Abstract
Enteropathogenic Escherichia coli (EPEC) is an important cause of infant diarrhea in developing countries. EPEC uses a type III secretory system to deliver effector proteins into the host cell. These proteins cause the characteristic attaching and effacing lesion on enterocytes. Lactoferrin, a glycoprotein present in human milk, inhibits EPEC adherence to mammalian cells. To determine the effect of lactoferrin on the initial host cell attachment step that is mediated by the type III secretory system, we focused on EPEC-induced actin polymerization in HEp2 cells, on the hemolytic activity, and on measurement of E. coli secreted proteins A, B, and D (EspABD). Lactoferrin blocked EPEC-mediated actin polymerization in HEp2 cells and blocked EPEC-induced hemolysis. The mechanism of this inhibition was lactoferrin-mediated degradation of secreted proteins necessary for bacterial contact and pore formation, particularly EspB. The proteolytic effect of lactoferrin was prevented by serine protease inhibitors. This disruption of the type III secretory system implies that lactoferrin could provide broad cross protection against the enteropathogens that share this mechanism.
Enteropathogenic Escherichia coli (EPEC) is one of the major causes of infant diarrhea in developing countries. EPEC usually produces an acute watery diarrhea, but it can produce chronic diarrhea and lead to malnutrition (39).
EPEC induces a distinctive histopathology known as the attaching and effacing lesion, which is characterized by the intimate attachment of bacteria to the epithelial surface and the effacement of host cell microvilli. There are three stages in EPEC pathogenesis: (i) initial adherence to the host cell through the bundle-forming pilus, (ii) production and translocation of bacterial proteins through a needle complex via a type III secretory system, and (iii) actin polymerization-associated intimate attachment and pedestal formation (20, 39, 53).
The type III secretory system is present in many pathogenic gram-negative bacteria (Salmonella, Shigella, and Yersinia spp., Shiga toxin-producing E. coli, and Pseudomonas spp.). Its function is to transport virulence proteins from the bacterial cytoplasm into the host cell plasma membrane and cytoplasm upon contact with target cells (14, 20, 24). In EPEC, the type III secretory system forms a needle complex made of E. coli secretion component F (EscF), which is anchored to the inner and outer membranes of the bacteria through an inner and outer ring. Multimers of E. coli secreted protein A (EspA) attach to the tip of the needle, forming a tube-like structure between the bacterium and the host cell. Bacterial proteins (EspB, EspD, translocated intimin receptor [Tir], and others) are introduced into the mammalian cell via this tube. EspB and EspD create pores in the eukaryotic cell membrane. Intimin, an outer membrane bacterial protein binds to its receptor Tir, following the translocation and surface expression of Tir on the host cell. Intimin-Tir binding triggers polymerization of actin and other cytoskeletal components at the site of attachment, which then disrupts the normal enterocyte microvilli, forming the distinctive pedestal (10, 33, 53).
Development of specific immunity to these E. coli secreted proteins may play a role in protecting against infection. Children infected with EPEC have serum immunoglobulin G (IgG) against intimin, EspA, and EspB (38). Moreover, human milk contains antibodies to intimin, EspA, EspB, and Tir (36, 37, 41, 42, 45). Breastfeeding protects infants from respiratory and intestinal infections (25, 26, 54), including EPEC (4). Incubation with colostrum and human milk (samples from Mexico and Brazil) inhibits the adherence of EPEC to cultured cells (7, 9, 13). There are limited data suggesting that immunoglobulins, free secretory components, and lactoferrin from human milk each contribute to the inhibition of EPEC adherence to HeLa cells (11).
Lactoferrin is a glycoprotein present in milk, in other mucosal secretions (tears, saliva, and vaginal secretions, etc.), and in the specific granules of neutrophils. It is an iron-binding protein with multiple physiological functions: antimicrobial, anti-inflammatory, and immunomodulatory, among others (6, 34, 57). In vivo and in vitro studies have shown a protective effect of lactoferrin against gram-negative infections. Lactoferrin protects mice from a lethal dose of parenterally administered E. coli (61), protects against endotoxin-induced lethal shock in piglets (35), neutralizes endotoxin (62), protects rats from gut-related E. coli systemic infections (17), protects rabbits from Shigella flexneri-induced inflammatory enteritis (22), and decreases invasiveness of S. flexneri in HeLa cells (23).
The purpose of this study was to determine the effect of lactoferrin on the type III secretory system of EPEC. We first evaluated the effect of lactoferrin on attachment and actin polymerization. To isolate the initial steps in pathogenesis (needle complex-dependent secretion of EspABD), we focused on the hemolytic activity of EPEC. This approach allowed us to determine the effect of lactoferrin on the events prior to intimin-Tir-induced actin polymerization.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type EPEC O127:H6 E2348/69, a well-characterized virulent strain, was used in this study. Bacteria were grown under conditions that are known to induce the formation of the needle complex (31). Bacteria from overnight growth in Luria broth were inoculated at a final concentration of 1.7 × 105 CFU/ml in Dulbecco's modified Eagle's medium (DMEM) with 25 mM HEPES, pH 7.4, and incubated at 37°C in a 5% CO2 incubator.
Lactoferrin.
Recombinant human lactoferrin purified from Aspergillus awamori was provided by Agennix Corporation (Houston, Tex.). This lactoferrin preparation was 11% iron saturated. Unless otherwise noted, all experiments were done with a concentration of 10 mg of lactoferrin/ml (0.125 mM), which is approximately the concentration present in human colostrum.
Adherence assay and induction of actin polymerization.
The ability of lactoferrin to block EPEC attachment and actin polymerization was evaluated in a HEp2 cell assay system. A subconfluent layer of HEp2 cells was infected with EPEC E2348/69 at bacterium-to-target ratios of 50:1 and 500:1 with or without lactoferrin (10 mg/ml) and incubated at 37°C in 5% CO2 for 4 h. HEp2 cells were then washed vigorously to remove nonadherent bacteria, fixed with 4% formalin, permeabilized with Triton X-100, and stained with BODIPY-phallacidin (for actin) and 4′,6′-diamidino-2-phenylindole (DAPI) (for cell nucleus and bacterial DNA). The percentage of cells showing fluorescent actin staining (FAS) was determined. A HEp2 cell was considered FAS positive if there was a microcolony with at least 10 bacteria with associated actin polymerization. A minimum of 200 HEp2 cells were counted on each slide.
Hemolysis assay.
The initial attachment of EPEC was studied by a hemolysis assay. An intact type III secretion system is required to cause hemolysis of red blood cells in vitro (29, 49, 56). Human erythrocytes were obtained from volunteers after informed consent. Erythrocytes were washed three times with 10 mM phosphate-buffered saline (PBS; pH 7.4) and added to EPEC (final concentration, 1.7 × 105 CFU/ml) in DMEM-HEPES; the erythrocytes were at a final concentration of 2% of the total volume. This preparation was incubated in the presence or absence of lactoferrin (0 to 10 mg/ml). After 6 h of growth in lactoferrin-DMEM-HEPES, erythrocytes and bacteria were removed by centrifugation (17,000 × g for 5 min) and supernatants were monitored for hemoglobin release by measuring the optical density at 543 nm (OD543). Myoglobin, an iron-containing protein, was used as a control.
Measurement of EspABD.
The effect of lactoferrin on the secretion of E. coli proteins that are responsible for hemolysis was determined. Bacteria were grown in DMEM-HEPES without erythrocytes in the presence or absence of lactoferrin (10 mg/ml) for a period of 7 h. Bacterial growth was monitored spectrophotometrically at the OD600. Aliquots were serially removed and centrifuged (17,000 × g for 10 min), and pellets and supernatants were prepared. The supernatants were assayed in an enzyme-linked immunosorbent assay (ELISA) to measure the amount of released proteins. Ninety-six-well microtiter plates (Immulon 2 HB; Thermo Labsystems, Franklin, Mass.) were coated with duplicate samples of 100 μl of each supernatant and incubated overnight at 4°C. Plates were then blocked with 5% bovine serum albumin for 1 h at 37°C. Affinity-purified anti-EspA from human milk (M. Noguera-Obenza and T. G. Cleary, unpublished data) and monoclonal antibodies against EspB (15) and EspD (49) diluted in 0.1% bovine serum albumin were used for the detection of secreted proteins. Peroxidase-conjugated anti-human secretory IgA was used for EspA detection, and peroxidase-conjugated anti-mouse IgG was used for EspB and EspD detection. The reaction was developed with o-phenylenediamine and H2O2, and it was stopped by adding 2.5 N sulfuric acid. The plates were read at the OD490.
Pellets obtained as described above were washed twice with PBS and resuspended in sample buffer (2-mercapthoethanol, sodium dodecyl sulfate [SDS], and 0.1% bromophenol blue). Samples were then resolved by SDS-12.5% polyacrylamide gel electrophoresis, transferred to nitrocellulose, blocked with 3% skim milk, and incubated overnight at 4°C with either affinity-purified anti-EspA or monoclonal antibodies to EspB and EspD and the conjugates listed above. Western blots were developed by using H2O2 and 0.3% 4-chloro-1-naphthol. The development was stopped by washing with distilled water.
Studies with purified EspB.
Purification of EspB was done by using E. coli M15 containing the plasmid encoding C-terminally histidine-tagged EspB (originally cloned from Shiga toxin-producing E. coli O26:H strain 413/89-1) (16). This strain was grown in Terrific broth (ENE Mate; ISC Bioexpress, Kaysville, Utah) until the OD600 was 0.7. Bacteria were induced to express EspB by adding isopropyl-β-d-thiogalactopyranoside (final concentration of 1 mM) and phenylmethylsulfonyl fluoride (1 mM) at 30°C for 3 h. The cells were harvested by centrifugation, and the pellet was lysed by lysozyme and sonication. The resulting supernatant was incubated with nickel nitrilotriacetic agarose (Qiagen, Valencia, Calif.) for 1 h at 4°C. The agarose was then poured into a column and washed with increasing concentrations of imidazole in PBS to elute the purified protein. All steps during the purification were performed under nondenaturing conditions according to the manufacturer's instructions. SDS-polyacrylamide gel electrophoresis was performed to confirm the purity of the eluted protein.
To determine the stability of purified EspB in lactoferrin, EspB (0.5 to 8 μg/ml) was incubated with various concentrations of lactoferrin (0 to 10 mg/ml) in DMEM-HEPES for 4 h at 37°C in a 5% CO2 incubator. EspB remaining after incubation with lactoferrin was assessed by ELISA and Western blotting. A standard curve was made by using defined concentrations of EspB to enable an exact quantitation of the amount of protein in unknown samples. There was a linear relation between the amount of EspB and the OD determined by ELISA (OD = 0.4231 EspB + 0.0395).
Pure EspB (2 μg/ml) was then incubated with lactoferrin (10 mg/ml) in the presence or absence of protease inhibitors, antipain (100 μM), aprotinin (800 nM), chymostatin (100 μM), and soybean trypsin inhibitor (5 mM), for 2 h at 37°C in a 5% CO2 incubator. The EspB remaining after incubation was assessed by Western blotting.
Statistical methods.
Data are expressed as means ± standard deviations (SD) unless otherwise noted. Regression analysis was used for comparison of secreted proteins over time in the presence or absence of lactoferrin. Paired data were analyzed by two-tailed Student's t test.
RESULTS
Lactoferrin inhibits EPEC-induced actin polymerization.
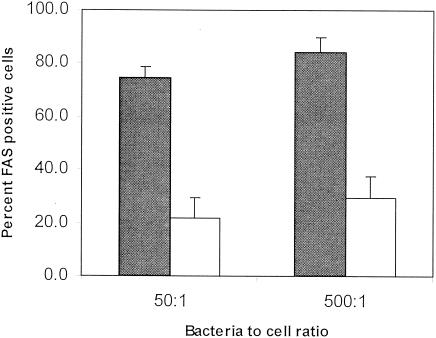
To determine the effect of lactoferrin on EPEC attachment and induction of actin polymerization in mammalian cells, a HEp2 cell model was used. FAS positivity was significantly decreased in the presence of lactoferrin (P < 0.01, n = 3 experiments). Lactoferrin inhibited EPEC-induced FAS by 70.4%, with a bacterium-to-target ratio of 50:1, and by 65.3%, with a bacterium-to-target ratio of 500:1 (Fig. 1). Since previous data with the Shigella model (21) suggested that lactoferrin disrupts the type III secretory system, we next focused on EPEC-induced hemolysis so that we could isolate the first steps in EPEC type III secretory system function.
FIG. 1.
Effect of lactoferrin on EPEC actin polymerization in HEp2 cells. HEp2 cells were incubated with EPEC at bacterium-to-cell ratios of 50:1 and 500:1 in the presence (white bars) or absence (filled bars) of lactoferrin (10 mg/ml). Actin polymerization was determined by the percentage of cells showing FAS. Lactoferrin significantly decreased FAS positivity (means ± SD of results from three experiments).
Lactoferrin blocked EPEC-induced hemolysis.
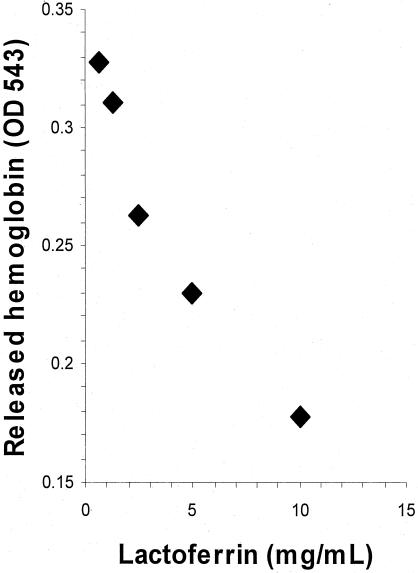
Lactoferrin blocked EPEC-induced hemolysis in a dose-dependent manner (r = 0.9637, P < 0.01) (Fig. 2). Lactoferrin (0.125 mM) reduced hemolysis by 43% ± 5%; this was significantly different from myoglobin (0.125 mM), which reduced hemolysis by 1% ± 6% (P < 0.02). This reduction in hemolysis could be explained by a possible effect of lactoferrin on EPEC's growth or an effect on the type III secretory system. We therefore next determined whether lactoferrin decreased bacterial growth under the assay conditions.
FIG. 2.
Effect of lactoferrin on human red blood cell hemolysis caused by EPEC. Lactoferrin at different concentrations (0 to 10 mg/ml), incubated with 2% erythrocytes and bacteria in DMEM-HEPES for 6 h, blocked EPEC-induced hemolysis in a dose-dependent manner.
Lactoferrin did not impair growth of EPEC.
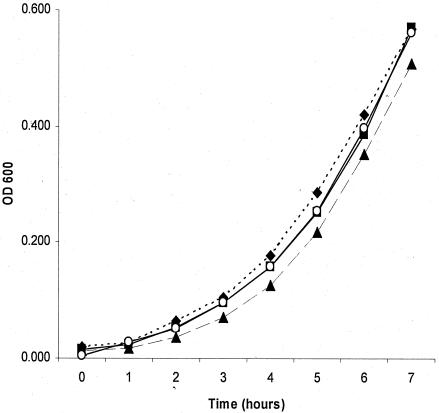
Lactoferrin, at a concentration of 10 mg/ml (37°C, 7-h incubation in DMEM-HEPES), did not affect the growth of EPEC. Growth was determined by hourly measurements of the OD600 for an initial inoculum of 1.7 × 105 CFU/ml. Saturation with ferric chloride at a 2:1 molar ratio of iron to lactoferrin had no effect on growth (Fig. 3). We next evaluated the effect of lactoferrin on type III secretory system secreted bacterial proteins that are critical for hemolysis.
FIG. 3.
Growth curve of EPEC in the presence of lactoferrin. Bacteria were incubated in DMEM-HEPES, pH 7.4, at 37°C in a 5% CO2 incubator for 7 h in the presence or absence of lactoferrin. Bacterial growth was monitored every hour spectrophotometrically at the OD600. Bacteria in DMEM-HEPES (♦) and DMEM-HEPES saturated with iron (▪) had growth similar to that of bacteria in DMEM-HEPES with lactoferrin (10 mg/ml) (▴) or DMEM-HEPES with lactoferrin saturated with iron (2:1 molar ratio of iron to lactoferrin) (○) (means of results of three experiments).
Lactoferrin decreased the ability to detect EspABD secreted into growth media.
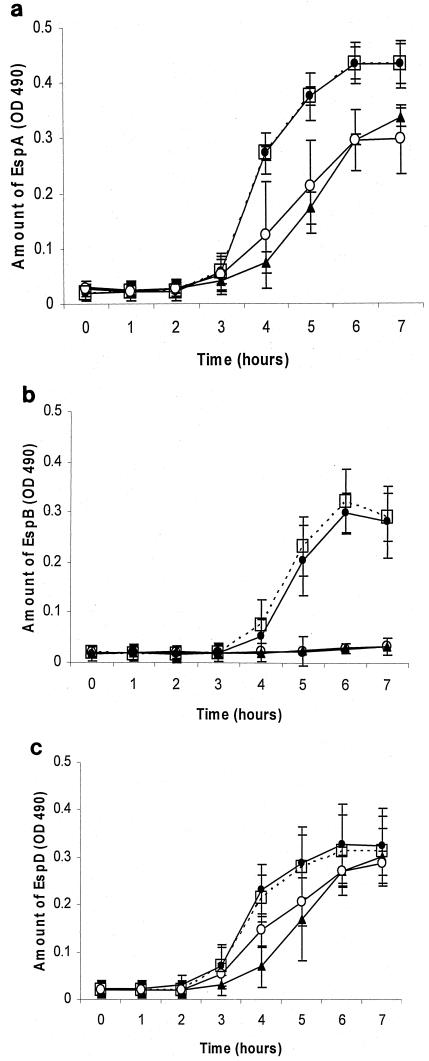
Bacteria in DMEM-HEPES, when not treated with lactoferrin, started secreting EspABD after 2 to 3 h of incubation, with a peak at 6 to 7 h. When bacteria were grown in the presence of lactoferrin, the amount of EspB detected by ELISA dramatically decreased in the supernatant (n = 3 experiments, P < 0.0001), as did the amount of EspA (P < 0.001). The effect of lactoferrin on EspD was less dramatic. Saturation with iron (2:1 molar ratio of iron to lactoferrin) did not affect these results (Fig. 4).
FIG.4.
Effect of lactoferrin on the amount of EspA (a), EspB (b),and EspD (c) secreted into the growth media. Bacteria in DMEM-HEPES (•) and DMEM-HEPES saturated with iron (□) begin secreting EspABD, measured by ELISA, at 2 to 3 h of incubation, with a peak at 6 to 7 h. Lactoferrin (10 mg/ml) (▴) decreased the detection of EspA and EspB in the supernatant and delayed the detection of EspD. Saturation of lactoferrin with iron (○) did not affect these results (means ± SD of results of three experiments).
Bacteria were then grown in DMEM-HEPES for 5 h, a time point at which there are large amounts of secreted proteins in the supernatant as described above. At 5 h, lactoferrin (10 mg/ml, final concentration) was added to the culture media, and 15 min later, the amount of EspABD in the supernatant was measured. Lactoferrin produced an immediate and significant decrease in the amount of EspB in the supernatant (96.4% ± 0.7% reduction, P < 0.03, n = 3 experiments). It also decreased EspA (61.1% ± 22.1%) and EspD (51% ± 27.8%).
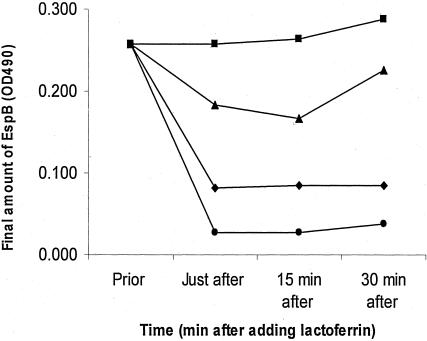
Since the most significant effect of lactoferrin was seen on EspB, we focused on this protein. Lactoferrin (0.01 to 10 mg/ml) was added to bacteria after growth in DMEM-HEPES for 5 h. Immediately after lactoferrin was added, as well as 15 and 30 min later, the amount of EspB present in the supernatant was measured by ELISA. Lactoferrin produced an immediate decrease in the amount of EspB in the supernatant in a dose-dependent manner (Fig. 5). This decrease in the detection of the proteins could be explained by a possible effect of lactoferrin blocking the ELISA plates, by binding of lactoferrin to the proteins, or by lactoferrin-mediated proteolytic digestion. To evaluate these possibilities, we assessed the effect of lactoferrin on these proteins by Western blot analysis.
FIG. 5.
Effect of lactoferrin added after 5 h of EPEC growth on supernatant concentration of EspB. Bacteria were grown in DMEM-HEPES for 5 h before lactoferrin was added at the following concentrations: 0.01 (▪), 0.1 (▴), 1 (♦), and 10 (•) mg/ml. Immediately after adding lactoferrin, 15 and 30 min later, the amount of EspB in the supernatant was measured by ELISA. Lactoferrin produced an immediate decrease in the amount of EspB in a dose-dependent manner.
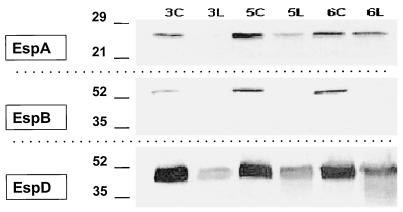
Lactoferrin decreased the amount of cell-associated EspABD in the bacterial pellet.
Bacteria in DMEM-HEPES started to have detectable amounts of cell-associated EspABD after 2 to 3 h of incubation, with a peak at 5 to 6 h (Fig. 6). Lactoferrin at a concentration of 10 mg/ml dramatically decreased the amount of cell-associated EspA, EspB, and EspD at 3, 5, and 6 h of incubation. The most striking change was with EspB. This reduction in the amount of the secreted proteins could be explained by a possible effect of lactoferrin on the synthesis of the proteins (down-regulation of the espA, espB, and espD genes) or on degradation of the proteins. Since it is known that lactoferrin has a proteolytic effect on proteins secreted by Shigella and Haemophilus spp. (21, 43), we focused on the latter possibility. For this purpose, we used purified EspB.
FIG. 6.
Composite Western blot showing the effect of lactoferrin on cell-associated EspA, EspB, and EspD. Bacteria were grown in DMEM-HEPES, and their pellets were evaluated for the presence of EspABD. In the control groups with DMEM-HEPES at 3, 5, and 6 h (3C, 5C, and 6C, respectively), EspA, EspB, and EspD were detected. In the lactoferrin groups at 3, 5, and 6 h (3L, 5L, and 6L, respectively), EspA and EspD were decreased and EspB was undetectable. At 6 h there was the suggestion that EspD breakdown products were also detected. Molecular mass markers are shown in kilodaltons on the left.
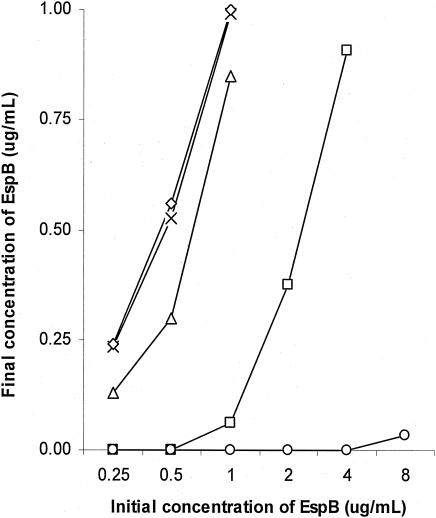
Lactoferrin caused degradation of EspB.
Pure EspB (0.25 to 8 μg/ml) was incubated with lactoferrin (0 to 10 mg/ml) for 4 h, at 37°C in a 5% CO2 incubator. The final concentration of EspB was measured by ELISA. Lactoferrin decreased the amount of EspB in a dose-dependent manner (Fig. 7).
FIG. 7.
Effect of lactoferrin on pure EspB. Purified EspB at various concentrations (0.25 to 8 μg/ml) was incubated with lactoferrin (0 [×], 0.01 [⋄], 0.1 [▵], 1 [□], and 10 [○] mg/ml) for 4 h at 37°C. The amount of EspB remaining was determined by ELISA. Lactoferrin decreased the amount of EspB in a dose-dependent manner.
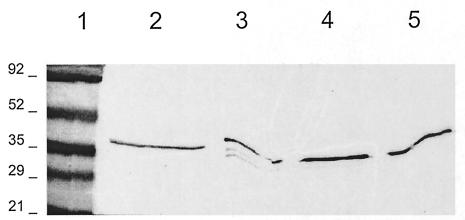
The above-described experiments suggested that lactoferrin was causing degradation of the secreted proteins. To confirm these findings, lactoferrin (10 mg/ml) was incubated with pure EspB (2 μg/ml) for 2 h and then Western blotting was performed; it showed that EspB was degraded into three bands. Since previous studies suggest that lactoferrin has a serine protease activity, the ability of serine protease inhibitors to block proteolysis was investigated. EspB degradation was inhibited by antipain, chymostatin, and soybean trypsin inhibitor (Fig. 8).
FIG. 8.
Proteolytic effect of lactoferrin on EspB. Pure EspB (2 μg/ml) was incubated with DMEM-HEPES (lane 2, control), lactoferrin (10 mg/ml) without protease inhibitors (lane 3), lactoferrin plus chymostatin (lane 4), or lactoferrin plus trypsin inhibitor (lane 5). Three degradation products of EspB are seen with lactoferrin that are not seen with the protease inhibitors. Lactoferrin plus antipain had the same effect (data not shown). Molecular mass markers in kilodaltons are shown in lane 1.
DISCUSSION
EPEC, like many bacterial pathogens, uses a type III secretion system to deliver effector proteins into host cells (30). The supermolecular structure of the EPEC type III secretory system has been extensively studied (10, 12, 27, 33, 48, 58). In this study, we have demonstrated that lactoferrin impairs the function of the type III secretory system. It blocks actin polymerization due to interference with the initial step in EPEC mammalian cell attachment. Lactoferrin blocked FAS and hemolysis by causing degradation of the proteins necessary for bacterial contact and pore formation.
The type III secretory system is necessary to cause hemolysis in vitro (29, 49, 56). The ability of lactoferrin to block EPEC-induced hemolysis implies an effect of lactoferrin on components of the type III apparatus, especially the secreted proteins EspA, EspB, and EspD, which are known to be key elements of EPEC hemolytic activity. In this study, the most dramatic effect of lactoferrin was observed on EspB, a protein that is critical for the development of the attaching and effacing lesion characteristic of EPEC infection. It has been demonstrated that in the absence of EspB no alterations in the cytoskeleton are observed, Tir does not become localized to the cell membrane, and flux of inositol phosphate is not observed in infected cells (19, 32). EspB is required for changes in short circuit current across polarized intestinal epithelial cells and for membrane depolarization in Caco-2 cells (8, 50). EspB is also required for induction of NF-κB activation, for interleukin-8 secretion, for transepithelial migration of neutrophils, and for a decrease in transepithelial electrical resistance, all of which may contribute to diarrhea (46, 47, 60).
The role of the type III secretory system proteins in disease has been investigated with animal models. EspA and EspB proteins were required for virulence and triggering formation of the attaching and effacing lesion in a rabbit EPEC model (1). Likewise, in the Citrobacter rodentium murine colonic hyperplasia model (mouse EPEC equivalent), EspB was necessary for signal transduction and for colonization (40). Thus, it has been confirmed that the type III secretory system events do, indeed, occur in vivo and are very likely to be critically important for development of disease.
EspB is also likely to be important in human infection. A randomized trial with adults showed that diarrhea was more common in volunteers who ingested a wild-type EPEC strain than in those who ingested an isogenic ΔespB mutant strain. Humoral and cell-mediated immune responses to EPEC antigens were stronger among the recipients of the wild-type strain (52). These results demonstrated that EspB is a critical virulence determinant of EPEC infection and suggest that EspB contributes to an immune response.
The effect of lactoferrin on EPEC secreted proteins found in this study has a parallel in other bacterial models. Similar observations were made regarding the effect of lactoferrin on S. flexneri (21). Lactoferrin induces release and causes degradation of two key virulence proteins, invasion plasmid antigens B and C (IpaB and IpaC), which are necessary for the invasion of mammalian cells by Shigella. The proteolytic effect of lactoferrin on IpaB and IpaC is partially blocked by serine protease inhibitors. Likewise, in Haemophilus influenzae, lactoferrin attenuates pathogenic potential by selectively inactivating IgA1 protease and Hap adhesin, two autotransported proteins that are presumed to facilitate colonization (43, 44). Lactoferrin removes IgA1 protease from the bacterial cell wall and proteolytically degrades Hap. Both actions are inhibited by serine protease inhibitors. Lactoferrin cleaves these Haemophilus surface proteins at arginine-rich sites (28).
Lactoferrin has been thought to protect against gram-negative bacteria in a variety of ways. It sequesters iron that is essential for bacterial growth (3). However, the antibacterial activity of lactoferrin is not due only to its iron-binding capacity (51, 55). A pepsin-derived fragment of lactoferrin has iron-independent bactericidal activity that is associated with release of lipopolysaccharide (LPS) (59). Lactoferrin binds to LPS via two sites: a high-affinity N-terminal domain and a low-affinity site in the C terminus (18). Lactoferrin binding to the lipid A portion of LPS on the cell surface disrupts the bacterial cell membrane (2, 5). Lactoferrin binding to the phosphate groups of lipid A makes the fatty acyl chains more rigid, so they become more tightly packed (5). The change in LPS packing induced by lactoferrin's N-terminal positive charges could disrupt interactions between LPS and proteins in or near the surface. We speculate that the binding of lactoferrin to LPS interferes with the type III secretory machinery, causing the secreted proteins to be released and then digested. Lactoferrin-induced release could explain why the cell-associated secreted proteins were also decreased in the bacterial pellets. Based on the Shigella model (21), we have hypothesized that lactoferrin's effect is a two-step phenomenon. First, the N-terminal domain of lactoferrin binds to LPS, causing an increased delivery of the secreted proteins to the cell surface. Second, lactoferrin induces digestion of the exposed proteins.
Type III secretion systems are conserved across bacterial species, and many components show similarities to proteins involved in flagellar biosynthesis. Disruption of the needle complex and type III secretion system in two type III secretory system models (EPEC and Shigella) strongly suggests that lactoferrin could provide broad cross protection against enteropathogens that share this fundamental mechanism.
Acknowledgments
This work was funded by PHS award PO1-HD 13021-25.
We thank Herbert DuPont and Pablo Okhuysen for helpful suggestions.
Editor: J. D. Clements
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., Y. Q. An, M. Geerts, B. G. Thijs, H. A. de Boer, D. M. MacLaren, J. de Graaff, and J. H. Nuijens. 1994. Lactoferrin is a lipid A-binding protein. Infect. Immun. 62:2628-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, R. R., M. F. Cole, and J. R. McGhee. 1977. A bactericidal effect for human lactoferrin. Science 197:263-265. [DOI] [PubMed] [Google Scholar]
- 4.Blake, P. A., S. Ramos, K. L. MacDonald, V. Rassi, T. A. Gomes, C. Ivey, N. H. Bean, and L. R. Trabulsi. 1993. Pathogen-specific risk factors and protective factors for acute diarrheal disease in urban Brazilian infants. J. Infect. Dis. 167:627-632. [DOI] [PubMed] [Google Scholar]
- 5.Brandenburg, K., G. Jurgens, M. Muller, S. Fukuoka, and M. H. Koch. 2001. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 382:1215-1225. [DOI] [PubMed] [Google Scholar]
- 6.Brock, J. H. 2002. The physiology of lactoferrin. Biochem. Cell Biol. 80:1-6. [DOI] [PubMed] [Google Scholar]
- 7.Camara, L. M., S. B. Carbonare, M. L. Silva, and M. M. Carneiro-Sampaio. 1994. Inhibition of enteropathogenic Escherichia coli (EPEC) adhesion to HeLa cells by human colostrum: detection of specific sIgA related to EPEC outer-membrane proteins. Int. Arch. Allergy Immunol. 103:307-310. [DOI] [PubMed] [Google Scholar]
- 8.Collington, G. K., I. W. Booth, M. S. Donnenberg, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic Escherichia coli virulence genes encoding secreted signaling proteins are essential for modulation of Caco-2 cell electrolyte transport. Infect. Immun. 66:6049-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravioto, A., A. Tello, H. Villafan, J. Ruiz, S. del Vedovo, and J. R. Neeser. 1991. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J. Infect. Dis. 163:1247-1255. [DOI] [PubMed] [Google Scholar]
- 10.Daniell, S. J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F. P. Booy, R. K. Shaw, S. Knutton, G. Frankel, and S. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3:865-871. [DOI] [PubMed] [Google Scholar]
- 11.de Araujo, A. N., and L. G. Giugliano. 2001. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 1:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delahay, R. M., S. Knutton, R. K. Shaw, E. L. Hartland, M. J. Pallen, and G. Frankel. 1999. The coiled-coil domain of EspA is essential for the assembly of the type III secretion translocon on the surface of enteropathogenic Escherichia coli. J. Biol. Chem. 274:35969-35974. [DOI] [PubMed] [Google Scholar]
- 13.Delneri, M. T., S. B. Carbonare, M. L. Silva, P. Palmeira, and M. M. Carneiro-Sampaio. 1997. Inhibition of enteropathogenic Escherichia coli adhesion to HEp-2 cells by colostrum and milk from mothers delivering low-birth-weight neonates. Eur. J. Pediatr. 156:493-498. [DOI] [PubMed] [Google Scholar]
- 14.DeVinney, R., A. Gauthier, A. Abe, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell. Mol. Life Sci. 55:961-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebel, F., C. Deibel, A. U. Kresse, C. A. Guzman, and T. Chakraborty. 1996. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect. Immun. 64:4472-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Deibel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 17.Edde, L., R. B. Hipolito, F. F. Hwang, D. R. Headon, R. A. Shalwitz, and M. P. Sherman. 2001. Lactoferrin protects neonatal rats from gut-related systemic infection. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1140-G1150. [DOI] [PubMed] [Google Scholar]
- 18.Elass-Rochard, E., A. Roseanu, D. Legrand, M. Trif, V. Salmon, C. Motas, J. Montreuil, and G. Spik. 1995. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 312:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foubister, V., I. Rosenshine, M. S. Donnenberg, and B. B. Finlay. 1994. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect. Immun. 62:3038-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 21.Gomez, H., T. Ochoa, L. Carlin, and T. Cleary. 2003. Human lactoferrin impairs virulence of Shigella flexneri. J. Infect. Dis. 187:87-95. [DOI] [PubMed] [Google Scholar]
- 22.Gomez, H., T. Ochoa, I. Herrera-Insua, L. Carlin, and T. Cleary. 2002. Lactoferrin protects rabbits from Shigella flexneri-induced inflammatory enteritis. Infect. Immun. 70:7050-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez, H. F., I. Herrera-Insua, M. M. Siddiqui, V. A. Diaz-Gonzalez, E. Caceres, D. S. Newburg, and T. G. Cleary. 2001. Protective role of human lactoferrin against invasion of Shigella flexneri M90T. Adv. Exp. Med. Biol. 501:457-467. [DOI] [PubMed] [Google Scholar]
- 24.Goosney, D. L., D. G. Knoechel, and B. B. Finlay. 1999. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg. Infect. Dis. 5:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson, L. A. 1999. Human milk and host defense: immediate and long-term effects. Acta Paediatr. Suppl. 88:42-46. [DOI] [PubMed] [Google Scholar]
- 26.Hanson, L. A., F. Jalil, R. Ashraf, S. Bernini, B. Carlsson, J. R. Cruz, T. Gonzalez, M. Hahn-Zoric, L. Mellander, Y. Minoli, et al. 1991. Characteristics of human milk antibodies and their effect in relation to the epidemiology of breastfeeding and infections in a developing country. Adv. Exp. Med. Biol. 310:1-15. [DOI] [PubMed] [Google Scholar]
- 27.Hartland, E. L., S. J. Daniell, R. M. Delahay, B. C. Neves, T. Wallis, R. K. Shaw, C. Hale, S. Knutton, and G. Frankel. 2000. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol. Microbiol. 35:1483-1492. [DOI] [PubMed] [Google Scholar]
- 28.Hendrixson, D. R., J. Qiu, S. C. Shewry, D. L. Fink, S. Petty, E. N. Baker, A. G. Plaut, and J. W. St. Geme III. 2003. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 47:607-617. [DOI] [PubMed] [Google Scholar]
- 29.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 33.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruzel, M. L., Y. Harari, D. Mailman, J. K. Actor, and M. Zimecki. 2002. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin. Exp. Immunol. 130:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, W. J., J. L. Farmer, M. Hilty, and Y. B. Kim. 1998. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 66:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loureiro, I., G. Frankel, J. Adu-Bobie, G. Dougan, L. R. Trabulsi, and M. M. Carneiro-Sampaio. 1998. Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia coli virulence-associated proteins: intimin, BfpA, EspA, and EspB. J. Pediatr. Gastroenterol. Nutr. 27:166-171. [DOI] [PubMed] [Google Scholar]
- 37.Manjarrez-Hernandez, H. A., S. Gavilanes-Parra, E. Chavez-Berrocal, A. Navarro-Ocana, and A. Cravioto. 2000. Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun. 68:5030-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez, M. B., C. R. Taddei, A. Ruiz-Tagle, L. R. Trabulsi, and J. A. Giron. 1999. Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J. Infect. Dis. 179:269-274. [DOI] [PubMed] [Google Scholar]
- 39.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguera-Obenza, M., T. J. Ochoa, H. F. Gomez, M. L. Guerrero, I. Herrera-Insua, A. L. Morrow, G. Ruiz-Palacios, L. K. Pickering, C. A. Guzman, and T. G. Cleary. 2003. Human milk secretory antibodies against attaching and effacing Escherichia coli antigens. Emerg. Infect. Dis. 9:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parissi-Crivelli, A., J. M. Parissi-Crivelli, and J. A. Giron. 2000. Recognition of enteropathogenic Escherichia coli virulence determinants by human colostrum and serum antibodies. J. Clin. Microbiol. 38:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plaut, A. G., J. Qiu, and J. W. St. Geme III. 2000. Human lactoferrin proteolytic activity: analysis of the cleaved region in the IgA protease of Haemophilus influenzae. Vaccine 19(Suppl. 1):S148-S152. [DOI] [PubMed] [Google Scholar]
- 44.Qiu, J., D. R. Hendrixson, E. N. Baker, T. F. Murphy, J. W. St. Geme III, and A. G. Plaut. 1998. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 95:12641-12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanches, M. I., R. Keller, E. L. Hartland, D. M. Figueiredo, M. Batchelor, M. B. Martinez, G. Dougan, M. M. Careiro-Sampaio, G. Frankel, and L. R. Trabulsi. 2000. Human colostrum and serum contain antibodies reactive to the intimin-binding region of the enteropathogenic Escherichia coli translocated intimin receptor. J. Pediatr. Gastroenterol. Nutr. 30:73-77. [DOI] [PubMed] [Google Scholar]
- 46.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 47.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw, R. K., S. Daniell, F. Ebel, G. Frankel, and S. Knutton. 2001. EspA filament-mediated protein translocation into red blood cells. Cell. Microbiol. 3:213-222. [DOI] [PubMed] [Google Scholar]
- 50.Stein, M. A., D. A. Mathers, H. Yan, K. G. Baimbridge, and B. B. Finlay. 1996. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect. Immun. 64:4820-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart, J., S. Norrell, and J. P. Harrington. 1984. Kinetic effect of human lactoferrin on the growth of Escherichia coli 0111. Int. J. Biochem. 16:1043-1047. [DOI] [PubMed] [Google Scholar]
- 52.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Victora, C. G., P. G. Smith, J. P. Vaughan, L. C. Nobre, C. Lombardi, A. M. Teixeira, S. C. Fuchs, L. B. Moreira, L. P. Gigante, and F. C. Barros. 1989. Infant feeding and deaths due to diarrhea. A case-control study. Am. J. Epidemiol. 129:1032-1041. [DOI] [PubMed] [Google Scholar]
- 55.Visca, P., C. Dalmastri, D. Verzili, G. Antonini, E. Chiancone, and P. Valenti. 1990. Interaction of lactoferrin with Escherichia coli cells and correlation with antibacterial activity. Med. Microbiol. Immunol. 179:323-333. [DOI] [PubMed] [Google Scholar]
- 56.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward, P. P., S. Uribe-Luna, and O. M. Conneely. 2002. Lactoferrin and host defense. Biochem. Cell Biol. 80:95-102. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, R. K., R. K. Shaw, S. Daniell, S. Knutton, and G. Frankel. 2001. Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli. Cell. Microbiol. 3:753-762. [DOI] [PubMed] [Google Scholar]
- 59.Yamauchi, K., M. Tomita, T. J. Giehl, and R. T. Ellison III. 1993. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 61:719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]
- 61.Zagulski, T., P. Lipinski, A. Zagulska, S. Broniek, and Z. Jarzabek. 1989. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 70:697-704. [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, G. H., D. M. Mann, and C. M. Tsai. 1999. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect. Immun. 67:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]