Abstract
Aims
To study the potential utility of caffeine based probes of CYP1A2 enzyme activity in predicting the pharmokinetics of tacrine in patients with Alzheimer's disease.
Methods
The pharmokinetics of a single 40 mg oral dose of tacrine were measured in 19 patients with Alzheimer's disease. Each patient also received 2 mg kg−1 [13C-3-methyl] caffeine orally and had breath and urine samples collected.
Results
Tacrine oral clearance (CL F−1 kg−1 ), which varied 15-fold among the patients, correlated significantly with the 2 h total production of 13CO2 in breath (r=0.56, P=0.01), and with each of two commonly used urinary caffeine metabolite ratios: the raxanthine/caffeine ratio’ (1,7X+1, 7U)/1,3,7X) (r=0.76, P=0.0002) and the ‘caffeine metabolic ratio’ (AFMU+1X+1U)/1, 7U)(r=0.76, P=0.0001).
Conclusions
These observations support a central role for CYP1A2 in the in vivo disposition of tacrine and the potential for drug interactions when tacrine treated patients receive known inducers or inhibitors of this enzyme. The magnitude of the correlations we observed, however, are probably not suffcient to be clinically useful in individualizing tacrine therapy.
Keywords: tacrine, caffeine, pharmacokinetics, metabolism, cytochrome P-450, Alzheimer's disease
Introduction
Tacrine, a reversible cholinesterase inhibitor, is an approved drug for the treatment of patients with Alzheimer's disease [1, 2]. It is recommended that patients begin treatment at a low dose of tacrine (40 mg day−1 in four divided doses), with dose escalation every 6 weeks until side effects are encountered, or until the maximal recommended dose of drug (160 mg day−1 ) is achieved [3]. Cholinergic symptoms (nausea and vomiting) are the most frequent side effects observed in patients during dose titration. However, many patients never experience cholinergic side effects. For these patients, the recommended titration sequence may be unnecessary and result in ineffective treatment for several months.
Cholinergic side effects of tacrine have been shown to correlate significantly with plasma levels of parent drug [4]. Monitoring of plasma tacrine levels might therefore allow more rapid dose titration and decrease the incidence of cholinergic side effects. This is not currently an option for most physicians prescribing tacrine, because plasma level assays are not commercially available.
Several recent observations have suggested that it might be possible to use caffeine as a surrogate ‘probe’ to predict tacrine kinetics in patients. Tacrine, like caffeine, is essentially completely absorbed from the digestive tract [5, 6]. Tacrine undergoes extensive first pass metabolism and has multiple metabolites. Only small amounts of tacrine appear as unchanged drug in urine with no single metabolite accounting for more than 5% of the dose excreted in urine. Both caffeine and tacrine are chiefly metabolized by CYP1A2 [7–9]. Studies have indicated that there is up to 60-fold interpatient variation in liver CYP1A2 catalytic activity [10]. Relatively simple assays of specific caffeine metabolites in urine or breath have been proposed as convenient estimates of in vivo CYP1A2 activity [11]. We reasoned that if CYP1A2 activity is rate limiting in tacrine clearance, the results of these caffeine tests might predict tacrine kinetics in patients. This hypothesis is supported by an incidental observation we made when investigating tacrine liver toxicity [12]. In 39 patients with Alzheimer's disease, we found that CYP1A2 activity, as measured using the [13C-3-N-methyl] caffeine breath test, did not identify the individuals most susceptible to tacrine induced liver injury. However, in 10 of the patients participating in this study, tacrine plasma concentrations were determined after 12 weeks of therapy. We found a highly significant correlation between these plasma levels and the caffeine breath test results that we had obtained before the patients started tacrine (P=0.003). These observations were retrospective and difficult to interpret since only single blood levels of tacrine had been obtained, and there had been considerable attrition in the number of patients participating in the study by the time the blood levels were determined.
In the current study, we administered caffeine to patients with Alzheimer's disease and then measured urine and breath metabolites to derive three commonly used estimates of CYP1A2 activity. The results of these tests were then correlated with the apparent oral clearance of tacrine determined in each patient to see if any of the caffeine based tests would be clinically useful tools in estimating tacrine kinetics in vivo.
Methods
Patient population
Patients with Alzheimer's disease considering tacrine therapy but not currently receiving the medication were recruited through the Cognitive Disorders Clinic in the Department of Neurology at the University of Michigan Medical Center (UMMC) for participation in this study. All patients met the NINCDS/ADRDA diagnostic criteria of possible or probable Alzheimer's disease of mild to moderate severity and were greater than 50 years old [13]. Male and female patients of varying ethnic backgrounds were recruited. A complete chart review, history and physical examination, screening blood work, and interview with prospective subjects and their care givers was undertaken by one of the physician investigators (RJF). Patients were selected on the basis of being non-smokers who were capable of providing sequential breath and urine samples. Patients also had to be capable of complying with dietary restrictions before and during the study. Patients were excluded if they were receiving any drugs known to be substrates, inducers, or inhibitors of CYP1A2 (i.e. theophylline, omeprazole, cimetidine) [14–17]. Patients with other degenerative brain disorders, significant active medical problems including hypertension, heart and lung disease, peptic ulcer or other gastrointestinal mucosal disease, liver and kidney disease, or known intolerance to caffeine or tacrine were excluded. Over 50 patients with Alzheimer's disease were contacted, and 22 patients agreed to be enrolled in the study. All patient testing and methods were approved by the UMMC Institutional Review Board. Informed consent was obtained before enrollment from the patient and/or their recognized guardian or principal care giver.
Patient protocol
Patients recruited for the study underwent all testing in the University of Michigan General Clinical Research Center (GCRC). A baseline ECG, urinalysis, CBC, serum electrolytes, and liver function tests were obtained as an outpatient within 2 weeks of the scheduled testing. Subjects were asked to abstain from methylxanthine containing foods and beverages for 72 h prior to admission and throughout the duration of the 3 day study. Compliance was checked by reviewing a 3 day diet diary recorded immediately prior to admission by the patient's care giver that included the amount, type, and time of all food and beverage intake. Subjects received a standardized, ‘inducer free’ isocaloric diet throughout the protocol (i.e. containing no chargrilled meats, methylxanthines, or fruits and vegetables) [18–20].
Patients were randomized to receive either caffeine or a single 40 mg dose of tacrine at 08:00 on Day 2 and the other drug at 08:00 h on Day 3 after an overnight fast. Breath and urine samples were periodically collected following caffeine administration for 24 h. Plasma samples were collected for 12 h after tacrine administration using a forearm heparin lock. Vital signs and frequent monitoring of all patients was conducted following drug administration. Subjects were instructed not to eat or drink any foods or beverages other than water for 4 h after drug administration. Outpatient prescription medications were administered at their usual doses no earlier than 4 h after ingestion of tacrine or caffeine. Patients and their care givers were allowed to ambulate as tolerated in the GCRC throughout the study.
Twenty-two patients were recruited and completed the protocol. The blood samples from the first two patients were inappropriately handled by our laboratory (serum was prepared instead of plasma) and a third patient's blood samples were lost in transit to the contract assay laboratory. The remaining 19 patients form the basis of this report.
Caffeine breath test (CBT)
Nineteen subjects received a 2 mg kg−1 dose of [13C-3-methyl] caffeine at 08:00 h on Day 2 or Day 3. The [13C 3-methyl] caffeine was supplied as part of a commercially available kit (Metabolic Solutions, Merrimack, NH). The 2 mg kg−1 dose was dissolved in 250 ml of water and administered to subjects after an overnight fast. The caffeine solution was followed by a 250 ml water rinse of the container. Subjects were allowed to drink tap water as desired following caffeine ingestion. Exhaled breath samples were collected at time 0, 30, 60, 90, and 120 min after caffeine administration using a Quintron Alveo Sampler device (Quintron, Milwaukee, WI). Four breath samples were obtained at baseline and duplicate breath samples were obtained at all other time points. Breath samples were stored in 10 ml evacuated glass tubes (Exetainers, Labco, U.K.) at room temperature. The 13CO2 content in exhaled breath samples was determined using gas chromatography mass spectral analysis at Metabolic Solutions facilities within 4 weeks of sample collection. Comparison to standard reference gas samples using previously established methods was employed [21]. The measured 13C/12C ratio in each sample was used to calculate the extent of demethylation of the parent compound. There were excellent intrapatient correlations between the total 13CO2 produced in the following intervals: 0–30, 0–60, 0–90, and 0–120 min (data not shown). The results were reported as the % of administered 13C dose exhaled over 2 h, as is customarily reported in the literature [21, 22].
Caffeine urine metabolite testing
Pre-dose urine samples and all urine collected from 0–4 h, 4–6 h, 6–12 h, and 12–24 h was quantified and stored. Within 24 h of sample collection, 3 ml aliquots of urine were saved and stored in sterile plastic containers at −20° C for future analyses. Metabolism of caffeine in humans involves an initial demethylation step that results in the formation of several dimethylxanthines that are then further metabolized. As was recently reviewed by Kalow, several non-invasive tests utilizing caffeine urinary metabolites have been reported to provide estimates of CYP1A2 activity in man[11]. In our study, the 4–6 h urine sample collected in all 19 patients was used to determine the ‘paraxanthine/caffeine ratio’ as recently described by Kadlubar [23]. The 12–24 h urine sample collected in all 19 patients was tested for the presence of 5-acetylamino-6-formyl-amino-3-methyluracil (AFMU), 1-methylxanthine (1 X), 1-methylurate (1 U), and 1,7-dimethylurate (1,7 U) to determine the ‘caffeine metabolic ratio’ as recently described by Kalow [11].
Paraxanthine/Caffeine ratio-(PCUR)
Urine samples collected 4–6 h after caffeine(1,3,7 X) ingestion, were assayed for the concentrations of 1,7-dimethylurate (1,7 X), 1,7 U, and 1,3,7-trimethyl xanthine, caffeine (1,3,7 X) in the Laboratory of Dr L. Kaminsky of Wadsworth Laboratories, Albany, NY using h.p.l.c. The ratio (1,7 X+1,7 U)/1,3,7 X was calculated as an index of CYP1A2 activity. Urine samples stored at −20° C, were transported on dry ice to Albany for analyses. Urine (4 ml) was solid phase extracted on a Lida DS column (6 ml). Recoveries were 88.0±5.3% for 1,3, 7 X, 91.8±2.6% for 1,7 X, and 49.3±6.5% for 1,7 U. H.p.l.c. analyses were conducted per previously reported methods [24] with a 0.045% acetic acid (A) solvent gradient modified as follows: 100% A (0 min), 80% A (12 min), 80% A (18 min), 50% A (20 min), 50% A (26 min), 100% A (28–52 min) at a flow rate of 1.1 ml min−1. The lower limit of detection was 1 ng ml−1.
Caffeine metabolic ratio (CMR)
Urine samples collected 12–24 h after caffeine ingestion, were sent on dry ice to the Laboratory of Dr Bing-Kou Tang at the University of Toronto for the analysis of concentrations of AFMU, 1 X, 1 U, and 1,7 U. The ratio (AFMU+1 X+1 U)/1,7 U (CMR) was calculated as an index of CYP1A2 activity. The caffeine metabolites were measured by h.p.l.c. as previously described [25]. In brief, the xanthine and urates were extracted by organic solvent from the acidified urine and then determined by h.p.l.c. with an Ultrasphere ODS caffeine column (Beckman Instruments, Inc., CA). AFMU was converted to 5-acetylamino-6-amino-3-methyluracil (AAMU) by a 10 min exposure to sodium hydroxide at pH 10. The total acetylated metabolite in urine in the form of AAMU was determined by exclusion chromatography with a TSK-GEL G2000PW column (TOSOHAAS, PA). All samples were run in duplicates.
Tacrine pharmacokinetics
All subjects received 40 mg tacrine (Cognex®, Parke-Davis Pharmaceuticals, Division of Warner-Lambert Co., Morris Plains, NJ) along with 250 ml of water at 08:00 h on Day 2 or Day 3 after an overnight fast. Subjects were NPO and only allowed to drink water for 4 h after tacrine ingestion. Blood samples were collected in heparinized tubes immediately before dosing and 0.5 to 12 h postdose. Plasma was stored frozen until assayed for tacrine and 1-OH tacrine according to a validated h.p.l.c./fluorescence assay by Oneida Research Services, Inc., Whitesboro, NY [26]. The lower limit of quantitation was 0.5 ng ml−1 for tacrine and 1 ng ml−1 for 1-hydroxytacrine. Precision (%RSD) based on quality control samples analyzed with study samples ranged from 3.5% to 8.4%; accuracy (%RE) of quality control samples ranged from −2.0% to 10%.
Pharmacokinetic parameters were calculated by noncompartmental analysis of plasma concentration-time data. Area under plasma concentration-time curve (AUC) was calculated using the linear trapezoidal method. AUC was calculated from time zero to infinity. Apparent elimination-rate constant (λz ) was determined by linear regression of the natural logarithm (ln) of plasma drug concentration over time during the elimination phase; apparent elimination half-life (t1/2 ) was calculated as 0.693/λz. Tacrine oral clearance (CL/F ) was calculated as dose/AUC.
Diet diary analysis
The diet diaries prospectively recorded for each patient 3 days prior to clinical testing were reviewed by a dietician. The mean daily calorie content, macronutrient composition, and presence of foods with known effects on human cytochrome P-450 expression were carefully analyzed (data not shown). Most patients complied with pre-testing dietary restrictions although meals containing small amounts of cooked cruciferous vegetables were noted in ∼50% of subjects. Four subjects had consumed grapefruit within 3 days of study testing but the results of their caffeine urine and breath tests did not appear to be different from the other subjects (not shown).
Statistical analysis
The strength of the linear relationships between the CBT, CMR, PCUR, and pharmacokinetic parameters was assessed with Pearson's product-moment correlation coefficient. Models for explaining variation in the pharmacokinetic parameters were constructed by use of linear regression with forward variable selection. Logarithmic transformation was performed when necessary to normalize data.
Results
Nineteen patients completed the study and had evaluable data (see Methods). All subjects had mild to moderate, possible or probable Alzheimer's disease of varying duration. The mean age of study participants was 71 years (range 54–87 years) with seven male and 12 female subjects successfully completing the study. Seventeen subjects were receiving stable doses of a variety of prescription medications while only two patients were receiving no additional medications (Mean number of medications=2.68/patient; range: 0–9 per patient). Serum creatinine values varied from 0.7 to 1.3 mg dl−1 and the estimated creatinine clearance, calculated using the Cockcroft-Gault method [27], varied over three fold among the nineteen patients with a mean value of 60.3 ml min−1 (range: 30.6–99.0 ml min−1 ).
The mean (±s.d.) CBT result in the 19 subjects was 4.15±1.64%/2 h, the mean PCUR result was 1.37±0.80, and the mean CMR result was 5.95±2.38. The degree of interindividual variation in test results was ∼5 fold for the CBT (1.35%/2 h—6.73%/2 h), ∼4 fold for the CMR (2.72–11.44), and ∼16 fold for the PCUR (0.22–3.63). The observed interindividual variation in the caffeine test results in this study, with the PCUR demonstrating the greatest variation, is consistent with findings from other studies involving healthy subjects [11, 28–30].
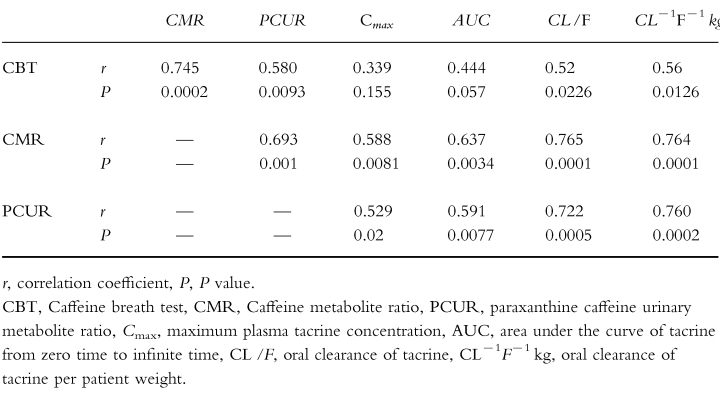
There were significant intraindividual correlations among the various caffeine test results (Table 1). The correlation between the CMR and CBT results (r=0.745, P=0.0002), the CMR and PCUR results (r=0.693, P=0.001), and the CBT and PCUR results (r=0.580, P=0.0093) were all significant. Each of these correlations remained significant (P<0.02) when a Spearman Rank (non-parametric) analysis was employed. It has recently been suggested that renal factors including urinary flow rate may influence the urinary caffeine metabolite ratios, particularly the PCUR [25]. In support of this, the PCUR but not the CMR test results correlated with the urinary flow rate measured during the corresponding collection interval (r=0.491, P=0.0328 and r=0.133, P=0.586 respectively, data not shown).
Table 1.
Correlation between CYP1A2 activity measures and tacrine pharmacokinetic parameters.
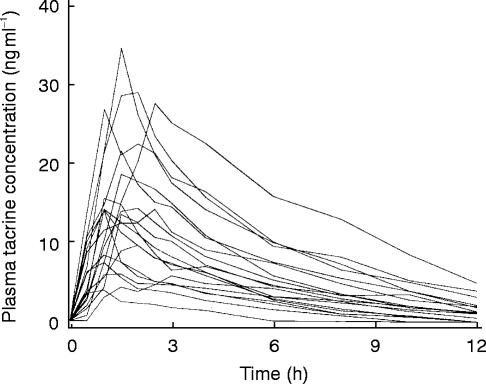
There was substantial interpatient variability in the observed pharmacokinetic parameters of tacrine (Figure 1 and Table 2). There was nearly 9 fold interpatient variation in the Cmax values and 14 fold interpatient variation in the calculated AUC and apparent oral clearance of tacrine. Absorption of drug from the gastrointestinal tract was fairly rapid, as previously reported, with tmax varying from 1.0 to 3.0 h. The apparent elimination half-life could be readily calculated in all cases and ranged from 2.0 to 4.2 h, consistent with previous reports [31–34].
Figure 1.
Individual (n=19) plasma tacrine concentrations after oral administration of 40 mg tacrine.
Table 2.
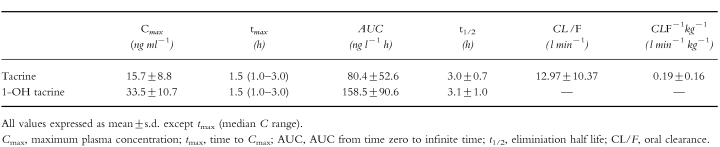
Pharmacokinetic parameters of parent tacrine and 1-OH tacrine in 19 patients.
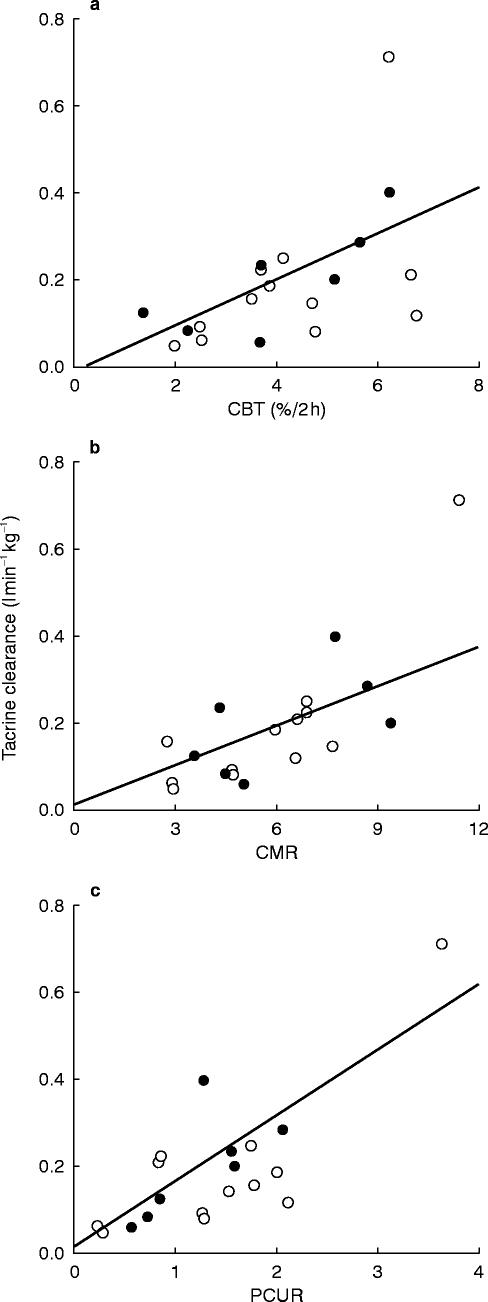
The aim of our study was to determine whether any of the three caffeine test results could significantly predict the apparent oral clearance of tacrine. Correlations between the clearance of tacrine and the CBT, CMR, and PCUR can be seen in Figure 2. In each case, the correlation was significant (Table 1), the correlation with the CMR being the best (r=0.765 P=0.0001). The correlations generally improved when the clearance of tacrine was corrected for subject weight. The ability of the tests to predict the Cmax of tacrine was less than that of predicting the oral clearance of tacrine.
Figure 2.
Individual (n=19) oral clearance of tacrine (CLF−1 kg−1) and estimates of CYP1A2 activity. a=Caffeine Breath Test (CBT), b=Caffeine metabolic ratio (CMR), c=Paraxanthine/caffeine urinary metabolite ratio (PCUR), open circle, female; solid circle, male.
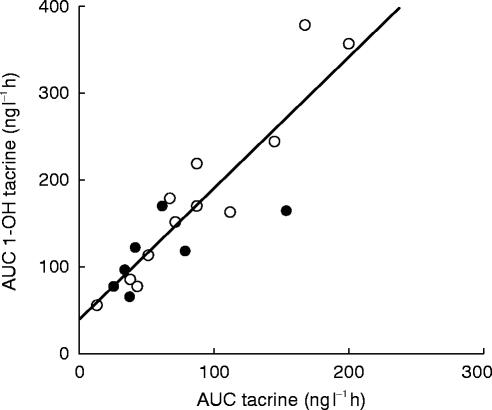
The formation of 1-OH tacrine was rapid as demonstrated by the early detection of this metabolite in plasma with tmax ranging from 0.5 to 3.0 h. Marked interindividual variability in the pharmacokinetic parameters of 1- OH tacrine was also observed (Table 2). The Cmax of 1-OH tacrine showed over four-fold variation and the mean Cmax of this metabolite exceeded the Cmax of parent tacrine by nearly two fold in every patient. There was nearly six-fold variation in the AUC and apparent oral clearance of 1-OH tacrine. The absolute value of the AUC 1-OH tacrine exceeded that of AUC tacrine in all 19 patients and the mean AUC 1-OH tacrine (158.5 ng ml−1 h) was nearly twice that of the mean AUC tacrine (80.4 ng ml−1 h). There were significant intraindividual correlations noted between the Cmax of tacrine and 1-OH tacrine (r=0.734, P=0.0003) and between the AUC of tacrine and 1-OH tacrine as well (r=0.892, P=0.0001) (Figure 3).
Figure 3.
Individual subject correlations of tacrine and 1-OH tacrine area under the curve (AUC) values, r=0.892, P=0.0001. open circle, female; solid circle, male.
Adverse events
Two patients complained of symptoms consistent with cholinergic side effects within the first 2 h following tacrine administration. Only one patient, number 8, experienced severe nausea with vomiting that required intravenous hydration. This patient had the lowest oral clearance of tacrine and low but not the lowest value for any of the caffeine tests. The other patient, number 16, developed only mild nausea following tacrine administration which spontaneously abated. This patient had an oral clearance of tacrine and caffeine test results near the means for the group.
Discussion
A significant amount of data obtained in in vitro systems supports a central role for CYP1A2 in tacrine metabolism [8, 9, 35]. Since both caffeine and tacrine appear to be largely metabolized by CYP1A2 [7, 8], we believed that the ability to metabolize caffeine should predict the apparent oral clearance of tacrine in patients with AD [12]. In support of our hypothesis, statistically significant correlations between each of the three caffeine test results and the oral clearance of tacrine were demonstrated (Table 1). The results of this study therefore support the conclusion that CYP1A2 activity is largely rate limiting in the elimination of tacrine. These results are consistent with other clinical observations of the in vivo metabolism of tacrine and known drug interactions involving tacrine. For example, CYP1A2 activity as measured by the CMR is reported to be lower in women than men [36] and this may account for why women tended to have higher tacrine blood levels in clinical trials [37]. In addition, it seems likely that induction of CYP1A2 activity by cigarette smoke [10] accounts for the observation that cigarette smokers have lower blood levels of tacrine in clinical trials [37]. Likewise, treatment with cimetidine or fluvoxamine, known inhibitors of CYP1A2 [17, 38–39], results in reduced oral clearance of tacrine in humans [40, 41].
We had hoped that at least one of the caffeine based tests would very accurately predict the oral clearance of tacrine, and hence that caffeine might be clinically useful in guiding physicians to a more efficient dose titration scheme for tacrine. However, none of the tests were excellent predictors. The magnitude of the correlation between the CMR and apparent oral clearance of tacrine is comparable with that reported between this test and plasma caffeine clearance (r=0.77) [25]. Since it is estimated that >90% of caffeine is cleared in vivo by CYP1A2 [11], the degree of correlation we observed may be expected. The relative poor predictiveness of the PCUR test could also have been anticipated, since recent observations suggest that the results of this test correlate poorly with direct measurements of caffeine clearance [42, 43]. The lack of accuracy of the PCUR has been attributed to the fact that the ratio depends on the rate of urinary elimination of unmetabolized caffeine, which appears to be dependent on urine flow rate [25]. Our data supports this idea since urine flow rate did correlate with the PCUR measurements we obtained. The relatively poor predictiveness of the CBT was unexpected. The CBT directly measures the 3-demethylation of caffeine, a reaction unequivocally attributed to CYP1A2 [7], and the CBT result correlates well with caffeine plasma clearance [44–46]. In retrospect, it would have been optimal to also measure plasma caffeine clearance in our study.
The suboptimal predictiveness of the caffeine based tests could be explained if tacrine itself is not be a perfect ‘probe’ for CYP1A2 activity [47]. The major metabolite of tacrine produced by liver microsomes, 1-OH tacrine, represents <5% of total tacrine metabolites recovered in urine [5, 35]. This is because 1-OH tacrine undergoes secondary metabolism in the liver and data obtained in liver microsomes suggest that this secondary metabolism is also catalyzed by CYP1A2 [8, 48]. Our observations with 1-OH tacrine are consistent with a major role for CYP1A2 in the elimination of this metabolite in vivo. This is because the amount of 1-OH tacrine formed after the oral dose must equal the amount eliminated from the body. This translates into the equation:
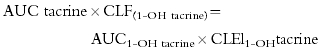 |
where CLF(1-OH tacrine) is the formation clearance of 1-OH tacrine and CLEl(1-OH tacrine) is the elimination clearance of 1-OH tacrine. This equation can be rewritten as follows:
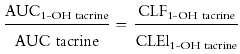 |
The excellent correlation we found between between AUC tacrine and AUC 1-OH tacrine (Figure 3) indicates that the CLEl1-OH tacrine and CLF1-OH tacrine varied in parallel among our patients (i.e., patients with high CLEl also had high CLF). This is consistent with both production and elimination of the metabolite being a reflection of a single enzyme, ie. CYP1A2. The importance of CYP1A2 in the elimination of both tacrine and 1-OH tacrine would also account for why treatment with cimetidine has been shown to cause increases in the AUC of 1-OH tacrine in addition to increases in the AUC of parent tacrine [40]. It seems reasonable to assume that competition between tacrine and its metabolites for CYP1A2 metabolism may complicate the relationship between tacrine clearance and CYP1A2 activity.
In summary, highly variable pharmacokinetics of tacrine and 1-OH tacrine were observed in this population of elderly patients with Alzheimer's disease receiving multiple medications. Each of the three tests of caffeine metabolism used in this study significantly correlated with the oral clearance of tacrine, supporting a rate limiting role of CYP1A2 in the in vivo elimination of the drug. However, the strength of the correlations observed are probably not sufficient to be clinically useful.
Acknowledgments
The authors would like to thank Laurie A. Bluemlein, M.S., R.N., and Debbie S. Blake, B.A., for their contributions in patient recruitment and coordination.
This work was supported in part by grants from the NIH (ESO4238, PBW), The University of Michigan Clinical Research Center (5-MO1-RR00002), The Michigan Alzheimer's Disease Research Center, NIH (P50-AG08671), and Parke-Davis Pharmaceutical Research Division, Warner Lambert Company, Ann Arbor, MI.
References
- 1.Farlow M. Management of Alzheimer's Disease: Today's Options and Tomorrow's Opportunities. Alz Dis Assoc Disord. 1994;8(Suppl 2):S50–S57. [Google Scholar]
- 2.Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. JAMA. 1994;271:985–991. [PubMed] [Google Scholar]
- 3.Parke-Davis Pharmaceuticals Warner Lambert. Cognex (tacrine) package insert. Parke Davis Pharm. 1994.
- 4.Ford JM, Truman CA, Wilcock GK, Roberts CJ. Serum concentrations of tacrine hydrochloride predict its adverse effects in Alzheimer's disease. Clin Pharmacol Ther. 1993;53:691–695. doi: 10.1038/clpt.1993.91. [DOI] [PubMed] [Google Scholar]
- 5.Hartvig P, Askmark H, Aquilonius SM, Wiklund L, Lindstrom B. Clinical Pharmacokinetics of intravenous and oral 9-amino-1,2,3,4-tetrahydroacridine, Tacrine. Eur J Clin Pharmacol. 1990;38:259–263. doi: 10.1007/BF00315027. [DOI] [PubMed] [Google Scholar]
- 6.Ahlin A, Adem A, Junthe T, Ohman G, Nyback H. Pharmacokinetics of tetrahydroamino-acridine: relations to clinical and biochemical effects in Alzheimer patients. Int Clin Psychopharmacol. 1992;7:29–36. [PubMed] [Google Scholar]
- 7.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci USA. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madden S, Woolf TF, Pool WF, Park K. An investigation into formation of stable,protein-reactive and cytotoxic metabolites from tacrine in vitro. Biochem Pharmacol. 1993;46:13–20. doi: 10.1016/0006-2952(93)90342-t. [DOI] [PubMed] [Google Scholar]
- 9.Woolf TF, Pool WF, Bjorge S, Chang T, Goel OP, Trager WF. Bioactivation and irreversible binding of the cognition activator tacrine using human and rat liver microsomal preperations. Drug Metab Dispos. 1993;21:1–9. [PubMed] [Google Scholar]
- 10.Sesardic D, Boobis AR, Edwards RJ, Davies DS. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1988;26:363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalow W, Tang BK. The use of caffeine for enzyme assays: a critical appraisal. Clin Pharmacol Ther. 1993;53:503–514. doi: 10.1038/clpt.1993.63. [DOI] [PubMed] [Google Scholar]
- 12.Fontana RJ, Turgeon DK, Woolf TF, Knapp MJ, Foster NL, Watkins PB. The caffeine breath test does not identify patients susceptible to tacrine hepatotoxicity. Hepatology. 1996;23:1429–1435. doi: 10.1002/hep.510230619. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Rost KL, Brosicke H, Brockmoller J, Scheffler M, Helg H, Roots I. Increase of cytochrome P450IA2 activity by omeprazole: evidence by the 13C-‘N-3-methyl’-caffeine breath test in poor and extensive metabolizers of S-mephenytoin. Clin Pharmacol Ther. 1992;52:170–180. doi: 10.1038/clpt.1992.126. [DOI] [PubMed] [Google Scholar]
- 15.Nousbaum JB, Berthou F, Carlhant D, Gouerou H, Riche C, Robaszkiewicz M. Four-week treatment with omeprazole increases the metabolism of caffeine. Am J Gastro. 1994;89:371–375. [PubMed] [Google Scholar]
- 16.Sarkar MA, Hunt C, Guzelian PS, Karnes HT. Characterization of human liver cytochromes P450 involved in theophylline metabolism. Drug Metab Dispos. 1992;20:31–37. [PubMed] [Google Scholar]
- 17.Knodell RG, Browne DG, Gwozdz GP, Brian WR, Guengerich FP. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology. 1991;101:1680–1692. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, Rothman N, Brown ED, et al. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome P4501a2 activity in humans. Cancer Res. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 19.Fuhr U, Klittich K, Staib AH. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1995;55:431–436. doi: 10.1111/j.1365-2125.1993.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juan D, Worwag EM, Schoeller DA, Kotake AN, Hughes RL, Frederiksen MC. Effects of dietary-protein on theophylline pharmacokinetics and caffeine and aminopyrine breath tests. Clin Pharmacol Ther. 1986;40:187–194. doi: 10.1038/clpt.1986.162. [DOI] [PubMed] [Google Scholar]
- 21.Kotake AN, Schoeller DA, Lambert GH, Baker AL, Schaffer DD, Josephs H. The caffeine CO2 breath test: dose response and route of N-demethylation in smokers and nonsmokers. Clin Pharmacol Ther. 1982;32:261–269. doi: 10.1038/clpt.1982.157. [DOI] [PubMed] [Google Scholar]
- 22.Wietholtz H, Voegelin M, Arnaud MJ, Bircher J, Preisig R. Assessment of the cytochrome P-448 Dependent liver enzyme system by a caffeine breath test. Eur J Clin Pharmacol. 1981;21:53–59. doi: 10.1007/BF00609588. [DOI] [PubMed] [Google Scholar]
- 23.Butler MA, Lang NP, Young JF, et al. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Kaminsky LS. Characterization of human cytochromes P450 involved in theophylline 8-hydroxylation. Biochem Pharmacol. 1995;50:205–211. doi: 10.1016/0006-2952(95)00120-o. [DOI] [PubMed] [Google Scholar]
- 25.Tang B-K, Zhou Y, Kadar D, Kalow W. Caffeine as a probe for CYP1A2 activity: potential influence of renal factors on urinary phenotypic trait measurements. Pharmacogenetics. 1994;4:117–124. [PubMed] [Google Scholar]
- 26.Haughey DB, McNaney CA, Collis MS, et al. Simultaneous determination of tacrine, 1-hydroxy-,2-hydroxy-, 4-hydroxy-tacrine in human plasma by high-performance liquid chromatography with fluorescence detection. J Pharm Sci. 1994;573:93–95. doi: 10.1002/jps.2600831113. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 28.Campbell ME, Spielberg SP, Kalow W. A urinary metabolite ratio that reflects systemic caffeine clearance. Clin Pharmacol Ther. 1987;42:157–165. doi: 10.1038/clpt.1987.126. [DOI] [PubMed] [Google Scholar]
- 29.Kalow W, Tang BK. Caffeine as a metabolic probe: Exploration of the enzyme-inducing effect of cigarette smoking. Clin Pharmacol Ther. 1991;49:44–48. doi: 10.1038/clpt.1991.8. [DOI] [PubMed] [Google Scholar]
- 30.Denaro CP, Benowitz NL. Caffeine metabolism: disposition in liver disease and hepatic-function testing. In: Watson RR, Clifton NJ, editors. Drug and alcohol abuse reviews, liver pathology and alcohol. Vol. 2. The Humana Press; 1991. pp. 513–539. [Google Scholar]
- 31.Cutler NR, Sedman AJ, Prior P, Underwood BA, Selen A, Gracon SI. Steady state pharmacokinetics of tacrine in patients with Alzheimer's disease. Psychopharmacology Bulletin. 1990;26:231–234. [PubMed] [Google Scholar]
- 32.Forsyth DR, Morgan R, Truman CA, Ford JM, Wilcock GK, Roberts CJC. Proceedings of the BPS. 19–21. 1998. Pharmacokinetics of tacrine hydrochloride in patients with dementia; pp. 674–675. [Google Scholar]
- 33.Forsyth DR, Wilcock GK, Morgan RA, Truman CA, Ford JM, Roberts CJ. Pharmacokinetics of tacrine hydrochloride in Alzheimers disease. Clin Pharmacol Ther. 1989;46:634–641. doi: 10.1038/clpt.1989.199. [DOI] [PubMed] [Google Scholar]
- 34.Wagstaff AJ, McTavish D. Tacrine: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in Alzheimer's Disease. Drugs and Aging. 1996;4:510–540. doi: 10.2165/00002512-199404060-00006. [DOI] [PubMed] [Google Scholar]
- 35.Truman CA, Ford JM, Roberts CJC. Comparison of the chromatographic characteristics of metabolites of tacrine hydrochloride in human serum and urine with those of in vitro metabolic products from hepatic microsomes. Biochem Pharmacol. 1991;42:956–959. doi: 10.1016/0006-2952(91)90061-9. [DOI] [PubMed] [Google Scholar]
- 36.Relling MV, Lin J, Ayers GD, Evans WE. Racial and gender differences in N-acetyl tranferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992;52:643–658. doi: 10.1038/clpt.1992.203. [DOI] [PubMed] [Google Scholar]
- 37.Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer's disease. JAMA. 1994;271:992–998. [PubMed] [Google Scholar]
- 38.Rasmussen BB, Maenpaa J, Pelkonen O, et al. Selective serotonin reuptake inhibitors and theophylline metabolism in human liver microsomes: potent inhibition by fluvoxamine. Br J Clin Pharmacol. 1995;39:151–159. doi: 10.1111/j.1365-2125.1995.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becquemont L, Le Bot MA, Riche C, Beaune P. Influence of fluvoxamine on tacrine metabolism in vitro: Potential implication for the hepatotoxicity in vivo. Fundam Clin Pharm. 1996;10:156–157. doi: 10.1111/j.1472-8206.1996.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 40.Forgue ST, Reece PA, Sedman AJ, deVries TM. Inhibition of tacrine oral clearance by cimetidine. Clin Pharmacol Ther. 1996;59:444–449. doi: 10.1016/S0009-9236(96)90114-9. [DOI] [PubMed] [Google Scholar]
- 41.Becquemont L, Ragueneau I, Le Bot A, Riche C, Brentano CF, Jaillon P. Influence of the CYP1A2 inhibitor fluvoxamine on tacrine pharmacokinetics in humans. Clin Pharmacol Ther. 1997;61:619–627. doi: 10.1016/S0009-9236(97)90095-3. [DOI] [PubMed] [Google Scholar]
- 42.Fuhr U, Rost KL, Engelhardt R, et al. Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 phenotyping in man by in vivo versus in vitro correlations. Pharmacogenetics. 1996;6:159–176. doi: 10.1097/00008571-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT. Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenetics. 1996;6:121–149. doi: 10.1097/00008571-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Renner E, Wietholtz H, Huguenin P, Arnaud MJ, Preisig R. Caffeine: a model compound for measuring liver function. Hepatology. 1984;4:38–46. doi: 10.1002/hep.1840040107. [DOI] [PubMed] [Google Scholar]
- 45.Pons G, Blais JC, Rey E. Maturation of caffeine N-demethylation in infancy: A study using the ‘13’CO2 breath test. Pediatr Res. 1988;23:632–636. doi: 10.1203/00006450-198806000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Rost KL, Roots I. Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: Coincidence with plasma clearance and breath test. Clin Pharmacol Ther. 1994;55:402–411. doi: 10.1038/clpt.1994.49. [DOI] [PubMed] [Google Scholar]
- 47.Spaldin V, Madden S, Adams DA, Edwards RJ, Davies DS, Park BK. (1995) Determination of human hepatic cytochrome P4501A2 activity in vitro-Use of tacrine as an isoenzyme-specific probe. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 1995:929–934. [PubMed] [Google Scholar]
- 48.Spaldin V, Madden S, Pool WF, Woolf TF, Park BK. The effect of enzyme inhibition on the metabolic activation of tacrine by human liver enzymes. Br J Clin Pharmacol. 1994;38:15–22. doi: 10.1111/j.1365-2125.1994.tb04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]