Abstract
Aims
Midazolam has good anxiolytic qualities and is a well established premedication agent before anaesthesia or short surgical procedures. The objective of the present study was to determine pharmacokinetic data from individual plasma concentration profiles obtained following intravenous and buccal administration of midazolam.
Methods
Eight young healthy volunteers received single doses of 5 mg midazolam i.v. and after a period of 1 week buccally in a cross over manner. Blood samples were obtained up to 480 min. The measurement of plasma midazolam concentrations was by gas-chromatography.
Results
The maximum plasma concentration was 55.9 ng ml−1 (range 35.6–77.9 ng ml−1 ) at 30 min (range 15–90 min) following buccal administration. AUC was calculated to be 15 016 ng ml−1 min (s.d. 3778 ng ml−1 min) following i.v. and 11191 ng ml−1 min (s.d. 1777 ng ml−1 min) following buccal midazolam. This gave a mean midazolam bioavailabilty of 74.5%.
Conclusions
The pharmacokinetic data presented in this study demonstrate a high bioavailability and reliable plasma concentrations following buccal midazolam. The clinical benefit of buccal midazolam may be in particular patient controlled premedication or sedation in adults.
Keywords: buccal, intravenous, midazolam, pharmacokinetics
Introduction
The water soluble benzodiazepine midazolam (Dormicum® ) is often used in anaesthesia. Due to its good anxiolytic qualities and reliable absorption following oral administration midazolam is a well established premedication before anaesthesia or short surgical procedures. Following oral administration peak plasma concentrations are found after 60 min [1–3]. The bioavailability following oral administration has been shown to be between 35 and 44% [3, 4].
Other routes of administration have been investigated especially in children. Recent studies have demonstrated good premedication or induction following intranasal administration of midazolam [5, 6]. The bioavailability in children following intranasal midazolam is 78% with peak plasma concentrations at 10 min [7].
The main advantage of intranasal application is avoidance of the hepatic first-pass-effect [8]. However, in individual cases a varying amount of the administered fluid may run postnasally into the oropharynx. Swallowed and gastrointestinally absorbed midazolam is submitted to the hepatic first pass effect which decreases the bioavailability.
The recommended dose of intranasal midazolam in children is said to be 0.2 mg kg−1 [5, 6, 9]. In adults (70 kg) an equivalent intranasal dose of ≈3 ml midazolam (5 mg ml−1 ) would have to be administered to achieve a sedative effect. This route of administration seems therefore not to be suitable in adults.
Another route of midazolam administration is the buccal route; however, this has not been well investigated. The absorption via the buccal mucosa also results in a high bioavailability due to lack of the hepatic first pass effect [10]. A greater volume could be administered buccally when the solution is well dispersed.
In the present study pharmacokinetic data were determined from individual plasma concentrations obtained following intravenous and buccal administration of 5 mg midazolam.
Methods
Following approval by the local ethics committee and written informed consent eight young healthy volunteers received single doses of 5 mg midazolam intravenously (i.v.) and after a period of 1 week buccally in a cross over manner. To guarantee a complete washout of midazolam between investigations a period of at least 7 days was maintained between the two forms of administration.
Only healthy volunteers (physical status ASA I or II) receiving no medication were included. Other exclusion criteria were chronic or acute drug or alcohol abuse, benzodiazepine allergy, women who were pregnant or breast feeding and patients with decreased liver or renal function.
For buccal administration the volunteers received 0.5 ml of midazolam (1 ml=5 mg) first on the right and then on the left buccal mucosa with an interval of 30 s between doses. The midazolam solution was administered using a 26 gauge plastic cannula with the tip removed. A 5 ml placebo was simultaneously administered i.v. via a 22 gauge intravenous plastic cannula. For i.v. administration the volunteers received 5 mg midazolam (1 ml=1 mg) via a 22 gauge plastic intravenous cannula over 30 s. The volunteers simultaneously received 0.5 ml of a bitter tasting placebo buccally as described above.
Blood samples were obtained at 3, 6, 9, 12, 15, 20, 30, 45, 60, 90, 120, 180, 240 and 480 min from the contralateral arm via a 18 gauge cannula following administration. The blood was centrifuged and the plasma was stored at −40° C until it was analysed.
At each sampling point the volunteers were questioned regarding buccal mucosa irritation, nausea and degree of sedation. During the whole investigation arterial oxygen saturation (BIOX, Fa. Ohmeda), heart rate and respiratory rate were registered and documented.
The measurement of plasma midazolam concentrations was by gas-chromatography. The method is a modification of that described by Greenblatt et al. [13].
The plasma midazolam concentration was linearly related to the peak height ratio of internal standard to midazolam for 2.5–200 ng midazolam. The sensitivity limit of the method was ≈1–2 ng midazolam ml−1. Relative standard deviations for replicate samples ranged from 2 to 3% at plasma concentrations of 25 ng ml−1 and 7–8% at 100 ng ml−1. Recovery of midazolam and internal standard was greater than 90%. Concentrations of midazolam in the samples were determined using the slope of the calibration curve. This was repeated with every series of plasma extracts, together with the area ratio of midazolam to that of the internal standard calculated from the chromatogram in each sample.
Based on the individual midazolam plasma concentration-time curves following i.v. and buccal administration, the maximal concentration (Cmax ) and the time to maximal concentration (tmax ) were determined.
The calculation of the AUC (0,480 min) was performed according to the linear trapezoidal rule. AUC (480,∞) was calculated from the plasma midazolam concentration at 480 min and the elimination rate constant (λz ). The elimination phase half-life was 0.693/λz.
The bioavailability after buccal administration (Fbuccal ) was calculated according to the equation: Fbuccal=AUCbuccal/AUC i.v.
Intergroup differences regarding buccal mucosa irritation, nausea and degree of sedation were determined using a chi-square-test. A statistically significant difference was assumed at P<0.05.
Results
The mean age of the volunteers was 25.9 years (range 22–30 years), the mean weight 68.3 kg (range 48–87 kg) and the mean height 179 cm (range 165–194 cm). Four female and four male volunteers were included.
No symptoms of a midazolam overdose like respiratory depression or cardiovascular depression were seen in any volunteer. No volunteer complained of nausea or vomiting. Following buccal midazolam two volunteers complained of buccal mucosa irritation for 15 and 30 min, respectively, following administration. Following i.v. midazolam, two volunteers complained of mucosa irritation for 6 and 20 min, respectively.
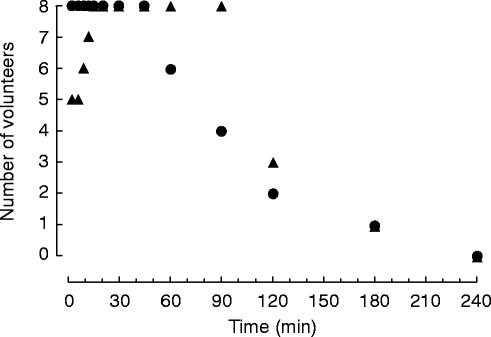
Within 15 min all volunteers either fell asleep or felt sedated. In one volunteer sedation continued for 240 min following buccal midazolam administration (Figure 1). Within 3 min following i.v. midazolam all volunteers either fell asleep or felt sedated. Sedation continued for 240 min in one volunteer (Figure 1).
Figure 1.
Volunteer sedation following intravenous (•) and buccal (▴) administration of 5 mg midazolam (1 ml=5 mg). Depicted are the number of volunteers who assessed themselves sedated.
No clinically relevant changes in arterial oxygen saturation, blood pressure and heart rate following both i.v. and buccal midazolam administration were demonstrated.
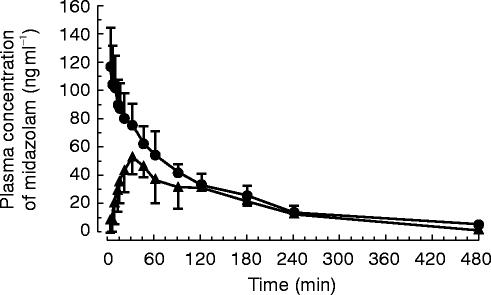
Mean midazolam plasma concentration curves following both modes of administration are depicted in Figure 2. The maximum midazolam plasma concentration was 55.9 ng ml−1 (range 35.6–77.9 ng ml−1 ) 30 min (range 15–90 min) following buccal administration (Table 1).
Figure 2.
Plasma concentration curves following intravenous (—•—) and buccal (—▴—) administration of 5 mg midazolam (mean±s.d.).
Table 1.
Pharmacokinetic data following intravenous and buccal administration of 5 mg midazolam. Values are mean±s.d. except where indicated.
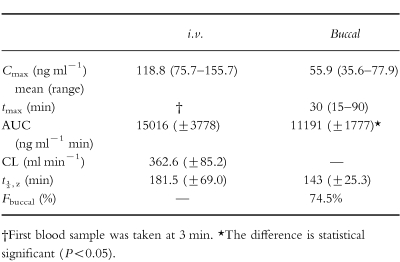
AUC was calculated to be 15017 ng ml−1 min (s.d. 3778 ng ml−1 min) following i.v. and 11191 ng ml−1 min (s.d. 1778 ng ml−1 min) following buccal midazolam (Table 1). The approximate 95% confidence interval was 11858 ng ml−1 min to 18174 ng ml−1 min and 9705 ng ml−1 min to 12677 ng ml−1 min for the AUC following i.v. and buccal midazolam, respectively. This gave a mean buccal midazolam bioavailability of 74.5% (Table 1). Mean plasma clearance following i.v. administration was 362.6 ml min−1 (s.d. 85.2 ml min−1 ).
Mean terminal half-life (t1/2,z ) following i.v. midazolam administration was 181.5±69.0 min and 143±25.3 min following buccal midazolam administration (Table 1). The approximate 95% confidence interval was 123.8 min to 239.2 min and 121.8 min to 164.2 min for the AUC following i.v. and buccal midazolam, respectively.
Discussion
Due to its anxiolytic and sedative qualities midazolam is usually used as a premedication prior to diagnostic procedures and elective anaesthesia. In children the intranasal administration of midazolam as premedication and induction of anaesthesia has already been described [5, 6]. The main advantage of intranasal midazolam is the high bioavailability resulting from absorption without a hepatic first pass effect. Walberg et al. demonstrated a midazolam bioavailability of 78% following intranasal administration in children [7]. The bioavailability of 74.5% following buccal midazolam is comparable. This is expected because buccally administered midazolam is also absorbed without a hepatic first pass effect [10].
The time to maximum plasma concentration (tmax ) was 30 min following buccal midazolam which is markedly prolonged in comparison to a tmax of 10 min following intranasal midazolam [7]. The possible reason for this is the increased salivation following buccal administration of midazolam due to its bitter taste. A variable amount of the midazolam solution is then swallowed and therefore not absorbed buccally. If it were possible to obtain a more concentrated midazolam solution which could be administered to the buccal mucosa in a more dispersed manner, a rapid and reliable buccal absorption could be achieved.
The present pharmacokinetic data are largely in agreement with previous studies [1, 3, 4, 11].
The difference in terminal half-lives following i.v. and buccal midazolam was not statistically significant. However there was a significant difference between the AUC of the two groups.
In three individuals the ratio of AUC gave values of >100% bioavailability; this is difficult to explain. The assays for individual subjects were handled in pairs and a contamination of the column could be excluded. The exact midazolam concentration for buccal administration was guaranteed because a common midazolam solution (5 mg ml−1 ) was used. As the syringes were not weighed before and after administration of the compound methodological errors could not be excluded totally although they seem to be very improbable.
It may be assumed that patient acceptance of the buccal mode of midazolam administration will be higher than that of the intranasal mode. Following intranasal midazolam, due to its bitter taste, children develop a distrust of further management [6]. In a direct comparison, sublingual midazolam was much better tolerated than intranasal midazolam [14]. An increased acceptance can be achieved by improving the taste of midazolam [14]. In the present study two volunteers complained of buccal mucosa irritation. Two volunteers who received placebo buccally also complained of mucosa irritation.
Single reports of sedation with intranasal midazolam in adults show that this mode of administration may be promising [15, 16]. However, to achieve a sufficient sedative effect large volumes of midazolam have to be administered intranasally. As large volumes are not well tolerated intranasally the buccal mode represents a more suitable way of midazolam administration in adults.
The clinical effect documented in the present study was based on the individual sedation assessed by the volunteers. 15 min following buccal midazolam all volunteers assessed themselves as sedated. In one volunteer this sedative effect lasted 240 min. Allonen et al. demonstrated a diminished hypnotic action at plasma midazolam concentrations below 40–60 ng ml−1 [4]. There was a linear correlation between the measured plasma levels and sedation index following i.v. midazolam [4]. As in the present study the assessment of sedation was not very specific; a correlation between plasma concentration and sedation was not calculated. However, it can be assumed that individual responses to midazolam plasma concentrations differ.
The main clinical use of buccal midazolam could be an ‘on demand’ administration. Both intranasal and oral midazolam administration are not suitable for this due to the large volumes that must be administered and the delayed onset of action. As maximum plasma concentrations are already reached 30 min following buccal midazolam an ‘on demand’ titration by repeated administration of small midazolam boli until the desired clinical effect is reached may be possible.
The principle of an ‘on demand’ medication is already known in the management of acute or chronic pain via PCA (patient controlled analgesia) or PCINA (patient controlled intranasal analgesia) [17, 18] and patient controlled sedation (PCS) [19, 20]. If a device could be used which would provide certain safety precautions such as a set time interval between two boluses and precisely defined bolus volumes, patient controlled ‘on demand’ buccal midazolam administration could be established in preoperative premedication or intraoperative sedation.
In conclusion, the pharmacokinetic data presented in this study demonstrate a high bioavailability and reliable plasma concentrations following buccal midazolam. The clinical benefit of buccal midazolam may be in particular patient controlled premedication or sedation in adults.
Acknowledgments
The authors would like to thank Dr Rose and Dr Weinreich from Hoffmann La Roche, Grenzach-Whylen, for making midazolam and diazepam available for determination midazolam plasma concentrations.
References
- 1.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender and obesity on midazolam kinetics. Anesthesiol. 1984;61:27–35. [PubMed] [Google Scholar]
- 2.Reeves JG, Fragen RJ, Vinik R, Greenblatt DJ. Midazolam. pharmacology and uses. Anesthesiol. 1985;62:310–324. [PubMed] [Google Scholar]
- 3.Smith MT, Eadie MJ, Brophy TO. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981;19:271–278. doi: 10.1007/BF00562804. [DOI] [PubMed] [Google Scholar]
- 4.Allonen H, Ziegler G, Klotz U. Midazolam kinetics. Clin Pharmacol Ther. 1981;30:653. doi: 10.1038/clpt.1981.217. [DOI] [PubMed] [Google Scholar]
- 5.Wilton NCT, Leigh J, Rosen DR, Pandit UA. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiol. 1988;69:972–975. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Tolksdorf W, Eick C. Rektale, orale und nasale Prämedikation mit Midazolam bei Kindern im Alter von 1–6 Jahren. Anaesthesist. 1991;40:661–667. [PubMed] [Google Scholar]
- 7.Walberg EJ, Wills RJ, Eckhert J. Plasma concentrations of midazolam in children following intranasal administration. Anesthesiol. 1991;74:233–235. doi: 10.1097/00000542-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hussain A. Mechanism of nasal absorption of drugs. J Pharm Sci. 1989;79:261–272. [PubMed] [Google Scholar]
- 9.Saint-Maurice C, Landais A, Delleur MM, Esteve C, MacGee K, Murat I. The use of midazolam in diagnostic and short surgical procedures in children. Acta Anaesthesiol Scand. 1990;34(Suppl 92):39–41. doi: 10.1111/j.1399-6576.1990.tb03180.x. [DOI] [PubMed] [Google Scholar]
- 10.De Boer AG, de Leede LGJ, Breimer DD. Drug absorption by sublingual and rectal routes. Br J Anaesth. 1984;56:69–82. doi: 10.1093/bja/56.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Malinovsky JM, Lejus C, Servin F, et al. Plasma concentrations of midazolam after i.v., nasal or rectal administration in children. Br J Anaesth. 1993;70:617–620. doi: 10.1093/bja/70.6.617. [DOI] [PubMed] [Google Scholar]
- 12.Rubio F, Miwa BJ, Garland WA. Determination of midazolam and two metabolites of midazolam in human plasma by gas chromatography- negative chemical-ionization mass spectrometry. J Chromatography. 1982;233:157–165. doi: 10.1016/s0378-4347(00)81742-9. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt DJ, Locniscar A, Ochs HR, Lauven PM. Automated gas chromatography for studies of midazolam pharmacokinetics. Aesthesiol. 1981;55:176–179. doi: 10.1097/00000542-198108000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Karl HW, Rosenberger JL, Larach MG, Ruffle JM. Transmucosal administration of midazolam for premedication of pediatric patients. Comparison of the nasal und sublingual routes. Anesthesiol. 1993;78:885–891. doi: 10.1097/00000542-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Moss ML, Buongiorno PA, Clancy VA. Intranasal midazolam for claustrophobia in MRI. J Computer Assisted Tomography. 1993;17:991–992. doi: 10.1097/00004728-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Cheng ACK. Intranasal midazolam for rapidly sedating an adult patient. Anesth Analg. 1993;76:904. doi: 10.1213/00000539-199304000-00043. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann KA. Patient-controlled intravenous analgesia for postoperative pain relief. In: Max MB, Portenoy RK, editors. Advances in pain research and therapy. Vol. 18. Laska: Raven Press; 1991. pp. 481–506. [Google Scholar]
- 18.Striebel HW, Koenigs D, Krämer J. Postoperative pain management by demand-adapted fentanyl titration. Anesthesiol. 1992;77:281–285. doi: 10.1097/00000542-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Osborne GA, Rudkin GE, Curtis NJ, Vickers D, Cracker AJ. Intra-operative patient-controlled sedation. Anaesthesia. 1991;46:553–556. doi: 10.1111/j.1365-2044.1991.tb09654.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudkin GE, Osborne GA, Curtis NJ. Intra-operative patient-controlled sedation. Anaesthesia. 1991;46:90–92. doi: 10.1111/j.1365-2044.1991.tb09345.x. [DOI] [PubMed] [Google Scholar]