Abstract
Aims
The aim of this study was to compare the cardiovascular effects of levobupivacaine with those of rac-bupivacaine following i.v. administration to 14 healthy male volunteers.
Methods
Drugs were infused (at 10 mg min−1 ) using a randomized, double-blind, complete crossover procedure with a washout period of at least 1 week. The administration of drug was discontinued on the appearance of defined CNS symptoms or when a total of 150 mg had been given. Parameters measured were arterial blood pressure, heart rate, ECG, ejection fraction, acceleration index, stroke index and cardiac index.
Results
The mean doses administered were 56.1 mg and 47.9 mg for levobupivacaine and rac-bupivacaine respectively and the maximum mean plasma concentrations were 2.62 and 2.25 μg ml−1 respectively. Despite the dose and plasma concentrations being comparable, levobupivacaine produced a statistically significant smaller reduction in mean stroke index (−5.14 vs−11.86 ml m−2, P=0.001), acceleration index (−0.09 vs−0.20 s−2, P=0.011) and the ejection fraction (−2.50 vs−4.29%, P=0.024). Both levobupivacaine (non significant) and rac-bupivacaine (significant) produced small increases in the PR interval and the corrected QT interval and although the effects of rac-bupivacaine appeared to be greater the difference between the two drugs was not significant.
Conclusions
In conclusion, this study has shown that following i. v. administration levobupivacaine produces significantly less effects on cardiovascular function than does rac-bupivacaine. In particular the negative inotropic effect for levobupivacaine was less than that for rac-bupivacaine as indicated by changes in stroke index, acceleration index and ejection fraction.
Keywords: levobupivacaine, rac-bupivacaine, cardiovascular function, S(−) enantiomer, volunteers
Introduction
Bupivacaine has a moderately rapid onset and a long duration of action as a local anaesthetic. These properties and its relative selectivity, at low concentrations, for sensory nerve fibers, have some practical advantages in clinical situations where maintenance of motor function is essential [1, 2].
The drug has a good safety record, but there have been reports of serious CNS and cardiovascular adverse reactions, including some deaths, in cases of inadvertent intravascular injection or after i.v. regional (Bier’s) anaesthesia. The occurrence of irreversible, fatal ventricular fibrillation without previous warning symptoms as well as evidence of increased toxicity during pregnancy have been of particular concern [3–6].
The possible occurrence of CNS symptoms is dose-limiting for many common uses of local anaesthetics, in particular prolonged epidural anaesthesia, infiltration anaesthesia of major regions and large plexus or multiple nerve blocks. Whereas all modern local anaesthetics have a similar therapeutic index with regard to CNS toxicity, bupivacaine appears to be more cardiotoxic than the other members of this group [3–7].
Bupivacaine is a rac-mixture (50:50) of the R(+) form and the S(−) enantiomer (levobupivacaine). Shortly after the introduction of bupivacaine to clinical practice a study demonstrated lower toxicity and longer duration of the anaesthetic effects in animals of the S(−) enantiomer as compared with the R(+) form and the rac-mixture (8). Since then it has been shown repeatedly that stereospecific factors are involved in the interaction of bupivacaine and most other amide-type local anaesthetics with the sodium channels of the pain-conducting nerve fiber [4, 9].
In different animal models levobupivacaine was less toxic than the R(+) enantiomer to the heart [10–13]. In the present study the tolerability and cardiovascular effects of levobupivacaine was compared with rac-bupivacaine in healthy human volunteers.
Methods
Subject selection and study protocol
The protocol was approved by the independent Ethics Review Committee of Inveresk Research International (IRI), Edinburgh. After giving written informed consent to participate 14 male subjects were included in the study. For these, the age range was 19–40 (mean 29.5) years, height range 165–185 (mean 173.1) cm and body weight 63.3–81.7 (mean 72.3) kg. All subjects were in good health as assessed by history, physical examination, routine laboratory tests and electrocardiography (12-lead ECG).
Approximately 1 week before their definitive inclusion in the study the subjects underwent a training session to familiarize themselves with the typical central nervous (CNS) effects of the local anaesthetics. Lignocaine was infused intravenously at a rate of 15 mg min−1 until the appearance of early symptoms of CNS toxicity, such as lightheadedness, tinnitus or numbness of the tongue. Only subjects who experienced such symptoms during the infusion of doses up to a maximum of 200 mg were admitted.
Study design
The study medication was a 0.5% solution of either levobupivacaine hydrochloride or rac-bupivacaine hydrochloride (Marcain). The experiment was designed as a randomized, crossover study in which each subject received both compounds on two separate occasions (sessions 1 and 2) with a washout period of at least 1 week between the treatments.
Infusion and monitoring
The subjects were admitted to the clinic and fasted overnight. In the morning of the following day they received levobupivacaine or rac-bupivacaine by intravenous infusion at a rate of 10 mg min−1 using a Unita S syringe drive (B. Braun). The subjects were closely observed and the ECG was monitored continuously from 1 h before to 6 h after dosing. Arterial pressure and pulse rate were recorded 30 and 5 min before and at regular intervals during the infusion and up to 24 h after it had been discontinued. Arterial pressure was measured using an automatic electronic sphygmomanometer (Takeda Digital Medical BP Meter UA 751) which was calibrated before and after use. The ECG method was automated and was a Hewlett Packard Page Writer XLi intepretitive ECG.
Cardiovascular function was measured using transthoracic electrical bioimpedance (TEB) (BoMed NCCOM3-R7). Slow mode recording was employed with mean values of each displayed variable over 16 accepted heart beats displayed. For each infusion session the difference from predose (‘response’) was calculated for each subject. For endpoints recorded at minute intervals using the BoMed, predose was defined as the time point 1 min before the commencement of the infusion. For the vital signs, predose was defined as the time point 5 min before the commencement of the infusion. For the ECG intervals, predose was defined as the time point 15 min before the commencement of the infusion.
Every minute after the start of the infusion the subjects were asked the question: ‘Do you have any symptoms?’. They were instructed specifically to report the appearance of any subjective CNS symptoms and grade them as mild, moderate or severe, and to continue to do so as long as the symptoms persisted after the infusion. The administration was discontinued on the appearance of clinically significant CNS toxicity or on completion of dosing (maximum 150 mg). The protocol also had provisions for stopping the infusion should one or several of the following changes occur: systolic pressure <100 mm Hg, pulse >100 beats min−1, ECG showing any degree of heart block or cardiac output falling by 20% or more.
Blood sampling and analysis
Venous blood samples (5 ml) were obtained from the arm contralateral to that used for the administration of the drugs immediately before the dose, at the end of the infusion and at different pre-established times after the start of the infusion (5, 10, 15, 20, 25, 30, and 45 min and 1, 2, 4, 6, 8, 12, and 24 h). The plasma was separated by centrifugation, transferred to clean prelabeled tubes and stored frozen at −20° C until analyzed.
The concentration of levobupivacaine and the R(+) enantiomer in plasma was measured using high performance liquid chromatography (h.p.l.c.). The drugs, together with the added internal standard (lignocaine), were extracted from alkalinized plasma into hexane, injected onto a 2×0.46 cm guard column (Lichrosorb SI 60, 10 Fμm) and separated on a 25×0.46 cm stainless steel chiral h.p.l.c. column (Pirckle-L-phenylglycine, 5 Fμm, Hichrom Ltd) coupled to a spectrophotometric detector (wavelength: 210 nm). The limit of determination (the amount giving a clearly discernible peak 3 times greater than the background noise) was 0.3 μg ml−1 for both levobupivacaine and the R(+) enantiomer. At plasma concentrations of either enantiomer in the range of approximately 0.5–8 μg ml−1 the accuracy of the method varied between −7.36% and 0.67% and the precision between 3.7 and 8.7%.
Pharmacokinetics
The concentrations of levobupivacaine and the R(+) enantiomer were entered into the SIPHAR pharmacokinetic program (Simed). It was planned to conduct a bi-exponential model adapted to intravenous infusion of drugs to calculate and tabulate the pharmacokinetic values: However, concentrations were measurable for only 120 min and meaningful data could not be obtained.
Data management and statistics
All study data recorded in the case record form, except cardiovascular function (TEB) and clinical chemistry and hematology measurements, were subjected to double entry in a clinical data management system. The TEB data were recorded directly onto the computer disc from the instrument and merged with the other observations in the data management system before processing.
For the purposes of sample size calculation, cardiac output and stroke volume were defined to be the primary endpoints of this study. It was assumed that the mean cardiac output in supine healthy volunteers was about 8.0 l min−1 and the study aimed to detect a 10% difference in cardiac output. As a relevant estimate of within subject variability was 0.9 l min−1, the power calculation indicated a minimum of 11 subjects (80% power, 5% significance). It was assumed that the mean stroke volume in supine healthy volunteers was about 126 ml and this study aimed to detect a 10% difference in stroke volume. Based on a relevant estimate of within subject variability (s.d.=14.3 mm) power calculations showed that a minimum of 12 subjects would be required (80% power, 5% significance). Allowing for study withdrawals, a total of 14 patients were entered into the study.
For statistical analysis, end of infusion values were compared with pre-dose values. Analysis of variance (ANOVA) was used to test the differences (Δ) between the two treatments. The results were presented as adjusted (least square) means. Estimates of the treatment effects (Δ) were calculated together with 95% confidence intervals. Comparisons yielding two-tailed probabilities of P<0.05 were considered statistically significant.
Results
Study performance
All 14 subjects completed both sessions of the experiment according to the protocol without experiencing serious adverse events. As expected, mild to moderate CNS symptoms occurred in most of the volunteers and were used as a criterion for stopping the infusion. However, in subject 1 dry mouth and a 20% fall in the cardiac index occurred almost simultaneously during both sessions. In subject 2 this limiting value of the cardiac index was exceeded only during the rac-bupivacaine session. On the other hand, subject 5 completed the levobupivacaine infusion (150 mg) without reporting any characteristic CNS toxicity symptoms, and there was also some doubt with regard to the consistency of the symptoms during the levobupivacaine session in subject 10.
A total of 11 other mild to moderately intense additional adverse events was reported by six subjects. However, only two of these (one case of fatigue on levobupivacaine and one case of somnolence on rac-bupivacaine) were considered likely to be related to the drugs.
Dose infused
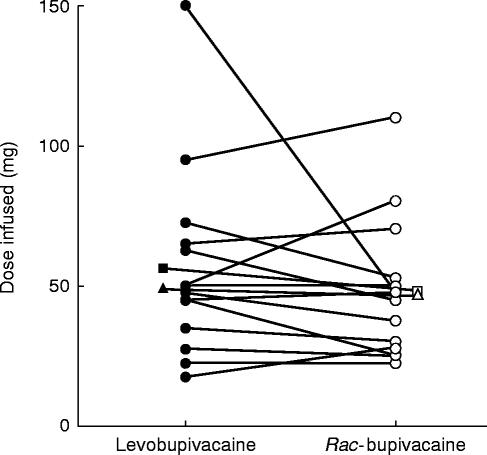
In Figure 1, the dose of levobupivacaine and rac-bupivacaine administered to each of the volunteers is shown. The duration of the levobupivacaine infusion varied from 2 to 15 min, whereas the rac-bupivacaine infusion lasted from 2 to 11 min. The mean dose administered was 56.1 mg (levobupivacaine) and 47.9 mg (bupivacaine). The median values were 48.8 mg and 46.3 mg, respectively.
Figure 1.
Amount of levobupivacaine (•) and rac-bupivacaine (○) infused in 14 subjects. The corresponding mean (▪-□) and median (▴-▵) values are shown.
Cardiovascular function
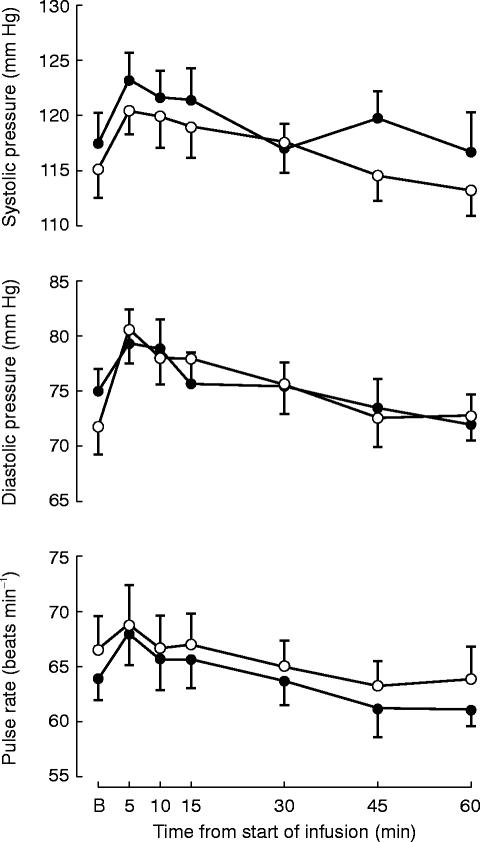
During both treatments systolic and diastolic pressure as well as pulse rate increased. The maximum ocurred at 5 min after the start of the infusion (Figure 2). The recordings returned to pretreatment values after 30–60 min. Rac-bupivacaine produced a greater increase of diastolic blood pressure from baseline to the end of the infusion (P=0.029). However, the fact that this variable showed a lower value before levobupivacaine than before rac-bupivacaine may have influenced the response (Figure 2).
Figure 2.
Vital signs at baseline (B) and at different times after the start of i.v. infusion of levobupivacaine (•) and rac-bupivacaine (○) in 14 healthy subjects (mean±s.e. mean).
Both levobupivacaine (non-significant) and rac-bupivacaine (significant) at end of infusion produced slight increases of the PR interval and the corrected QT interval (QTc), but the difference between the drugs did not reach statistical significance (Table 1). The time-course of the mean values of the cardiac index, stroke index, acceleration index and ejection fraction from immediately before the start of the administration (−1 min) until 15 min after the end of the infusion is shown in Figure 3. All variables were decreased at the end of the infusion, but cardiac function recovered rapidly to approach pre-treatment values after 15 min. The change of the stroke index, acceleration index and the ejection fraction from baseline to the end of the infusion was significantly greater with rac-bupivacaine compared with levobupivacaine (Figure 3).
Table 1.
Cardiovascular variables (mean±s.d.) before and after i.v. infusion of levobupivacaine and racemic bupivacaine to healthy subjects (n=14).
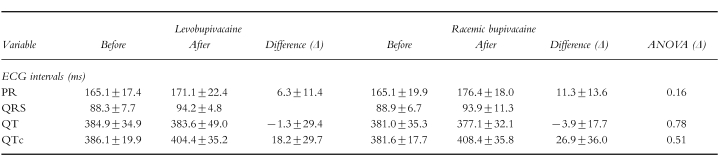
Figure 3.
Cardiovascular variables as assessed by transthoracic electrical bioimpedance at baseline (B) and at different times after the end (E) or the start (S) of the infusion of levobupivacaine (•) and rac-bupivacaine (○) in 14 healthy subjects (mean±s.e. mean).
Pharmacokinetics
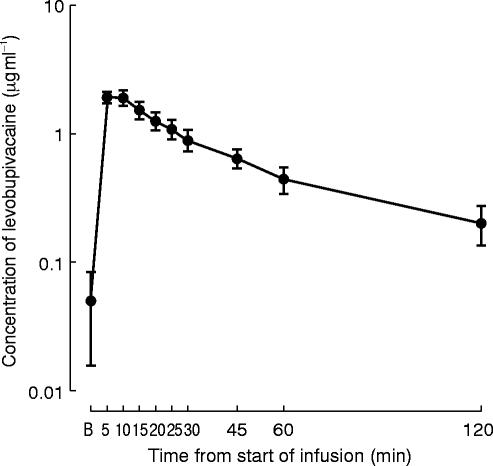
The mean maximum plasma concentration of levobupivacaine at the end of the infusion or shortly thereafter was 2.62 μg ml−1, whereas the corresponding value for the sum of the two enantiomers administered as rac-bupivacaine was 2.25 μg ml−1. For levobupivacaine (Figure 4) a maximum mean concentration of 1.93 μg ml−1 (range 0.91–3.54 μg ml−1 ) occurred at 5 and 10 min after the start of the infusion. The peak level was followed by a rapid decay of the plasma concentration which decreased to values of approximately 0.3–0.6 μg ml−1 in all the subjects after 45–120 min.
Figure 4.
Semilogarithmic plot of the plasma concentrations (mean±s.e. mean) in 14 healthy subjects at different times after the start of the infusion of levobupivacaine (mean dose: 56.1 mg).
Discussion
In the present study symptoms indicating CNS toxicity and leading to discontinuation of the infusion appeared in 13 out of the 14 subjects after either levobupivacaine or rac-bupivacaine. The mean and median threshold doses were similar for both drugs and thus no significant differences were shown with regard to their subjective CNS tolerability. The effect of levobupivacaine on the heart was less marked than that of rac-bupivacaine.
Because no placebo session was included in the protocol, the time-course of the increase in systolic and diastolic pressure and pulse rate after either local anaesthetic should be interpreted cautiously. However, there was a significantly less pronounced increase of the diastolic pressure with levobupivacaine than with the rac-compound and a lesser prolongation of the PR interval with levobupivacaine although the difference between the two drugs was not statistically significant (NS) when end infusion values were considered. The consistent reduction of heart contractility at the end of the infusion in the present study and the finding of less depression with levobupivacaine, lends further support to the contention that the negative inotropic effect of bupivacaine has an enantiomer-specific component. A differential effect has previously been shown in the anaesthetized rat (arrhythmogenicity) and in guinea-pig isolated papillary muscle (electrophysiology), whereas it is not observed in the guinea-pig isolated heart (contractility) [10, 11, 14].
The overall acute toxicity of levobupivacaine in the mouse and the rabbit was lower than that of the R(+) enantiomer [8]. However, most published animal and clinical studies of the specific CNS toxic effects have been carried out with the rac-compound which hitherto has been the only commercially available bupivacaine product [4, 5, 7, 15–17]. A number of studies in different animal models have addressed the differences between the two enantiomers and rac-bupivacaine with regard to their cardiovascular effects [10–12, 14].
In the isolated guinea-pig heart, levobupivacaine produced less retardation of the AV-conduction time [14]. Moreover, in the rabbit isolated heart, levobupivacaine causes less QRS widening and arrhythmias than the R(+) enantiomer [12].
When compared with the baseline values, the magnitude of the negative inotropic effect of rac-bupivacaine in the present study was −21.4% for the stroke index, −14.6% for the acceleration index and −6.6% for the ejection fraction. With levobupivacaine the change of these indices was significantly less, i.e. −9.7%, −6.6%, and −3.9%, respectively. In clinical terms these differences are small but they do indicate that levobupivacaine may be a safer drug than rac-bupivacaine for procedures requiring high doses of the local anaesthetic and in patients at special risk of suffering cardiovascular complications.
The cardiotoxic effects of bupivacaine may arise either by a direct action on the heart or through a reflex mechanism involving the nervous system [16, 19–21]. The present study was designed to measure haemodynamic variables in the presence of mild CNS toxicity at the end of the infusion, and hence the findings would not distinguish between direct and indirect cardiovascular drug effects.
In summary, levobupivacaine caused less negative inotropic effects than rac-bupivacaine. Because the maximum mean blood concentrations of the two drugs were similar, we conclude that levobupivacaine is less cardiotoxic than than rac-bupivacaine.
References
- 1.Covino BG. Clinical pharmacology of local anaesthetic agents. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anaesthesia and management of pain. 2. Philadelphia: JB Lippincott Co.; 1988. pp. 111–144. [Google Scholar]
- 2.Brennum J, Nielsen PT, Horn A, et al. Quantitative sensory examination of epidural anaesthesia and analgesia in man; dose-response effect of bupivacaine. Pain. 1994;56:315–326. doi: 10.1016/0304-3959(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 3.Crandell JT, Kotelco DM. Cardiotoxicity of local anaesthetics during late pregnancy. Anesth Analg. 1985;64:204. [Google Scholar]
- 4.de Jong RH. Local anaesthetics. St Louis: Mosby—Year Book; 1994. [Google Scholar]
- 5.Strichartz GR, Berde CB. Local anaesthetics. In: Miller RD, editor. Anaesthesia. 4. New York: Churchill Livingstone; 1994. [Google Scholar]
- 6.Santos AC, Pedersen H. Current controversies in obstetric anaesthesia. Anesth Analg. 1994;78:753–760. doi: 10.1213/00000539-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Scott DB, Lee A, Fagan D, et al. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–569. [PubMed] [Google Scholar]
- 8.Åberg G. Toxicological and local anaesthetic effects of optically active isomers of two local anaesthetic compounds. Acta Pharmacol Toxicol. 1972;31:273–286. [PubMed] [Google Scholar]
- 9.Lee-Son S, Wang GK, Concus A, et al. Stereoselective inhibition of neuronal sodium channels by local anaesthetics. Anesthesiol. 1992;77:324–335. doi: 10.1097/00000542-199208000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Vanhoutte F, Vereecke J, Verbeke N, Carmeliet E. Stereoselective effects of the enantiomers of bupivacaine on the electrophysiological properties of the guinea-pig papillary muscle. Br J Pharmacol. 1991;103:1275–1281. doi: 10.1111/j.1476-5381.1991.tb12336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denson DD, Behbehani MM, Gregg RV. Enantiomer-specific effects of an intravenously administered arrhythmogenic dose of bupivacaine on neurons of the nucleus tractus solitarius and the cardiovascular system in the anesthetized rat. Reg Anesth. 1992;17:311–316. [PubMed] [Google Scholar]
- 12.Mazoit JX, Boico O, Samii K. Myocardial uptake of bupivacaine: II. Pharmacokinetics and pharmacodynamics of bupivacaine enantiomers in the isolated perfused rabbit heart. Anesth Analg. 1993;77:477–482. [PubMed] [Google Scholar]
- 13.Graf BM, Bosnjak ZJ, Martin E, et al. Stereoselectivity of bupivacaine on cardiac Na+ channels is dependent on c-AMP. Anesthesiol. 1995;83:304. [Google Scholar]
- 14.Graf BM, Vicenzi MN, Kwok WM, et al. Enantiomer-specific component of bupivacaine alters only AV-conduction in isolated hearts. Anesthesiol. 1994;81:749. [Google Scholar]
- 15.Liu S, Paul GE, Carpenter RL, et al. Prolonged PR interval is a risk factor for bradycardia during spinal anaesthesia. Reg Anesth. 1995;20:41–44. [PubMed] [Google Scholar]
- 16.Rutten AJ, Nancarrow C, Mather LE, et al. Hemodynamic and central nervous system effects of intravenous bolus doses of lignocaine, bupivacaine, and ropivacaine in sheep. Anesth Analg. 1989;69:291–299. [PubMed] [Google Scholar]
- 17.McCaughey W. Adverse effects of local anaesthetics. Drug Safety. 1992;7:178–189. doi: 10.2165/00002018-199207030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Bernhards CM, Artru AA. Hexamethonium and midazolam terminate dysrhythmias and hypertension caused by intracerebroventricular bupivacaine in rabbits. Anesthesiol. 1991;74:89–96. doi: 10.1097/00000542-199101000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Butterworth JF, Brownlow RC, Leith JP, et al. Bupivacaine inhibits cyclic-3′,5′-adenosine monophosphate production. A possible contributing factor to cardiovascular toxicity. Anesthesiol. 1993;79:88–95. doi: 10.1097/00000542-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Coyle DE, Porembka DT, Sehlhorst CS, Wan L, Behbehani MM. Echocardiographic evaluation of bupivacaine cardiotoxicity. Anesth Analg. 1994;79:335–339. doi: 10.1213/00000539-199408000-00024. [DOI] [PubMed] [Google Scholar]