Abstract
Aims
To determine whether topical ocular chloramphenicol increases the risk of aplastic anaemia and to estimate the magnitude of this risk, if any.
Methods
Population-based prospective case-control surveillance of aplastic anaemia in a community of 4.2 million inhabitants from 1980 to 1995 (67.2 million person-years) plus case-population estimate of the risk, based on sales figures of ocular chloramphenicol in the study area during the study period.
Results
One hundred and forty-five patients with aplastic anaemia and 1,226 controls were included in the analysis. Three cases (2.1%) and 5 controls (0.4%) had been exposed to ocular chloramphenicol during the relevant etiological period. The adjusted odds ratio was 3.77 (95% confidence interval, 0.84–16.90). Two cases had also been exposed to other known causes of aplastic anaemia. The incidence of aplastic anaemia among users of ocular chloramphenicol was 0.36 cases per million weeks of treatment. The incidence among non users was 0.04 cases per million weeks.
Conclusions
An association between ocular chloramphenicol and aplastic anaemia cannot be excluded. However, the risk is less than one per million treatment courses.
Keywords: chloramphenicol, aplastic anaemia, ocular
Introduction
It has been postulated that the risk of aplastic anaemia associated with ocular chloramphenicol may be similar to that after oral administration, and a recommendation has been made to restrict its use [1]. This view has been criticised, but no specific epidemiological data have been published [2–6].
An exhaustive Medline search coupled with review of the references in the published studies shows that in the last 30 years, only 10 cases of aplastic anaemia attributed to ocular chloramphenicol have been described [7–16]. In addition, by 1993 the American Register of Drug-Induced Ocular Side Effects included 12 cases of aplastic anaemia and six of pancytopenia, plus 11 additional blood dyscrasias attributed to topical ocular chloramphenicol, but clinical details on these patients are not available [17]. Moreover, despite widespread use of ocular chloramphenicol in Great Britain, from 1966 to 1994 the Committee on Safety of Medicines had only received 11 reports of haematological reactions (all non-fatal) suspected to have been caused by it [4, 6].
Since 1980 we have been running a scheme for the case-control surveillance of aplastic anaemia in a population of 4.2 million inhabitants [18–20]. We present information on the cases and the controls exposed to ocular chloramphenicol, and we give estimates of the relative and absolute risks of aplastic anaemia associated with its use.
Methods
From 1980 to 1986, data were collected as part of the International Agranulocytosis and Aplastic Anemia Study (IAAAS), a multicentre case-control study carried out to assess the risk of these blood dyscrasias associated with the use of drugs [21]. Although the IAAAS ended in 1986, we carried on with the surveillance scheme of both dyscrasias. The present data refer to the period January 1980 to December 1995.
Briefly, in order to detect all cases of aplastic anaemia in the study region, our centre maintains regular contact with all hospitals in the Metropolitan Area of Barcelona (4.2 million inhabitants) through weekly or 2-weekly visits or telephone calls to a designated contact person (see Acknowledgements). Potential cases are patients with at least two of the following criteria: white blood cell count ≤3.5×109 l−1, platelets ≤50×109 l−1, haemoglobin <100 g l−1 or haematocrit of <30%; if the latter was one of the two fulfilled, a reticulocyte count of ≤30×109 l−1 was also required. The bone marrow biopsy has to be compatible with the diagnosis. It also has to be established that the condition is not due to neoplastic or granulomatous disease involving the bone marrow, systemic lupus erythematosus, hypersplenism or other conditions associated to pancytopenia. Patients exposed to antineoplastic chemotherapy or radiotherapy are excluded. A case can be accepted in the absence of a bone marrow biopsy if there are at least two typical marrow aspirates and if the clinical presentation and progression are typical [21].
Cases and controls are interviewed during hospital admission with a structured questionnaire administered by trained interviewers. Detailed information about drug use in the 6 months before admission is obtained, by means of an open question about previous use of drugs, and a list of common symptoms often prompting drug use (including a specific question on any ocular topical application). Clinical and laboratory data are collected. A haematologist confirms the diagnosis, by examining the clinical and laboratory data, including bone marrow aspirates and biopsy specimens. This review is carried out without knowledge of previous drug use.
Initially, for each case of agranulocytosis or aplastic anaemia, up to four controls admitted to the participating hospitals within 3 months of the index case were selected according to a list of admission diagnoses judged to be independent of the reason for use of most groups of drugs (e.g., acute traumatic injuries, non symptomatic conditions leading to surgery, acute infection). However, in order to increase statistical power, all controls for both series of patients (agranulocytosis and aplastic anaemia) are included in the present analysis and an unconditional model has been used (see below).
The definition of drug exposure is decided taking into account that for most cases the bone marrow injury could have occurred between 6 months and 1 month before admission. Therefore the standard exposure window for cases and controls is the 5-month period ending 1 month before admission, including short exposures.
The odds ratio was calculated after controlling for confounding factors by means of a multiple regression model including the following terms, which were selected a priori: age, sex, interviewer, use of acetylsalicylic acid, use of NSAIDs, use of trimethoprim-sulphamethoxazole, a collective term including drugs used for the treatment of rheumatic diseases which have been associated with aplastic anaemia (i.e., corticosteroids, penicillamine, and gold salts), an additional collective term including other drugs known to be risk factors for aplastic anaemia (i.e., ticlopidine, antithyroid drugs, carbamazepine, and allopurinol), and exposure to ocular chloramphenicol. As the number of exposed patients was low, and with the aim of confirming the estimates obtained with the multiple regression model, we performed a stratified analysis with two strata, defined according to the presence or absence of simultaneous exposure to the drugs known to induce aplastic anaemia listed above. BMDP software (BMDP Statistical Software, Inc., Cork, Ireland, 1990) was used.
In order to know the magnitude of the absolute risk of aplastic anaemia among users, the consumption of topical ocular chloramphenicol in the study area was examined and the absolute risk (and 95% confidence interval according to the Poisson distribution) was estimated, assuming a ‘worst case’ situation i.e., that all exposed cases were due to the drug of interest. Figures on sales of ocular chloramphenicol were kindly supplied by Laboratorios Cusí SA, the main manufacturer of ocular topical preparations in our country, and by the General Directorate of Drugs, Ministry of Health. Data from both sources were consistent.
Results
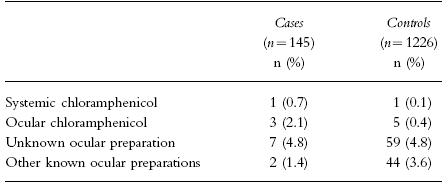
During the study period, 145 cases of aplastic anaemia fulfilling the inclusion criteria were identified and interviewed. They were compared with 1,226 controls. Three cases (2.1%) and five controls (0.4%) had been exposed to topical ocular chloramphenicol (see Table 1). All three cases had been treated with chloramphenicol eye drops. Two controls had been exposed to eye drops, one to eye drops and ointment, one to ointment, and one to ointment or eye drops (unknown). One case (0.7%) and one control (0.1%) had been exposed to systemic but not ocular chloramphenicol, and two controls had been exposed to chloramphenicol-containing skin ointment. Two cases (1.4%) and 44 controls (3.6%) had used ocular preparations containing drugs other than chloramphenicol. Seven cases (4.8%) and 59 controls (4.8%) had been exposed to unknown ocular topical preparations.
Table 1.
Exposure of cases of aplastic anaemia to systemic and ocular chloramphenicol and to other ocular topical drugs.
Of the three cases exposed to ocular chloramphenicol, two had also been exposed to other drugs known to be associated with aplastic anaemia. One had applied the drug 2–3 days per week during 2 years, ending 1 month before the diagnosis; she had also taken trimethoprim-sulphamethoxazole (2-3 days per week, during 1 month ending 5 months before the diagnosis), phenylbutazone (intermitttent short courses, ending 5 months before the diagnosis) and tiethylperazine (1 day per week during 5 years). Another had used ocular chloramphenicol in 2–3 courses of 2–3 days per month during 3 months, ending 2 weeks before the diagnosis; she had also taken gold salts (sodium aurothiomalate, 1 day per week for 3 months ending 8 days before the diagnosis). The third exposed case had applied ocular chloramphenicol during 7 days, ending 2 months before the diagnosis; she had not taken any other drugs known to be associated with aplastic anaemia, and she recovered 6 months later. The other two exposed cases died within 2 months after the diagnosis. Among the five controls exposed to ocular chloramphenicol, one had taken it in a fixed-dose combination with sulphacetamide, and she had also taken indomethacin one to five times per month during 10 years, ending 6 weeks before hospital admission.
The multivariate odds ratio for the association between ocular chloramphenicol and aplastic anaemia was 3.77 (95% confidence interval, 0.84–16.90). The stratified estimate was 3.52 (95% confidence interval, 0.80–15.47).
During the study period 5,851,881 units of ocular chloramphenicol were sold in the study area. Assuming the worst case, i.e., that the three exposed cases can be attributed to ocular chloramphenicol, the incidence would be 0.5 cases per million units (95% confidence interval, 0.10–1.50). However, if only one case is attributed to ocular chloramphenicol, the incidence would be 0.18 (95% confidence interval, 0.005–1.00). Each flask contains enough drug for 10 days of treatment, and this gives an incidence of 0.36 cases of aplastic anaemia per million weeks of treatment (0.12 cases per million weeks if only one case is considered). These figures compare with an incidence of 0.04 per million inhabitants and per week among the non-exposed population.
Discussion
Only three out of 145 consecutive and unselected cases of aplastic anaemia had been exposed to ocular chloramphenicol, and in two of them other causes were present. In addition, the case-control analysis, where confounding factors were taken into account, gave a non-significant association. However, the lower limit of the 95% confidence interval was close to one, and an association cannot be excluded. On the other hand, even if all exposed cases were attributed to ocular chloramphenicol, the risk would be less than one per million treatment courses on the worst assumption.
The proportions of cases and controls who were unable to remember the names of ocular preparations taken during the relevant period did not differ, and this suggests that recall bias can reasonably be excluded. However, the interpretation of our results is difficult, because the prevalence of use of the drug of interest was low and two out of three cases had been exposed to other known causes of aplastic anaemia.
Our results should be considered with regard to previous evidence suggesting a risk of aplastic anaemia associated with chloramphenicol. Since 1965, 10 patients with bone marrow aplasia attributed to ocular chloramphenicol have been described [7–16]. The first [7] was a marrow hypoplasia not fully documented with a bone marrow biopsy, and the patient had also taken an antihistamine preparation during the relevant time period. The second [8] had also been exposed to ocular sulphonamides, at doses far greater than those of chloramphenicol. The third had abnormal results of liver function tests [9]. The fourth had also taken eight additional different drugs [10]. The fifth [11] had also received antithyroid medication and sulphonamides intermittently. In the sixth case [12] no alternative causes were identified, but the diagnosis of aplastic anaemia was not fully documented with a bone marrow biopsy and the haematological recovery took only 3 weeks. The seventh [13] had been concomitantly treated with eight additional drugs, among them acetazolamide, a well established cause of aplastic anaemia [22]. The eighth [14] was a pure red cell hypoplasia rather than aplastic anaemia. No alternative aetiological explanations were found in the ninth case [15]. The tenth patient had also been exposed to seven additional drugs, among them acetazolamide [16]. In summary, these ten anecdotal reports add little evidence in favour of a causal association between ocular chloramphenicol and aplastic anaemia. In addition, a large case-control surveillance programme of aplastic anaemia did not find any case of the disease exposed to ocular chloramphenicol [23].
Metabolism by intestinal bacteria plus individual susceptibility may play a role in the pathogenesis of chloramphenicol-induced aplastic anaemia [24]. Intestinal bacteria can convert chloramphenicol into its dehydro derivative, which is absorbed. Dehydrochloramphenicol can induce DNA damage in intact bone marrow cells, and these cells can catalyze its nitroreduction to nitroso-derivatives which are 20 times more cytotoxic on DNA than chloramphenicol. The marrow of the predisposed host may possess a greater nitroreduction capacity and thus generate more toxic intermediates, or, alternatively, the host stem cells DNA may be inherently more sensitive to the offending metabolite or may have a decreased repair capacity [24]. When administered by topical application, ocular chloramphenicol is readily passed through the nasal lacrimal ductus onto the nasal mucosa, where it can be partly or totally absorbed without being subject to bacterial biotransformation [25]. Since the total amount absorbed must be several orders of magnitude less, this could determine a lower risk when the drug is applied topically on the eye.
We conclude that if there is an association between ocular chloramphenicol and aplastic anaemia, the absolute risk is very low, and results in very few cases. This low number of exposed cases adds difficulty for accurately estimating the magnitude of the association.
Acknowledgments
We are most grateful to all patients who accepted to contribute to the study; to Xavier Carné, Joan Juan, and Eulàlia Pérez for data collection, and to the following haematologists for their collaboration in case reporting: E. Abella, L. Andreu, R. Ayats, C. Besses, A. Bosch, F. Brichs, N. Crespo, I. De Diego, A. Domingo, J. Estella, J. Flores, B. Font, M. García, C. Guanyabens, D. Irriguible, A. Jaén, A. Julià, M. López, P. Marín, M. Melo, F. Millà, J. Moll, Ll. Monner, B. Nomdedéu, T. Olivé, L. Ortega, E. Pérez, I. Roig, R. Soto, T. Toll and S. Woessner.
The local network of the International Study on Agranulocytosis and Aplastic Anaemia was funded by Hoechst AG (Frankfurt) from 1980 to 1986. From 1986 to 1995 the study has been funded by Institut Català de la Salut and by Institut Català de Farmacologia.
References
- 1.Doona M, Walse JB. Use of chloramphenicol as topical eye medication: time to cry halt? Br Med J. 1995;310:1217–1218. doi: 10.1136/bmj.310.6989.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulla RJ, Barnes E, Rogers TR. Is it time to stop using chloramphenicol on the eye? Fears are based on only six cases. Br Med J. 1995;311:450. doi: 10.1136/bmj.311.7002.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley RJK, Kirkness CM, Kanski JJ, Ridgway AEA, Tullo AB, Watson PG. Is it time to stop using chloramphenicol on the eye? Safe in patients with no history of blood dyscrasia. Br Med J. 1995;311:450. doi: 10.1136/bmj.311.7002.450a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AV, Das SS, Tabaqchali S. Is it time to stop using chloramphenicol on the eye? Risk is low in short courses. Br Med J. 1995;311:450–451. doi: 10.1136/bmj.311.7002.450b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayner SA, Buckley RJ. Ocular chloramphenicol and aplastic anaemia. Is there a link? Drug Safety. 1996;14:273–276. doi: 10.2165/00002018-199614050-00001. [DOI] [PubMed] [Google Scholar]
- 6.McGhee CNJ, Anastas CN. Widespread ocular use of topical chloramphenicol: is there justifiable concern regarding idiosyncratic aplastic anaemia? Br J Ophthalmol. 1996;80:182–184. doi: 10.1136/bjo.80.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal RL, Blackman A. Bone-marrow hypoplasia following use of chloramphenicol eye drops. JAMA. 1965;191:148–149. doi: 10.1001/jama.1965.03080020064025. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter G. Chloramphenicol eye-drops and marrow aplasia. Lancet. 1975;2:326. doi: 10.1016/s0140-6736(75)92766-x. [DOI] [PubMed] [Google Scholar]
- 9.Abrams SM, Degnan TJ, Vinciguerra V. Marrow aplasia following topical application of chloramphenicol eye ointment. Arch Intern Med. 1980;140:576–577. [PubMed] [Google Scholar]
- 10.Fraunfelder FT, Bagby GC, Kelly DJ. Fatal aplastic anemia following topical administration of ophthalmic chloramphenicol. Am J Ophthalmol. 1982;93:356–360. doi: 10.1016/0002-9394(82)90540-2. [DOI] [PubMed] [Google Scholar]
- 11.Polak BCP. Chlooramfenical in de oogheelkunde. Geneesmiddelenbulletin. 1984;18:31–33. [Google Scholar]
- 12.Issaragrisil S, Piankijagum A. Aplastic anemia following topical administration of ophthalmic chloramphenicol: report of a case and review of the literature. J Med Ass Thailand. 1985;68:309–312. [PubMed] [Google Scholar]
- 13.Elberg JJ, Hansen WH. Kloramfenikoløjendråber og aplastisk anaemi. Ugeskr Laeger. 1986;148:2227–2228. [PubMed] [Google Scholar]
- 14.Fernández de Sevilla T, Alegre J, Vallespí T, Falcó V, Martínez-Vázquez JM. Adult pure red cell aplasia following topical ocular chloramphenicol. Br J Ophthalmol. 1990;74:640. doi: 10.1136/bjo.74.10.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky E, Biger Y, Zfidan Z, Schneider M. Topical application of chloramphenicol eye ointment followed by fatal bone marrow aplasia. Israel J Med Sci. 1989;25:54. [PubMed] [Google Scholar]
- 16.McWhae JA, Chang J, Lipton JH. Drug-induced fatal aplastic anemia following cataract surgery. Can J Ophthalmol. 1992;27:313–315. [PubMed] [Google Scholar]
- 17.Flegg P, Cheong I. Welsby PD. Chloramphenicol. Are concerns about aplastic anaemia justified? Drug Safety. 1992;7:167–169. doi: 10.2165/00002018-199207030-00001. [DOI] [PubMed] [Google Scholar]
- 18.International Agranulocytosis and Aplastic Anemia Study. The design of a study of the drug etiology of agranulocytosis and aplastic anemia. Eur J Clin Pharmacol. 1983;24:833–836. doi: 10.1007/BF00607096. [DOI] [PubMed] [Google Scholar]
- 19.Laporte JR, Capellà D, Juan J. Agranulocytosis induced by cinepazide. Eur J Clin Pharmacol. 1990;38:387–388. doi: 10.1007/BF00315580. [DOI] [PubMed] [Google Scholar]
- 20.Ibáñez L, Juan J, Pérez E, Carné X, Laporte JR. Agranulocytosis associated with aprindine and other antiarrhythmic drugs: an epidemiological approach. Eur Heart J. 1991;12:639–641. doi: 10.1093/oxfordjournals.eurheartj.a059953. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman DW, Kelly JP, Levy M, Shapiro S. The drug etiology of agranulocytosis and aplastic anemia. New York: Oxford University Press; 1991. [Google Scholar]
- 22.Keisu M, Wiholm B-E, Öst A, Mortimer O. Acetazolamide-associated aplastic anaemia. J Intern Med. 1990;228:627–632. doi: 10.1111/j.1365-2796.1990.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 23.Wiholm B-E, Kelly JP, Kaufman D, Issaragrisil S, Levy M, Anderson T, Shapiro S. Does use of ocular chloramphenicol cause aplastic anaemia? Br Med J. 1998;316:666. doi: 10.1136/bmj.316.7132.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yunis AA. Chloramphenicol. Relation of structure to activity and toxicity. Ann Rev Pharmacol Toxicol. 1988;28:83–100. doi: 10.1146/annurev.pa.28.040188.000503. [DOI] [PubMed] [Google Scholar]
- 25.Scruggs J, Wallace T, Hanna C. Route of absorption of drug and ointment after application to the eye. Ann Ophthalmol. 1978;10:267–271. [PubMed] [Google Scholar]


