Abstract
A 25-kDa cryptococcal deacetylase (d25) was found here to induce cell proliferation, as well as secretion of interleukin 2 and gamma interferon, but not interleukin 4, in spleen cells from d25-immunized or Cryptococcus neoformans-infected mice. The gamma interferon, but not the interleukin 2, response was required for the protective activities of d25 immunization in a murine cryptococcosis model.
The encapsulated fungus Cryptococcus (Filobasidiella) neoformans is an opportunistic pathogen that often infects patients with immune defects, although cryptococcosis has been reported also in individuals with an intact immune system (12, 22). Cryptococcal infections are particularly frequent in AIDS patients, often requiring lifelong suppressive therapy to prevent a relapse (19). Nevertheless, conventional antifungal therapy has toxic side effects, and survivors may progress to fatal disseminated disease despite long-term treatment (24). These observations indicate the need to develop alternative strategies to control cryptococcosis, including active, passive, or adoptive immunotherapy. Type 1 cytokines, such as interleukin 12 (IL-12) and gamma interferon (IFN-γ), are necessary for the development of immune-protective responses (4, 7, 20). In contrast, type 2 cytokines, such as interleukin 4 (IL-4) and interleukin 5, are not protective and may be responsible for destructive lung pathology (11, 13). While many studies have focused on capsular glucuronoxylomannan, the main virulence factor of C. neoformans, little is known of protein antigens capable of inducing cell-mediated immunity (1, 21). At least four cryptococcal proteins with T-cell-stimulating properties have been identified, and the corresponding genes have been cloned (2, 10, 15, 18). Two of them, with respective molecular masses of 98 and 25 kDa, have a polysaccharide deacetylase domain (2, 15). Immunization with the 25-kDa deacetylase (d25) was shown to protect mice from lethal experimental cryptococcosis (2).
The present study was undertaken to assess the ability of d25 to produce T1-type responses, as measured by lymphocyte proliferation and cytokine production, and to assess the role of such responses in the protective activities of d25 immunization. Mice used in this study were housed under specific-pathogen-free conditions in enclosed filter top cages of the Department of Pathology and Experimental Microbiology of the University of Messina (Messina, Italy). The mice were fed clean food and water ad libitum. All of the procedures described in this work were in agreement with the guidelines of the National Institute of Health for handling of laboratory animals. The studies performed here have been approved by relevant national and institutional committees.
Recombinant and natural d25 (rd25 and nd25, respectively) were produced and purified as previously described (2). Endotoxin was removed from rd25 preparations using repeated treatments with polymyxin-agarose beads (Bio-Rad Laboratories, Milan, Italy), according to the instructions of the manufacturer. Endotoxin concentration in rd25 or nd25 was <50 pg/mg as assessed by a Limulus amebocyte lysate assay kit (Associates of Cape Cod Inc.; distributed by PBI International, Milan, Italy). A group of BALB/c mice was infected intravenously (i.v.) with a sublethal dose (2.5 × 103 CFU) of the highly virulent C. neoformans H99 strain (ATCC 208821, obtained from the American Type Culture Collection, Manassas, Va.), grown in a synthetic medium (3) to mid-log phase. Mice from another group were immunized subcutaneously (s.c.) with 0.2 ml of a water in oil emulsion containing rd25 (100 μg) in complete Freund's adjuvant (CFA) (Sigma, Milan, Italy). Spleens were removed aseptically from these animals at the indicated times. Spleen cells were washed three times in phosphate-buffered saline (PBS) (0.01 M phosphate, 0.15 M NaCl [pH 7.4]) and resuspended in a culture medium containing RPMI 1640, 5% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from Invitrogen, Life Technologies, San Giuliano Milanese, Italy) and 5 × 10−3 M 2-mercaptoethanol (Sigma). Cells were incubated at 37°C in 5% CO2 in the presence or absence of stimuli in the flat-bottom wells of 96-well plastic plates (Nunc; distributed by Mascia Brunelli, Milan, Italy). Eighteen hours before the end of each incubation, 1 μCi of tritiated thymidine ([3H]TdR; ICN, Milan, Italy) was added. At the end of the incubation period, the cells were collected onto filter paper using a cell harvester (PBI International). The dried filters were counted in a liquid scintillation β-counter (Beckman, Milan, Italy), and the results were expressed as mean counts per minute ± the standard error of the mean (SEM) of triplicate samples. Initial experiments, performed in order to determine the optimal conditions required for splenocyte proliferation, showed that the rd25 antigen was able to induce lymphoproliferation in splenocytes, at concentrations between 2.5 and 50 μg/ml and at any time point after immunization or infection (7, 14, and 28 days) (Fig. 1). In contrast, splenocytes from mice injected with PBS in CFA did not proliferate (Fig. 1). Various numbers of splenocytes were assayed in order to determine the optimal conditions for proliferation. A significant response was achieved when greater than 4 × 105 cells/well were present, while a maximum peak of [3H]TdR incorporation occurred at 6 × 105 cells/well (Fig. 1). Higher cell density led to decreased proliferation over that observed at 6 × 105 cells/well. Collectively, these data indicated that rd25 could induce significant cell proliferation in immunized mice under different experimental conditions.
FIG. 1.
Proliferation of splenocytes from C. neoformans-infected (left) or rd25-immunized (right) mice after rd25 stimulation. Upper panels, spleens were collected at 14 days after immunization or infection, and splenocytes (6 × 105/well) were cultured for 72 h in the presence of various concentrations of rd25 before the addition of [3H]TdR. Eighteen hours later the cells were collected onto filter paper, and the dried filters were counted in a liquid scintillation β-counter. Middle panels, spleens were collected at 14 days after immunization or infection, and various numbers of splenocytes were cultured in the presence of rd25 (10 μg/ml). Lower panels, spleens were collected at 7, 14, and 28 days after immunization or infection, and splenocytes (6 × 105/well) were cultured in the presence of rd25 (10 μg/ml). Negative controls consisted of splenocytes from mice injected with PBS-CFA (white bars). Values represent the means ± SEM from three different experiments, each performed in triplicate. Asterisk, P < 0.05 relative to results for cells from PBS-CFA-injected mice by one-way analysis of variance and the Student-Keuls-Newman test.
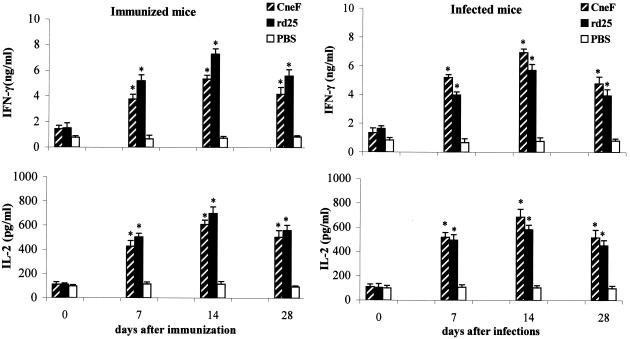
Next, it was of interest to investigate whether splenocytes (5 × 106/ml) from rd25-immunized or C. neoformans-infected mice were able to release significant amounts of type 1 (IL-2 and IFN-γ) or type 2 (IL-4) cytokines. To measure the production of IL-2, IL-4, and IFN-γ, commercially available enzyme-linked immunosorbent assay kits (Euroclone, Wetherby, United Kingdom) were used, according to the manufacturer's instructions. The results reported in Fig. 2 show that a significant secretion of both IL-2 and IFN-γ was observed in cultures of splenocytes from C. neoformans-infected or rd25-immunized mice after in vitro stimulation with the rd25 or with crude concentrated culture supernatants (CneF), obtained as previously described (2). Maximal cytokine secretion was observed in splenocytes obtained at 14 days after immunization (Fig. 2). As expected, splenocytes from PBS-injected mice were not able to induce type-1 cytokine release when stimulated with rd25 protein. No significant differences were detected between the rd25 and the nd25 protein in IL-2 and IFN-γ secretion by splenocytes (data not shown). When the supernatants from the experiments shown in Fig. 2 were tested for IL-4 concentration, no significant increases in the levels of this cytokine were observed under any of the experimental conditions used (not shown).
FIG. 2.
rd25-induced IFN-γ and IL-2 production in cultures of splenocytes from rd25-immunized or C. neoformans-infected mice. Spleens were collected at 7, 14, and 28 days after immunization or infection (see the legend to Fig. 1), and splenocytes (5 × 106/ml) were stimulated with CneF (10 μg/ml), rd25 (10 μg/ml), or PBS. Cytokine levels were assayed at 24 h (IL-2) or 48 h (IFN-γ) after the addition of the stimuli. Data are expressed as means ± SEM for three experiments, each performed in triplicate. Asterisk, P < 0.05, relative to results with cells from PBS-CFA-injected mice by one-way analysis of variance and the Student-Keuls-Newman test.
CD4+ T cells appear to be the main determinant of protection against disseminated cryptococcosis (8). In order to assess which T-cell subset (CD4+ or CD8+) was responsible for d25-induced IFN-γ and IL-2 production, CD4+ and CD8+ T cells were positively selected from spleen cells by means of a panning procedure using anti-murine CD4+ and CD8+ monoclonal antibodies, respectively (16). These monoclonal antibodies were purified from GK 1.5 and 2.43 hybridomas (ATCC) (17) and coated on polystyrene tissue culture plates. CD4+ and CD8+ T cells were obtained as described previously (16) after first removing B cells from the cell suspension (5). By immunofluorescence, >95% of the resulting cells were CD4+ or CD8+. These cells (5 × 106/ml) were resuspended in complete RPMI and cultured in the presence of splenic adherent cells (5 × 105/ml) from normal mice (5). After stimulation with rd25 (10 μg/ml), supernatants were harvested and assayed for IFN-γ and IL-2 production. Levels of both cytokines were significantly elevated in rd25-stimulated, but not in unstimulated, CD4+ T cells from C. neoformans-infected mice (Fig. 3). In addition, significant elevations of both cytokines were measured upon stimulation of CD4+ cells with CneF. In contrast, CD8+ T cells did not produce IFN-γ or IL-2 at significant levels (Fig. 3). These data demonstrate that CD4+ but not CD8+ lymphocytes are the cell type responsible for increased type 1 cytokine production in response to rd25 stimulation.
FIG. 3.
rd25-induced IFN-γ and IL-2 production in cultures of CD4+ or CD8+ cells from C. neoformans-infected mice. Spleens were collected at 14 days after infection, and CD4+ or CD8+ T cells (5 × 106/ml), separated by means of a panning procedure (16), were cultured in the presence of splenic adherent cells (5 × 105/ml) and CneF (10 μg/ml), d25 (10 μg/ml), or PBS. Cytokine levels were assayed as indicated in the legend to Fig. 2. Data are expressed as means ± SEM for three experiments, each performed in triplicate. Asterisk, P < 0.05, relative to results with cells from PBS-CFA-injected mice by one-way analysis of variance and the Student-Keuls-Newman test.
Since IL-12 and tumor necrosis factor alpha (TNF-α) have been reported to be essential for the development of protective T1 immunity to C. neoformans infection (4, 7, 9), the effects of neutralization of these cytokines on d25-induced production of IFN-γ and IL-2 were assessed. Mice were injected intraperitoneally (i.p.) with 0.2 mg of polyclonal goat anti-mouse TNF-α immunoglobulin G (IgG) (R&D Systems, distributed by Space Import Export, Milan, Italy), goat anti-mouse IL-12 IgG (17), or normal goat IgG at 1 h prior to immunization with rd25. IFN-γ and IL-2 levels were measured in rd25-stimulated splenocyte cultures at 7 and 14 days after immunization. At both time points, IFN-γ was significantly (P < 0.05) reduced in cultures from anti-TNF-α- or anti-IL-12-treated mice, whereas the latter treatments did not affect production of IL-2 (Fig. 4). These data indicated that both TNF-α and IL-12 were required for optimal IFN-γ, but not IL-2, production after rd25 immunization.
FIG. 4.
Role of IL-12 and TNF-α in the development of cytokine responses to d25. Spleens were collected at 7 and 14 days after immunization with rd25 (100 μg per mouse in CFA), and cultures of splenocytes (5 × 106/ml) were stimulated with rd25 (10 μg/ml). Cytokine levels were assayed at 24 h (IL-2) or 48 h (IFN-γ) after the addition of the stimulus. Experimental groups included immunized mice that were injected i.p. with 0.2 mg of goat polyclonal anti-mouse TNF-α IgG, goat anti-IL-12 IgG, or normal goat IgG at 1 h prior to infection. Data are expressed as means ± SEM for three experiments, each performed in triplicate. Asterisk, significantly (P < 0.05) different from normal IgG control group by one-way analysis of variance and the Student-Keuls-Newman test.
Next, we determined whether the IL-2 and IFN-γ responses observed after d25 immunization played a role in the protective effects of the latter. To assess the role of IL-2, groups of d25-immunized and nonimmunized mice were treated with polyclonal goat anti-mouse IL-2 IgG (R&D Systems) or normal goat IgG and challenged with C. neoformans (7.5 × 103 CFU). Figure 5 shows that for nonimmunized animals treatment with anti-IL-2 IgG did not significantly affect survival time relative to that for normal IgG-treated controls (12.2 ± 1.1 days versus 13.4 ± 1.0 days; P = 0.51). Similarly, in d25-immunized mice, anti-IL-2 treatment had no effects on survival time relative to that for normal IgG-treated controls (19.2 ± 1.2 days versus 20.7 ± 1.5 days; P = 0.38) (Fig. 5A). These data indicated that IL-2 production was not apparently required for the protective effects of d25 immunization. It is unlikely that the lack of effects of anti-IL-2 treatment we observed was due to insufficient in vivo IL-2 neutralization. In fact, mouse sera collected at 1, 2, and 4 weeks after anti-IL-2 treatment could totally neutralize, at 1:20 to 1:80 dilutions, the biological effects of recombinant IL-2. In these experiments, anti-IL-2 activity in mouse sera was detected by measuring inhibition of proliferation of CTLL-2 cell (ATCC) in the presence of 0.2 ng of rIL-2 (R&D Systems), exactly as described previously (6).
FIG. 5.
Role of IL-2 and IFN-γ in the protective effects of d25 immunization. Panels A and B show the effects of, respectively, IL-2 blockade and IFN-γ deficiency on C. neoformans-induced lethality in d25-immunized and unimmunized mice. (A) Eight-week-old female BALB/c mice were immunized s.c. with rd25 (100 μg per mouse in CFA) or injected with PBS-CFA. Mice belonging to each of these two subgroups were injected i.p. at the time of immunization and 15 days later with 0.5 mg of goat polyclonal anti-mouse IL-2 IgG or normal goat IgG. All mice were challenged i.v. with 7.5 × 103 viable C. neoformans cells (strain H99) at 1 week after immunization, and lethality was observed daily for 30 days. (B) Eight-week-old female IFN-γ−/− mice on a C57BL/6 background and control WT C57BL/6 mice were immunized with d25 in CFA or injected with PBS-CFA as described above. After 1 week, mice were challenged i.v. with 3 × 104 viable C. neoformans cells (strain H99), and lethality was observed daily for 30 days. Survival data were analyzed with Kaplan-Meier survival plots followed by the log rank test (JMP Software; SAS Institute, Cary, N.C.) on an Apple Macintosh computer.
Little is known of the role of IL-2 in C. neoformans infection. The addition of anti-IL-2 antibodies decreased the ability of human peripheral blood mononuclear cells to kill C. neoformans in vitro (20). To our knowledge, the role of endogenous IL-2 has not been studied in experimental cryptococcosis. Our data suggest that at least under the experimental conditions we used, IL-2 blockade has no effect on C. neoformans-induced lethality. This may reflect the complex role of IL-2 as a T-cell growth factor capable of enhancing both Th1 and Th2 responses (23). However, further studies are required to assess the role of IL-2 in anticryptococcal host defenses.
Next, we investigated the role of the IFN-γ response in the protective effects of d25 immunization. Female IFN-γ−/− (genetic background, C57BL/6) and their wild-type (WT) control mice (C57BL/6) were purchased from The Jackson Laboratory (Bar Harbor, Maine). IFN-γ-deficient mice were immunized with rd25 or PBS and challenged with C. neoformans (3 × 104 CFU i.v.). Results were compared with those observed for identically treated wild-type mice. Figure 5B shows that, as expected (14), nonimmunized IFN-γ-deficient mice succumbed more rapidly to C. neoformans infection than WT controls (P < 0.05). Moreover, the protective effects of d25 immunization observed in WT mice were totally abrogated in IFN-γ-deficient mice (Fig. 5B). These data indicate that IFN-γ has an obligatory role in the protective effects of d25 immunization.
In conclusion, our data show that the 25-kDa cryptococcal protein stimulates CD4+ T cells to develop a protective Th1 response associated with increased IFN-γ and IL-2 production. The IFN-γ response, but not the IL-2 response, is apparently required for the protective effects of d25 immunization. There are considerable data to suggest that cell-mediated immunity in general, and T1 responses in particular, are important for host defenses against cryptococcosis (7, 9). Results presented here indicate that d25 is the target for a CD4+-T-cell-mediated, IFN-γ-dependent protective immune response and support this antigen as a promising candidate for active or adoptive immunization strategies.
Acknowledgments
This work was supported by AIDS grants from the ISS (“Project AIDS” contract no. 50 C. 31 and 50 D. 30) and, in part, from MURST (“Progetti di Rilevanza Nazionale” contract no. 2001061479-006), CNR (“Progetto Finalizzato Biotecnologie”), and the European Commission (“HOSPATH” contract no. QLK2-CT-2000-00336).
Editor: T. R. Kozel
REFERENCES
- 1.Abrahams, J., and T. G. Gilleran. 1960. Studies on actively acquired resistance to experimental cryptococcosis in mice. J. Immunol. 85:629-635. [Google Scholar]
- 2.Biondo, C., C. Beninati, D. Delfino, M. Oggioni, G. Mancuso, A. Midiri, M. Bombaci, G. Tomaselli, and G. Teti. 2002. Identification and cloning of a cryptococcal deacetylase that produces protective immune responses. Infect. Immun. 70:2383-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan, K. L., and J. W. Murphy. 1993. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect. Immun. 61:2854-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decken, K., G. Kohler, K. Palmer-Lehmann, A. Wunderlin, F. Mattner, J. Magram, M. K. Gately, and G. Alber. 1998. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect. Immun. 66:4994-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777-2782. [PubMed] [Google Scholar]
- 6.Granelli-Piperno, A., L. Andrus, and E. Reich. 1984. Antibodies to interleukin 2. Effects on immune responses in vitro and in vivo. J. Exp. Med. 160:738-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herring, A. C., J. Lee, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill, J. O., and K. M. Aguirre. 1994. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J. Immunol. 152:2344-2350. [PubMed] [Google Scholar]
- 9.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 10.Huang, C., S. H. Nong, M. K. Mansour, C. A. Specht, and S. M. Levitz. 2002. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-cell responses. Infect. Immun. 70:5485-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 12.Kaplan, M. H., P. P. Rosen, and D. Armstrong. 1977. Cryptococcosis in a cancer hospital: clinical and pathological correlates in forty-six patients. Cancer 39:2265-2274. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami, K., Q. M. Hossain, T. Zhang, Y. Koguchi, Q. Xie, M. Kurimoto, and A. Saito. 1999. Interleukin 4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon gamma inducing cytokines. Cell Immunol. 197:55-61. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami, K., Y. Koguchi, M. H. Qureshi, A. Miyazato, S. Yara, Y. Kinjo, Y. Iwakura, K. Takeda, S. Akira, M. Kurimoto, and A. Saito. 2000. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-gamma production by NK cells. J. Immunol. 165:941-947. [DOI] [PubMed] [Google Scholar]
- 15.Levitz, S. M., S. Nong, M. K. Mansour, C. Huang, and C. A. Specht. 2001. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T-cell responses to Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 98:10422-10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mage, M. G. 1999. Fractionation of T cells and B cells, p. 3.5.1.-3.5.6. In J. E. Coligan et al. (ed.), Current protocols in immunology, vol. 1. John Wiley & Sons, Inc., New York, N.Y.
- 17.Mancuso, G., V. Cusumano, F. Genovese, M. Gambuzza, C. Beninati, and G. Teti. 1997. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect. Immun. 65:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandel, M. A., G. G. Grace, K. I. Orsborn, F. Schafer, J. W. Murphy, M. J. Orbach, and J. N. Galgiani. 2000. The Cryptococcus neoformans gene DHA1 encodes an antigen that elicits a delayed-type hypersensitivity reaction in immune mice. Infect. Immun. 68:6196-6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell, T. G., and J. R. Perfect. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody, C. H., C. L. J. Spurrell, and C. J. Wood. 1998. Interleukin-15 induces antimicrobial activity after release by Cryptococcus neoformans-stimulated monocytes. J. Infect. Dis. 178:803-814. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy, J. W. 1992. Cryptococcal immunity and immunostimulation. Adv. Exp. Med. Biol. 319:225-230. [DOI] [PubMed] [Google Scholar]
- 23.Spaccapelo, R., G. Del Sero, P. Mosci, F. Bistoni, and L. Romani. 1997. Early T cell unresponsiveness in mice with candidiasis and reversal by IL-2: effect on T helper cell development. J. Immunol. 158:2294-2302. [PubMed] [Google Scholar]
- 24.Spitzer, E. D., S. G. Spitzer, L. F. Freundlich, and A. Casadevall. 1993. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet 341:595-596. [DOI] [PubMed] [Google Scholar]