Abstract
Aims
To investigate whether results from case control and cross sectional studies which suggest an association between laxative use and other drug use could be confirmed in a cohort study of nursing home patients.
Methods
A prospective cohort study of 2355 nursing home patients aged 65 years and over was performed to estimate the incidence relative risk of constipation associated with drug use. The study was conducted with prescription sequence analysis of each resident's detailed pharmacy records and data on morbidity and mobility.
Results
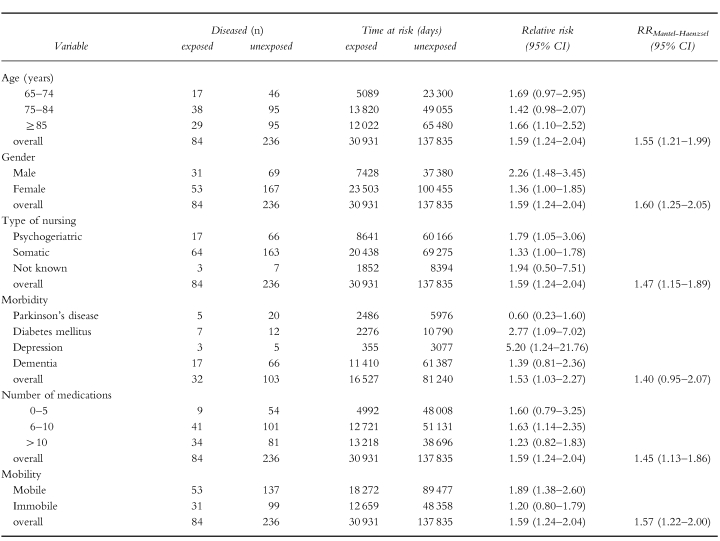
Use of drugs, which according to the summaries of product characteristics (SPC) and the literature on adverse drug effects have moderately to strongly constipating properties, was associated with a relative risk of 1.59 (95% CI 1.24–2.04) for the occurrence of constipation during exposure time. Use of drugs with mildly to moderately constipating effects was not associated with laxative use (RR 1.13; 95% CI 0.93–1.38). Stratification on the level of age, gender, type of nursing (psychogeriatric or somatic), morbidity, number of medications taken and mobility showed no confounding effects of these variables on outcome measurements. These variables all acted as effect modifiers. Effect of age and number of medications taken on the relative risk was nonlinear.
Conclusions
Although an association between drugs that exhibit moderately to strongly constipating effects and occurrence of constipation was found, the risk was not as high as seen in previous studies. The high prevalence of constipation in nursing home patients is only partly due to adverse drug effects.
Keywords: adverse effects, cohort study, constipation, laxatives, nursing home patients, prescription sequence analysis
Introduction
Many studies have reported laxative use in the elderly to be disturbingly high and it has been suggested that improved pharmacotherapy might reduce the prevalence of constipation [1]. The prevalence of constipation in ambulatory elderly over age 65 years varies from 16% to 41% [2, 3]. Chronic constipation may lead to complications such as faecal impaction, stercoral ulceration, bowel obstruction, sigmoid volvulus and even syncope [2]. The prevalence of constipation among institutionalized elderly has been reported to be even higher [4, 5]. In a population of 784 nursing home patients in the Netherlands, 53% were prescribed one or more laxatives daily [5]. Long-term use of stimulant laxatives may lead to abdominal cramps, fluid and electrolyte disturbances, malabsorption and cathartic colon [6].
In view of these unwanted effects and to improve the quality of life of the elderly it is worthwhile to study whether laxative use can be reduced in this population. Polypharmacy is an important risk factor for constipation, especially in nursing homes where levels of medication use are high [7]. Drugs which are commonly associated with constipation are opioids, iron salts, calcium channel blockers and drugs with anticholinergic/antimuscarinic effects [5]. The last group is also responsible for other potentially dangerous adverse effects in the elderly such as urinary retention, memory problems, delirium and acute glaucoma [8, 9].
In pharmacoepidemiological studies, laxative administration is used as a proxy for constipation because laxative use has been shown to correlate well with constipation [1]. The association between laxative use and other drug use has been assessed in several studies [1–3, 10, 11]. In most of these studies, only some subgroups of drugs were considered and the majority of these studies used cross-sectional study designs.
To investigate whether the suggested causal association between laxative use and comedication could be confirmed in a cohort of nursing home residents in the Netherlands we carried out a prospective study using prescription sequence analysis. If any such causal association exists, recommendations can subsequenly be given for alternative pharmacotherapy in order to reduce laxative use in the elderly.
Methods
Design
A prospective cohort study was performed to estimate the incidence relative risk of constipation as an adverse effect of drug use. The study was conducted with prescription sequence analysis of computerized pharmacy records.
Prescription sequence analysis is a method to determine side-effects of drugs through individual medication histories. It is based on the observation that a side-effect of drug A is followed by the prescription of drug B (a ‘proxy’ drug) if drug B is used to counteract the side-effect caused by drug A [12]. In this study, laxative drugs (all drugs in the Anatomical Therapeutic Chemical (ATC) classification A06 and A02AA02 [13]) were considered proxy drugs for constipation.
Setting
The study was undertaken in six nursing homes for long-term care with a 1030-bed capacity in the northern part of the Netherlands. In these nursing homes medical care is provided by nursing home physicians, who give medical care on a daily basis. Specialists’ medical input is obtained on demand.
A distinction is made between care for psychogeriatric residents and care for somatic residents. Nursing-, physician- and pharmacist care is comparable between the nursing homes. In each nursing home nursing staff defined constipation as not having defaecated for more than 3–5 days. Fluid- and fibre intake was comparable between the nursing homes.
Data collection
For each resident, pharmacy records of a 2 year period and individual morbidity and mobility data were collected. Pharmacy data included the generic name, strength, dosage, the frequency of use and the route of administration of the drugs, the prescription length (in days) and the following patient characteristics: age, gender, date of admission and date of discharge. Drugs were classified according to the ATC classification system [13]. Dermatological preparations were excluded from the analyses. Pharmacy records were linked with a national information system on nursing homes (SIVIS) [14], to collect the following data: type of nursing (psychogeriatric or somatic), morbidity and mobility.
Study population
All nursing home residents from six nursing homes were initially included in the cohort. The study population consisted of 2772 residents aged 65 years and over who were present at any time during the 2 year study period from 1 October 1993–1 October 1995. We excluded patients who could not be linked to data from the SIVIS-system (14.2%), and subsequently patients whose period of residence could not be defined as a result of missing data (1%). This resulted in a final study population of 2355 patients. Of these patients 65% were newly admitted during the study period.
Exposure definition
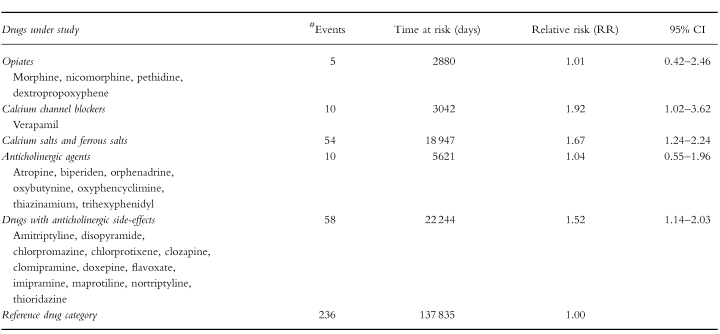
Drugs were classified into three categories: category 2: drugs that exhibit moderately to strongly constipating effects (see Appendix A), category 1: drugs that exhibit mildly to moderately constipating effects (see Appendix B) and a reference category which contained all other drugs. For each drug the summary of product characteristics (SPC) edited and approved by the Dutch Medicines Evaluation Board [15], provided the main source for the classification of the constipating effects of the drugs used by the study population, together with specific information on adverse drug effects from the literature [16, 17]. Each resident's exposure time was defined as the duration of drug use from category 1 or 2, respectively. To control for residual effects we performed both a study in which we defined exposure time as the duration of drug use plus the first 14 days after every exposure period and a study in which we excluded the first 14 days after every exposure period from both exposure time and nonexposure time. To investigate if any differences in constipating properties exist between certain subgroups of drugs from category 2, we performed subgroup analyses on pharmacological subgroups (see Table 4). Non-exposure time was defined as the remainder of the period of stay during the study period. Exposure days to category 2 drugs, to category 1 drugs, and nonexposure days were aggregated over the study population.
Table 4.
Subgroup analyses of drug groups from category 2.
Case definition
The occurrence of constipation was identified by the start of a laxative, which is considered a proxy drug. When the start of a laxative coincided with the start of a drug from category 2 or 1, the start was considered as a prophylactic start; these starts were not considered as cases. When the start of a laxative coincided with the date of admission to the nursing home or with the first day of the study period, the start was not considered as a case either. Patients were considered to be ‘at risk’ for constipation during the period of stay in which they did not use a laxative.
Analysis
Incidence rates during exposure and nonexposure time, respectively, were calculated by dividing the number of starts of laxative use by the total number of person-days at risk both during exposure time and during nonexposure time at risk. The incidence relative risk is determined as Iexp/Inonexp. Mantel-Haenszel relative risks were calculated to control for potential confounding effects of age, gender, morbidity (Parkinson's disease, diabetes mellitus, depression and dementia), type of nursing (psychogeriatric or somatic), number of medications taken and mobility. All statistical analyses were performed in SPSS for Windows [18]. Incidence relative risks were calculated with corresponding 95% confidence intervals (95% CI).
Results
Population characteristics
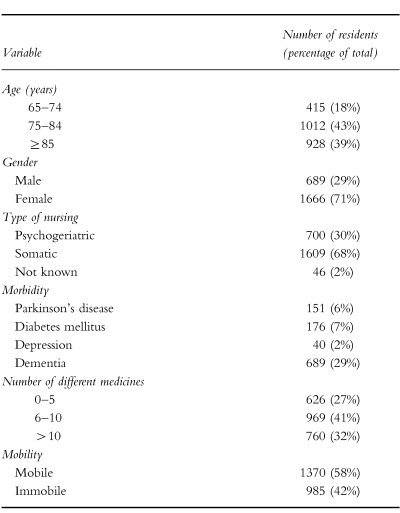
The mean age of the study population was 82 years (s.d. 7.3). The average residence time during the study period was 257 days (s.d. 260). The average number of different medicines (based on ATC-codes) per person was 8.9 during residence in the nursing home (s.d. 4.9; dermatological preparations excluded). The average number of different medicines per patient per day 4.9. Most drugs were used for more than 50% of the duration of stay in the nursing home.
In Table 1, characteristics of the study population are given. Of the mobile residents 35% were diagnosed with dementia, while 22% of the immobile residents were diagnosed with dementia. Forty-six percent of the study population used a drug from category 2; 57% of the study population used a drug from category 1.
Table 1.
Characteristics of the study population (n=2355).
Laxative use
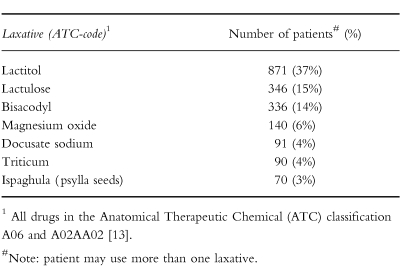
Fifty-six percent of the study population used a laxative at some time during stay at the nursing home. An overview of the laxatives used by the study population is given in Table 2. Seventy-four percent of the residents with Parkinson's disease used a laxative. At the entry of the study period, 416 (18%) of the patients used a laxative. After the study entry date 1109 (47%) patients started a laxative for one or more periods. Of these patients 233 (21%) used a laxative for a period of less than 30 days, and 876 (79%) patients used a laxative for a period of 30 days or more. The average duration of laxative use was 154 days (s.d. 192). On average, people who were on a laxative drug used it for more than 77% of their nursing home stay. Relatively high dosages of laxatives were used.
Table 2.
Laxatives used by the study population (n=2355).
Incidence rate ratios
The results from the cohort study are presented in Table 3. Use of drugs from category 2 (moderately to strongly constipating drugs) was associated with a relative risk of 1.59 (95% CI 1.24–2.04) for the occurrence of constipation and the incidence relative risk of exposure to category 1 drugs (mildly to moderately constipating drugs) was 1.13 (95% CI 0.93–1.38) compared with nonexposure. To control for residual effects we performed both a study in which we defined exposure time as the duration of drug use plus the first 14 days after every exposure period and a study in which we excluded the first 14 days after every exposure period from both exposure time and nonexposure time. When exposure time was defined as the duration of category 2 drug use plus the first 14 days after this period, the incidence relative risk was slightly higher (RR 1.69; 95% CI 1.33–2.15), indicating a carry-over effect from category 2 drug exposure in the nonexposure period. To exclude this carry-over effect, we deleted the first 14 days after exposure time from both exposure and nonexposure time, which resulted in an incidence relative risk of 1.60 (95% CI 1.25–2.06). Results of the subgroup analyses are given in Table 4. Point estimates varied from 1.01 (opiates) to 1.92 (verapamil) but the differences were not all statistically significant. Ninety-six percent of the people who received opiates received a laxative drug prior to the initiation of opiate use. Statistical analysis of possible confounding effects of the variables given in Table 1 showed no confounding effects from these variables as shown in Table 5. Gender, morbidity and mobility acted as effect modifiers. There was a nonlinear association with age and with the number of medications taken. Residents with depression and residents with diabetes mellitus were more at risk for the occurence of constipation as an adverse drug effect while residents with Parkinson's disease showed a markedly lower risk. Residents who were relatively mobile showed a higher risk for the occurrence of drug-induced constipation.
Table 3.
Relative risks for the occurrence of constipation associated with different drug categories.
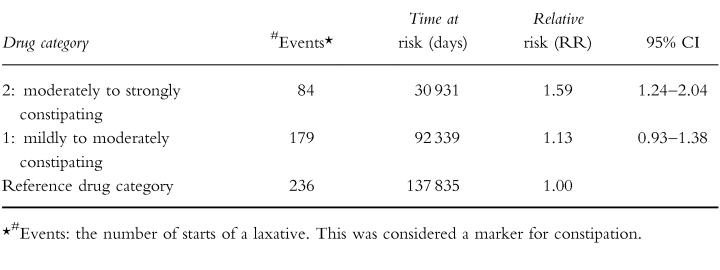
Table 5.
Relative risks for the occurrence of constipation associated with exposure to category 2 drugs, stratified for age, gender, type of nursing, mobility, number of medications and morbidity.
Discussion
Our study confirms earlier findings of a risk of constipation as a consequence of drug use. However, from this cohort study the relative risk appears to be lower than has been suggested in previous case control and cross-sectional studies. In 2355 nursing home patients, the use of drugs that exhibit moderately to strongly constipating effects was slightly but significantly associated with the start of a laxative (RR 1.59; 95% CI 1.24–2.04). When the first 14 days after every exposure period were added to the exposure time, the relative risk was slightly higher (RR 1.69; 95% CI 1.33–2.15), which indicates residual effects of these drugs depending on the elimination half-life. The results show that drugs which according to the summaries of product characteristics and to the literature on adverse drug effects exhibit a moderately to strongly constipating effect, in practice are only marginally associated with the occurrence of constipation. However, the fact that drugs from category 2 are used by nearly half of the study population at least once during the study period suggests that this side-effect could be clinically relevant in daily practice because it concerns many residents. Drugs that have been reported to have mild to moderate constipating effects were not associated significantly with constipation (RR 1.13; 95% CI 0.93–1.38). This means that although constipation is mentioned as a possible side-effect in the summaries of product characteristics and in the literature, the high prevalence of constipation is probably not due to use of drugs from this category. Subgroup analyses demonstrated that the use of the calcium channel blocker verapamil and the use of calcium- and ferrous salts, especially, was associated with a high risk for the occurrence of constipation. The fact that laxatives are given prophylactically with this drug category, explains why use of opiates was not associated with a higher risk. Several reports support our results. Mikus et al. recently showed that the constipating effect of codeine is only seen in extensive metabolizers (CYP2D6 phenotype) [20]. In a literature search (1965–94) concerning adverse events associated with antidepressant drugs, constipation did not belong to one of the 27 most frequently reported adverse events [21]. In a community based study no significant association was found between antidepressant drug use and use of laxatives [22].
Previous studies
In the study of Stewart et al. [2], a positive correlation was demonstrated between self-reported constipation and the total number of drugs used in an ambulatory elderly population, but no specific drug groups were correlated with constipation. Talley et al. [3] demonstrated that use of nonsteroidal anti-inflammatory drugs was a significant risk factor in elderly subjects with both functional constipation and outlet delay. However, whether this was a causal association remained unclear. In a cross-sectional study Monane et al. [1] found a strong association between laxative use and the use of highly anticholinergic antidepressants (OR 3.12; 95% CI 1.21–8.03) in nursing home patients. Also in a cross-sectional study Harari et al. [11] demonstrated that the use of iron supplements and calcium channel blockers was significantly associated with laxative use (OR 2.2 and OR 1.9, respectively) in elderly people residing in a long-term care setting. To our knowledge, our study is the first that uses a cohort design to determine the association between medication use and laxative administration in a nursing home population. In a cohort design, laxative use as a result of medication use (sequential use) can be properly assessed with prescription sequence analysis. With cross-sectional methods, temporal sequences of prescribing are more difficult to assess [12].
Possible bias
Selection bias
We excluded all nursing home residents for whom data were incomplete or invalid. Since they represented a small proportion of the population, this is not likely to have infuenced the results. Information for each resident was obtained from the same data set. Therefore it is unlikely that selection bias played a role in this study.
Information bias
Information bias might occur when a laxative is prescribed for an indication other than constipation. This could lead to a bias away from the null in both the exposed and nonexposed group. As the only other indication for the prescription of lactulose is hepatic (pre)coma, a very rare disease, it is not likely that it leads to differential misclassification. Also a laxative could be withheld from a patient suffering from constipation. This could lead to a bias towards the null. This kind of bias is not likely to occur often because residents are frequently monitored by nurses or carers, so constipation will be noticed at an early stage. However, this kind of bias could be relevant when the problem of constipation is considered (more constipated residents), but probably is not relevant when drug-induced constipation is considered (bias will occur in both exposed and nonexposed groups). Medication use of the individual nursing home resident is registrated centrally in one of the three computerized hospital pharmacies involved in our study. Dispensing of drugs takes place when the registration of medication is complete. Therefore information bias is not likely to occur.
Confounding bias
We tried to control for possible confounding patient characteristics such as age, gender, type of nursing, number of medications taken, morbidity and mobility. None of these variables was considered a confounder although some of the variables was considered effect modifiers (see below). No marked differences were seen in overall fluid- and fibre intake among the different nursing homes. Because we could not collect data on fluid and fibre intake at individual patient level, this may still confound our results. The influence of fluid and fibre intake on constipation has been assessed in only few studies. Although dietary fibre is often diminished in the elderly, no clear association has been made with true clinical constipation [7]. There is no data on dehydration as a risk factor for constipation in the elderly although the beneficial effect of fluid intake has been proven in young male volunteers [7]. A community based cohort study by Towers et al. showed that constipation was related to caloric intake rather than fibre consumption or fluid intake [19]. In our study a resident may develop constipation as a consequence of low fluid and fibre intake. When this patient is using drugs from category 1 or 2, this patient would be wrongly considered as a ‘case’. This might lead to a overestimation of the relative risk but it does not change our conclusion. Other possible confounders such as age and comorbidity did not play a role in our study. Obviously, we can never rule out confouding effects of factors that we are not aware.
Effect modifiers
Several other factors have been reported to be associated with constipation in the elderly. Bowel frequency is decreased with certain neurological and endocrine disorders such as Parkinson's disease and diabetes mellitus. Older people and women are reported to be more at risk for constipation. The type of nursing, psychogeriatric or somatic, can influence outcome. In several studies, inactivity and immobility have been identified as risk factors for constipation. Although data in the elderly are scarce, Towers et al. [19] showed that constipated elderly tend to report less regular activity and exercise. Therefore we stratified for the variables age, gender, type of nursing, morbidity, number of medications taken and mobility to control for possible confouding effects. Gender, morbidity and mobility acted as effect modifiers. The nonlinear association with age and number of medications taken suggests a combined effect of effect modification and confounding by these variables. Men were at a higher risk for the occurrence of constipation during category 2 drug exposure in comparison with women. Residents suffering from Parkinson's disease showed a markedly lower relative risk, probably because these residents are already constipated. Patients with depression and diabetes mellitus showed a higher risk for the occurence of constipation as an adverse drug effect. Finally, residents who were relatively mobile were more at risk for the occurrence of constipation during category 2 exposure. When pharmacotherapy is needed, the possible constipating effects of category 2 drugs should be taken into account with special reference to the patients’ gender, comorbidity, number of medications taken and mobility. In these risk groups in particular the use of category 2 drugs is associated with the occurrence of constipation. Furthermore, alternative pharmacotherapy could be considered with certain subgroups of category 2 drugs. For example, the necessity of verapamil, calcium salts and ferrous salts should be (re-)evaluated carefully. The use of newer antidepressants (such as selective serotonin reuptake inhibitors and reversible monoamine oxidase type A inhibitors) and antipsychotics with minor or no anticholinergic activity could be considered as alternatives to antidepressants and antipsychotics with anticholinergic side-effects.
In conclusion, this study demonstrates that medication, which according to their SPCs and the literature on adverse drug effects exhibit moderately to strongly constipating effects, is associated with the occurrence of constipation in a cohort of nursing home patients. However, the risk is not as high as previous studies suggest. Drugs which were classified as mildly to moderately constipating showed no increased risk for the occurrence of constipation. The high prevalence of laxative use in nursing home patients is only partly due to adverse drug effects. To minimize the risk for constipation, alternative pharmacotherapy could be considered for certain subgroups of drugs.
Acknowledgments
We express our gratitude to D. A. Bloemhof, pharmacist, for supplying pharmacy data; SIG Informatics on Health and Welfare, Utrecht, for supplying patient morbidity and mobility data; and nursing, medical and pharmacy staff from all participating nursing homes for their cooperation.
Appendix A
Drugs classified as moderately to strongly constipating [15-17].

Appendix B
Drugs classified as mildly to moderately constipating [15-17].
References
- 1.Monane M, Avorn J, Beers MH, Everitt DE. Anticholinergic drug use and bowel function in nursing home patients. Arch Intern Med. 1993;153:633–638. [PubMed] [Google Scholar]
- 2.Stewart RB, Moore MT, Marks RG, Hale WE. Correlates of constipation in an ambulatory elderly population. Am J Gastroenterol. 1992;87:859–864. [PubMed] [Google Scholar]
- 3.Talley NJ, Fleming KC, Evans JM, et al. Constipation in an elderly community: a study of prevalence and potential risk factors. Am J Gastroenterol. 1996;1:19–25. [PubMed] [Google Scholar]
- 4.Harari D, Gurwitz JH, Avorn J, Choodnovskiy I, Minaker KL. Constipation: assessment and management in an institutionalized elderly population. J Am Geriatr Soc. 1994;42:947–952. doi: 10.1111/j.1532-5415.1994.tb06585.x. [DOI] [PubMed] [Google Scholar]
- 5.Brouwers JRBJ, Tytgat G. Laxatives for elderly people with constipation. Formulary criteria, precautions and complications. Pharm Weekbl. 1993;128:1483–1487. [Google Scholar]
- 6.Wald A. Constipation in elderly patients. Pathogenesis and management. Drugs Aging. 1993;3:220–231. doi: 10.2165/00002512-199303030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Harari D, Gurwitz JH, Minaker KL. Constipation in the elderly. J Am Geriatr Soc. 1993;41:1130–1140. doi: 10.1111/j.1532-5415.1993.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging. 1993;3:335–348. doi: 10.2165/00002512-199303040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Riedel WJ, Van Praag HM. Avoiding and managing anticholinergic effects of antidepressants. CNS Drugs. 1995;3:245–259. [Google Scholar]
- 10.Jones RH, Tait CL. Gastrointestinal side-effects of NSAIDs in the community. Br J Clin Pract. 1995;49:67–70. [PubMed] [Google Scholar]
- 11.Harari D, Gurwits JH, Avorn J, Choodnovskiy I, Minaker KL. Correlates of regular laxative use by frail elderly persons. Am J Med. 1995;99:513–518. doi: 10.1016/s0002-9343(99)80228-9. [DOI] [PubMed] [Google Scholar]
- 12.Petri JL, De Vet HCW, Naus J, Urquhart J. Prescription sequence analysis: a new and fast method for assessing certain adverse reactions of prescription drugs in large populations. Statis Med. 1988;7:1171–1175. doi: 10.1002/sim.4780071110. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. Anatomical Therapeutic Chemical (ATC ) classification index. Oslo: WHO collaborating centre for drug statistics methodology; 1994. [Google Scholar]
- 14.SIG. Informatics in Health and Welfare. Utrecht, The Netherlands.
- 15.Dutch Medicines Evaluation Board. Rijswijk, The Netherlands.
- 16.Informatorium Medicamentorum. 1994. Royal Dutch Society for the Advancement of Pharmacy, The Hague, The Netherlands.
- 17.Dukes MNG, editor. Meyler's side effects of drugs. 12. Amsterdam: Elsevier Science Publishers; 1992. [Google Scholar]
- 18.Norusis. MJSPSS. 6.1. Guide to data analysis. New Jersey: Prentice-Hall Inc. Englewood Cliffs; [Google Scholar]
- 19.Towers AL, Burgio KL, Locher JL, Merkel IS, Safaeian M, Wald A. Constipation in the elderly: influence of dietary, psychological, and physiological factors. J Am Geriatr Soc. 1994;42:701–706. doi: 10.1111/j.1532-5415.1994.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 20.Mikus G, Trausch B, Rodewald C, et al. Effect of codeine on gastrointestinal motility in relation to CYP2D6 phenotype. Clin Pharmacol Ther. 1997;61:259–266. doi: 10.1016/S0009-9236(97)90196-X. [DOI] [PubMed] [Google Scholar]
- 21.Egberts ACG, Koning de GHP, Bakker A, Leufkens HGM. Pharmacoepidemiologic approaches to the evaluation of antidepressant drugs (thesis) Utrecht: Utrecht University; 1997. Adverse events associated with antidepressant drugs published in the medical literature. In Egberts ACG. [Google Scholar]
- 22.Egberts ACG, Leufkens HGM, Launer LJ, Grobbee DE, Hofman A, Hoes AW. Pharmacoepidemiologic approaches to the evaluation of antidepressant drugs (thesis) Utrecht: Utrecht University; 1997. Characteristics of older subjects using antidepressant drugs. In Egberts ACG. [Google Scholar]


