detect significant levels of Ig in sera of any B-cell recipient mice (data not shown). The inconsistent phenotype among mice that received immune B cells may be attributed to the difficulties, as has been described previously (30), associated with achieving B-cell reconstitution in μMT mice. Further studies will be required to explain the underlying mechanisms that lead to partial immunity in immune B-cell recipients and also the deletion of adoptively transferred B cells in μMT mice.
A possible explanation for the inability of transferred immune sera or B cells to fully restore mucosal immunity to infected μMT mice is that resistance to C. rodentium infection at the epithelium is actually independent of antibody. Instead, immunity may be dependent upon a population of CD4+ T cells that produce proinflammatory cytokines that activate effector mechanisms at the epithelium. If this hypothesis is correct, then these CD4+ T cells must be entirely defective in μMT mice but susceptible to depletion by the anti-CD4 GK1.5 MAb. This scenario is not implausible, since T-cell-associated immunological defects in μMT mice have been reported previously. Typically, these T-cell defects have been described in the context of systemic infection with intracellular pathogens and range from a reduced capacity to maintain long-term memory T-cell responses (4, 22) and to switch from a Th1 to a Th2 response following parasite infection (19). In disagreement with this T-cell hypothesis is the observation that mice deficient in the T-cell-expressed inflammatory cytokines gamma interferon and tumor necrosis factor or the T-cell-polarizing cytokine interleukin 12, although more susceptible, nevertheless resolve infection with C. rodentium with identical (tumor necrosis factor and gamma interferon) or only moderately delayed (interleukin 12) kinetics (13, 28). Coinciding with resolution of disease in these inflammatory cytokine-deficient animals is the development of robust antibody responses to C. rodentium (28). These data suggest that cytokine-mediated inflammatory responses at the mucosa can contribute to resolution of infection, but ultimately other effectors, of which antibodies are candidates, are critical for pathogen eradication. Further studies will be required to define the precise mechanisms through which CD4+ T cells and B cells contribute to immunity to C. rodentium.
This study is the first to describe the relative importance of T-cell subsets and B cells in immunity to the mucosal pathogen C. rodentium. A significant finding was the critical role of CD4+ T cells and B cells in immunity to primary infection. A better understanding of the mechanisms through which the host resolves infections with A/E pathogens should assist in the rational development of vaccines and therapeutics against these agents.
Acknowledgments
This work was supported by grants from The Wellcome Trust and EU.
Editor: B. B. Finlay
REFERENCES
- 1.An, H., J. M. Fairbrother, J. D. Dubreuil, and J. Harel. 1997. Cloning and characterization of the eae gene from a dog attaching and effacing Escherichia coli strain 4221. FEMS Microbiol. Lett. 148:239-245. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W. 1980. The microbiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 30:167-173. [PubMed] [Google Scholar]
- 3.Barthold, S. W., G. L. Coleman, R. O. Jacoby, E. M. Livestone, and A. M. Jonas. 1978. Transmissible murine colonic hyperplasia. Vet. Pathol. 15:223-236. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575-1583. [DOI] [PubMed] [Google Scholar]
- 5.Beutin, L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 30:285-298. [PubMed] [Google Scholar]
- 6.China, B., E. Jacquemin, A. C. Devrin, V. Pirson, and J. Mainil. 1999. Heterogeneity of the eae genes in attaching/effacing Escherichia coli from cattle: comparison with human strains. Res. Microbiol. 150:323-332. [DOI] [PubMed] [Google Scholar]
- 7.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., C. O. Tacket, G. Losonsky, G. Frankel, J. P. Nataro, G. Dougan, and M. M. Levine. 1998. Effect of prior experimental human enteropathogenic Escherichia coli infection on illness following homologous and heterologous rechallenge. Infect. Immun. 66:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425-431. [DOI] [PubMed] [Google Scholar]
- 10.Elson, C. O., Y. Cong, S. Brandwein, C. T. Weaver, R. P. McCabe, M. Mahler, J. P. Sundberg, and E. H. Leiter. 1998. Experimental models to study molecular mechanisms underlying intestinal inflammation. Ann. N. Y. Acad. Sci. 859:85-95. [DOI] [PubMed] [Google Scholar]
- 11.Gansheroff, L. J., M. R. Wachtel, and A. D. O'Brien. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 67:6409-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves, N. S., M. Ghaem-Maghami, G. Monteleone, G. Frankel, G. Dougan, D. J. Lewis, C. P. Simmons, and T. T. MacDonald. 2001. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect. Immun. 69:6651-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerse, A. E., K. G. Gicquelais, and J. B. Kaper. 1991. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect. Immun. 59:3869-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E., and S. W. Barthold. 1979. The ultrastructure of transmissible murine colonic hyperplasia. Am. J. Pathol. 97:291-313. [PMC free article] [PubMed] [Google Scholar]
- 17.Klapproth, J. M., I. C. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langhorne, J., C. Cross, E. Seixas, C. Li, and T. von der Weid. 1998. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. USA 95:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macpherson, A. J., A. Lamarre, K. McCoy, G. R. Harriman, B. Odermatt, G. Dougan, H. Hengartner, and R. M. Zinkernagel. 2001. IgA production without mu or delta chain expression in developing B cells. Nat. Immunol. 2:625-631. [DOI] [PubMed] [Google Scholar]
- 21.Manjarrez-Hernandez, H. A., S. Gavilanes-Parra, E. Chavez-Berrocal, A. Navarro-Ocana, and A. Cravioto. 2000. Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun. 68:5030-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastroeni, P., C. Simmons, R. Fowler, C. E. Hormaeche, and G. Dougan. 2000. Igh-6(−/−) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect. Immun. 68:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 24.Reece, S., C. P. Simmons, R. J. Fitzhenry, M. Batchelor, C. Hale, S. Matthews, A. D. Phillips, G. Dougan, and G. Frankel. 2002. Mutagenesis of conserved tryptophan residues within the receptor-binding domain of intimin: influence on binding activity and virulence. Microbiology 148:657-665. [DOI] [PubMed] [Google Scholar]
- 25.Reece, S., C. P. Simmons, R. J. Fitzhenry, S. Matthews, A. D. Phillips, G. Dougan, and G. Frankel. 2001. Site-directed mutagenesis of intimin alpha modulates intimin-mediated tissue tropism and host specificity. Mol. Microbiol. 40:86-98. [DOI] [PubMed] [Google Scholar]
- 26.Schauer, D. B., and S. Falkow. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schauer, D. B., B. A. Zabel, I. F. Pedraza, C. M. O'Hara, A. G. Steigerwalt, and D. J. Brenner. 1995. Genetic and biochemical characterization of Citrobacter rodentium sp. nov. J. Clin. Microbiol. 33:2064-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-γ. J. Immunol. 168:1804-1812. [DOI] [PubMed] [Google Scholar]
- 29.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L. A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190-200. [DOI] [PubMed] [Google Scholar]
- 30.Smelt, S. C., S. E. Cotterell, C. R. Engwerda, and P. M. Kaye. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 164:3681-3688. [DOI] [PubMed] [Google Scholar]
- 31.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]