Abstract
Aims
The aim of this study was to compare the effects of the ACE-inhibitor lisinopril and the angiotensin II receptor antagonist losartan on insulin sensitivity in the treatment of non diabetic hypertensives.
Methods
Twenty-five non diabetic subjects with mild to moderate hypertension, 11 females and 14 males, aged 44–63 years, after a 4-week wash-out period on placebo, were randomized to receive lisinopril 20 mg once daily or losartan 50 mg once daily for 6 weeks. Following another 4-week wash-out period, patients were crossed to the alternative regimen for further 6 weeks. At the end of the placebo and of the active treatment periods, blood pressure (BP) was measured (by standard mercury sphygmomanometer, Korotkoff I and V) and insulin sensitivity was assessed by the euglycaemic hyperinsulinaemic clamp technique. Glucose infusion rate (GIR) during the last 30 min of clamp and total glucose requirement (TGR) were evaluated.
Results
Both lisinopril and losartan significantly reduced SBP (by a mean of 20.2 and 17.2 mmHg, respectively) and DBP (by a mean of 15.2 and 12.3 mmHg, respectively), with no difference between the two treatments. GIR, used as an indicator of insulin sensitivity, was significantly increased by lisinopril (+1.5 mg min−1 kg−1, P < 0.05 vs baseline) but not by losartan (+0.42 mg min−1 kig−1, NS), the difference between the two drugs being statistically significant (P < 0.05). TGR was increased by lisinopril (+7.3 g, P < 0.05 vs baseline), whereas losartan did not significantly modify it (+1.9 g, NS).
Conclusions
In conclusion, with all cautions due to an absence in this study of a randomized placebo phase, our findings suggest that lisinopril improved insulin sensitivity whereas losartan did not affect it.
Keywords: hypertension treatment, insulin sensitivity, lisinopril, losartan
Introduction
Insulin resistance has been shown in subjects with essential hypertension and has been proposed as a metabolic link between hypertension, noninsulin-dependent diabetes mellitus (NIDDM), obesity, dyslipidaemia and atherosclerotic cardiovascular disease [1, 2]. Therefore, in the treatment of hypertension it is argued that consideration should be given to the effect of antihypertensive agents on insulin sensitivity.
Commonly used antihypertensive drugs, such as thiazide diuretics and β-adrenoceptor blockers, have been reported to impair insulin sensitivity, despite effectively lowering blood pressure [3, 4]. On the other hand, the angiotensin converting enzime inhibitors (ACE-I) have been found generally to have neutral or even favourable effects on glucose metabolism and on insulin sensitivity in both non diabetic [3, 5] and diabetic hypertensive patients [6–8].
Recently, specific antagonists of angiotensin II (Ang II) have been introduced into clinical practice for the treatment of hypertension and congestive heart failure [9] and may be useful tools for better understanding the role of the renin-angiotensin system (RAS) in the influence of ACE-I on insulin sensitivity. To date contrasting results have been reported in both experimental and clinical studies about the effect of Ang II antagonists on insulin sensitivity with some studies showing no influence [10–12] and other an improvement in insulin sensitivity [13–15].
The aim of this study was to evaluate the effects on insulin sensitivity of the ACE-I lisinopril [16] as compared to the Ang II antagonist losartan [17] in the treatment of nondiabetic patients with essential hypertension. We choose to test lisinopril because a few studies comparing the effects of different ACE-I in the treatment of non diabetic hypertensives indicated that all the ACE-I-tested produced a slight improvement in glucose and lipid metabolism, but lisinopril displayed the greatest metabolic response [6, 18].
Methods
This was a randomized, double-blind, cross-over trial. Subjects included in the study were 25 (14 males and 11 females) non diabetic, non obese (BMI < 30) outpatients, aged 42–63 years (mean age: 55 (2 year)), with mild to moderate, uncomplicated essential hypertension (DBP ≥95 and ≤114 mmHg).
Patients were excluded if they had severe target organ damage, active ischaemic heart disease, renal failure (serum creatinine > 114.9 (mol l−1)), evidence of chronic liver disease, active peptic ulcer or any gastrointestinal disease that may affect absorbtion, pregnancy or lactation. The study protocol was approved by the local Ethics Committee and informed consent was obtained from each subject at the time of enrolment.
After an initial 4-week wash-out period, during which antihypertensive medications, if any, were discontinued and placebo was administered, patients were randomized to receive lisinopril 20 mg once daily or losartan 50 mg once daily for 6 weeks. Following another 4-week wash-out period on placebo, patients were crossed to the alternative regimen for a further 6 weeks. From the time of enrollment until completion of the study, each participant maintained his or her diet, usual level of physical activity and avoided a change in body weight.
At the end of each placebo and active treatment period blood pressure (BP), body weight and insulin sensitivity were evaluated. BP measurements were obtained from each subject (right arm) in the seated position, using a standard mercury sphygmomanometer (Korotkoff I and V) with a cuff of appropriate size. Measurements were taken in the morning, after the subject had rested for 10 min in a quiet room. Three successive BP readings were obtained at 1 min intervals and averaged.
Insulin sensitivity was assessed by the euglycaemic, hyperinsulinaemic clamp, according to the technique of De Fronzo et al. [19]. At 08.00 h, after the subjects had fasted 12 h overnight, an intravenous catheter was placed in an antecubital vein for infusion of insulin and 20% glucose. A second catheter was inserted into a brachial artery for blood sampling. A 10 min priming infusion of insulin (Actrapid, HM, Novo Industries, Copenhagen, Denmark), calculated as the amount required to raise plasma insulin concentration to 100 μU ml−1 during the insulin clamp, was followed by a constant infusion of 40 mU min−1 m−2 of body surface area for 110 min. During insulin infusion, normal fasting blood glucose levels were maintained by adjustement of the infusion of a 20% glucose solution. The amount of glucose taken up (mg kg−1of body weight min−1) was calculated for each 10 min interval after the first 20 min of the clamp. Insulin sensitivity was calculated from the mean glucose uptake rate for the last 30 min of the clamp and expressed as the amount of glucose infused during that time (glucose infusion rate-GIR) in mg kg−1min−1. The total amount of exogenous glucose required to maintain a steady state blood glucose level in response to a defined increase in plasma insulin concentration (total glucose requirement-TGR) was also evaluated. Metabolic parameters including fasting plasma glucose and insulin, total cholesterol (TC), high-density-lipoprotein cholesterol (HDL-C) and total triglycerides (TG) were measured at the end of the placebo and active treatment periods.
Blood glucose in the fasting state and during glucose clamp studies was measured by the glucose oxidase method (Beckman Auto-Analywer, Fullerton, USA). Plasma insulin concentrations were determined by radioimmunoassay (r.i.a.). TC and TG levels were assayed by automated enzymatic methods.
Data are presented as means±standard deviations. The statistical analysis of the data was performed by analysis of variance (anova) for repeated measures; 95% confidence intervals of the estimated differences between treatments and placebo were performed for the BP TGR and GIR values. The level of significance was set as P < 0.05.
In order to verify the basic assumptions of cross-over design, besides the estimation of period effect, the presence of carry-out or sequence effect was also investigated [20]. However, a period effect or, more significantly, a sequence effect was not found for any variable.
Results
The main results of the study are shown in Table 1 and Figures 1 and 2.
Table 1.
Mean values of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean glucose infusion rate (GIR) over the last 30 min of the clamp, total glucose requirement (TGR) to hold glucose level constant during the clamp, baseline fasting plasma insulin (before the insulin infusion was started), total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG) and body mass index (BMI) during treatment with lisinopril and losartan.
Figure 1.
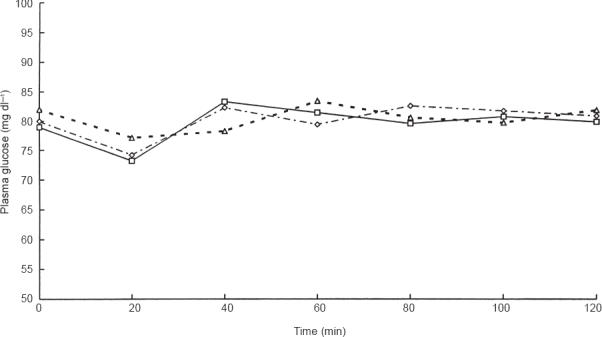
Plasma glucose levels (mg dl−1) during euglycaemic hyperinsulinaemic clamp under placebo (◊), lisinopril (□) and losartan (▵) treatment.
Figure 2.
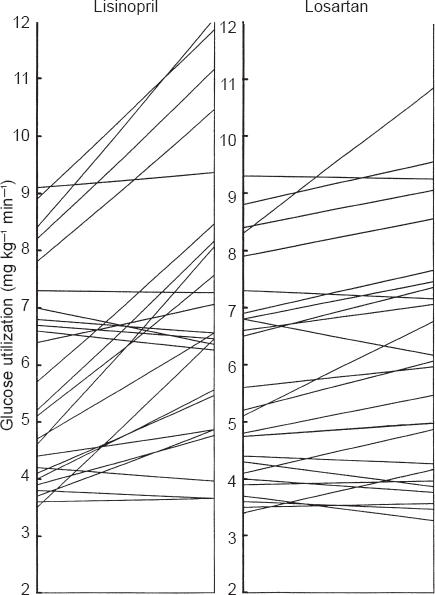
Insulin stimulated glucose utilization determined by clamp (last 30 min) presented for each subject at the end of the placebo treatment and at the end of lisinopril and losartan treatment.
Both drugs significantly reduced SBP: lisinopril by a mean of 20.2 mmHg (P < 0.0001 vs placebo), losartan by a mean of 17.2 mmHg (P < 0.0002 vs placebo). DBP was lowered by a mean of 15.2 mmHg with lisinopril (P < 0.001 vs placebo) and by a mean of 12.3 mmHg with losartan (P < 0.01 vs placebo). The mean difference in the response to lisinopril and losartan was 2.68 mmHg (95% CI: −1.57, 6.93) for SBP and 2.68 mmHg (95% CI: −0.32, 5.68) for DBP. The 95% CI showed no statistical difference between the two drugs although an advantage for lisinopril of almost 7 over 6 mmHg could not be excluded.
During the euglycaemic clamp, plasma glucose levels were maintained constant in the placebo as well in the two treatment groups (Figure 1). Fasting plasma insulin levels rose acutely and remained at steady state plateau (mean value: 108±5 μU ml−1 during placebo, 104±3 μU ml−1 during lisinopril and 106±4 μU ml−1 during losartan). The total amount of exogenous glucose required to hold glucose level constant during the clamp was not significantly modified by losartan as compared with placebo (mean difference: 1.92±2.58 g, 95% CI: −0.06, 3.83 NS), whereas it was significantly increased by lisinopril (by a mean of 7.36±3.75 g, 95% CI: 5.42, 9.30 P < 0.05 vs placebo), the difference between the two treatments being statistically significant (5.44 g, 95% CI: 3.61, 7.27). The mean rate of glucose uptake for the last 30 min of the clamp (GIR) was significantly increased by lisinopril (by a mean of 1.47±0.33 mg min−1 kg−1, 95% CI: 1.27, 1.67 P < 0.05 vs placebo) but not by losartan (0.42±0.30 mg min−1 kg−1, 95% CI: −0,27, 0,69, NS), the difference between the two drugs being statistically significant (0.99 mg min−1 kg−1, 95% CI: 0.81, 1.17).
Figure 2 provides the mean insulin stimulated glucose use in each subject at the end of the placebo period and at the end of lisinopril and losartan treatment.
There was no modification in body mass index (BMI), fasting blood glucose and plasma insulin levels in either the lisinopril and losartan treated subjects (Table 1). TC, HDL-C and TG did not change significantly during treatment with both drugs (Table 1).
Discussion
The results of this study show that, in nondiabetic patients with mild to moderate essential hypertension, monotherapy with both lisinopril 20 mg once daily and losartan 50 mg once daily significantly lowered BP values, with no statistical difference between the two treatments, which is in keeping with previous reports [16, 21–23].
Despite their equivalent BP lowering effect, lisinopril improved insulin sensitivity whereas losartan did not influence it. With all the caution due to the absence in this study of a randomized placebo phase, this finding suggests that the decrease in endogenous Ang II induced by ACE-inhibition may not contribute to the improvement in insulin sensitivity produced by lisinopril and supports a major contribute of increased kinin levels to this effect, although other mechanisms such as increased skeletal muscle blood flow and reduction in sympathetic tone, cannot obviously be excluded. Besides, 95% CI for differences between the two drugs in BP response suggested a possible advantage for lisinopril of almost 7 over 6 mmHg. Such a difference, although not statistically significant, might be clinically relevant and contribute to the different effect on insulin sensitivity.
The precise mechanism whereby kinins may exert a beneficial effect on insulin sensitivity remains inclear. However, kinins have been supposed to increase glucose and insulin delivery to tissues by inducing vasodilation, increasing vascular permeability and preventing vascular rarefaction [10, 24, 25].
The findings of the study are consistent with those reported by Tomiyama et al. [10] and Laakso et al. [11]. Similar results were obtained also by Moan et al. [12] using losartan in patients selected solely by blood pressure criteria (mild hypertension), whether they had insulin resistance or not.
However, the same authors in a previous study performed in five patients with severe hypertension, found that losartan improved insulin sensitivity with a decrease in plasma noradrenaline and blood viscosity [13]. Furthermore, in a study comparing the effects of an ACE-I, delapril, and another Ang II antagonist, TCV-116, on insulin sensitivity in an insulin resistant hypertensive rat model (fructose fed rats) and in essential hypertensive patients, Iimura et al. observed that both treatments were equally effective in improving insulin sensitivity in both rats and hypertensive humans, thus suggesting that the improvement of insulin resistance induced by the ACE-I might depend on Ang II action [14]. Similarly, Iyer & Katovich observed that both acute and chronic losartan treatment improved insulin sensitivity and glucose tolerance in the same experimental model (fructose fed rats) [15].
The discrepancy in the results of these studies might depend on several factors, which make them poorly comparable. Thus, for example, Tomiyama et al. used young SHR at an early stage of hypertension, whereas both Iimura and Iyer studied insulin resistant hypertensive rats, i.e. an animal model in which the potential of losartan for improving insulin sensitivity was maximized. Besides, patients with severe hypertension, like those studied by Moan et al. [13] may have more marked structural vascular changes in precapillary arteries and thus a greater potential for improvement to be induced by a vasodilatory compound such as losartan (given at high dosage) than patients with mild hypertension like those studied by Moan [12] and by us.
On the other hand, as suggested also by other authors [10], we cannot conclude that the renin angiotensin system and Ang II antagonists do not influence insulin sensitivity. In fact, the suppression or activation of the renin-angiotensin system may affect other neuroendocrine factors, which may influence insulin sensitivity. Besides, unlike ACE-I, AT1 antagonist therapy results in a feedback increase in the concentrations of Ang II which is able to act on AT2 receptors, the role of which is not clear. Furthermore, the potential effects of both ACE-I and Ang II antagonists on the local renin-angiotensin system, which might also influence insulin sensitivity, have not been evaluated. Thus, further studies are needed to clarify the effects of the renin-angiotensin system, including Ang II antagonists, on insulin sensitivity.
References
- 1.Ferrannini E, Haffner SM, Stern MP. Essential hypertension: an insulin resistant state. J Cardiovasc Pharmacol. 1990;15(Suppl 5):S18–S25. [PubMed] [Google Scholar]
- 2.De Fronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Pollare T, Lithell H, Berne C. A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med. 1989;321:868–873. doi: 10.1056/NEJM198909283211305. [DOI] [PubMed] [Google Scholar]
- 4.Pollare T, Lithell H, Selinus I, Berne C. Sensitivity to insulin during treatment with atenolol and metoprolol: a randomized, double-blind study of effects on carbohydrate and lipoprotein metabolism in patients with hypertension. N Engl J Med. 1989;321:868–873. doi: 10.1136/bmj.298.6681.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkner B, Canessa M, Anzalone D. Effect of angiotensin converting enzyme inhibitor (lisinopril) on insulin sensitivity and sodium transport in mild hypertension. Am J Hypertens. 1995;8:454–460. doi: 10.1016/0895-7061(95)00018-K. [DOI] [PubMed] [Google Scholar]
- 6.Paolisso G, Gambardella A, Verza M, et al. ACE-inhibition improves insulin sensitivity in aged insulin resistant hypertensive patients. J Human Hypertens. 1992;6:175–179. [PubMed] [Google Scholar]
- 7.Torlone E, Britta M, Rambotti AM. Improved insulin action and glycemic control after long-term angiotensin converting enzyme inhibition in subjects with arterial hypertension and type II diabetes. Diabetes Care. 1993;16:1347–1355. doi: 10.2337/diacare.16.10.1347. [DOI] [PubMed] [Google Scholar]
- 8.Shamiss A, Carrol J, Peleg E, Grossman E, Rosenthal T. The effect of enalapril with and without hydrochlorothiazide on insulin sensitivity and other metabolic abnormalities of hypertensive patients with NIDDM. Am J Hypertens. 1995;8:276–281. doi: 10.1016/0895-7061(94)00181-A. [DOI] [PubMed] [Google Scholar]
- 9.Lee RJ, Brunner HR. Clinical experience with angiotensin II receptor antagonists. J Human Hypertension. 7:36. [PubMed] [Google Scholar]
- 10.Tomiyama H, Kushiro T, Abeta H, et al. Kinins contribute to the improvement of insulin sensitivity during treatment with angiotensin converting enzyme inhibitor. Hypertension. 1994;23:450–455. doi: 10.1161/01.hyp.23.4.450. [DOI] [PubMed] [Google Scholar]
- 11.Laakso M, Karjalaainen L, Lempiainen-Kuosa P. Effects of losartan on insulin sensitivity in hypertensive subjects. Hypertension. 1996;28:392–396. doi: 10.1161/01.hyp.28.3.392. [DOI] [PubMed] [Google Scholar]
- 12.Moan A, Hoieggen A, Seljeflot I, Risanger T, Arnesen H, Kjeldsen SE. The effect of angiotensin II receptor antagonism with losartan on glucose metabolism and insulin sensitivity. J Hypertens. 1996;14:1093–1097. doi: 10.1097/00004872-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Moan A, Hoieggen A, Nordby G, Eide K, Kjeldsen SE. Effects of losartan on insulin sensitivity in severe hypertension: connections through sympathetic nervous system activity. J Human Hypertension. 1995;9(Suppl 5):S45–S50. [PubMed] [Google Scholar]
- 14.Iimura O, Shimamoto K, Matsuda K, et al. Effects of angiotensin receptor antagonist and angiotensin converting enzyme inhinitor on insulin sensitivity in fructose fed hypertensive rats and essential hypertensives. Am J Hypertens. 1995;8:353–357. doi: 10.1016/0895-7061(94)00245-7. [DOI] [PubMed] [Google Scholar]
- 15.Iyer S, Katovich M. Effect of acute and chronic losartan treatment on glucose tolerance and insulin sensitivity in fructose fed rats. Am J Hypertens. 1996;9:662–668. doi: 10.1016/0895-7061(96)00035-0. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster SG, Todd PA. Lisinopril. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension use in hypertension and congestive heart failure. Drugs. 1988;35:646–669. doi: 10.2165/00003495-198835060-00003. [DOI] [PubMed] [Google Scholar]
- 17.Goa KL, Nagstaff AJ. Losartan potassium. A review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. Drugs. 1996;51:820–845. doi: 10.2165/00003495-199651050-00008. [DOI] [PubMed] [Google Scholar]
- 18.Oksa A, Gajdos M, Fedelosova V, Spustova V, Dzurik R. Effects of angiotensin converting enzyme inhibitors on glucose and lipid metabolism in essential hypertension. J Cardiovasc Pharmacol. 1994;23:78–86. doi: 10.1097/00005344-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 19.DE, Fronzo RA, Tobin JA, Andres B. Glucose clamp technique, a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Senn SJ. Cross-over trials in clinical research. New York-London: John Wiley and Sons; 1993. [Google Scholar]
- 21.Nelson E, Merril D, Sweet C, et al. Efficacy and safety of oral MK-954 (DuP-753), an angiotensin II antagonist, in essential hypertension. J Hypertens. 1991;9(Suppl 6):S468–S469. [Google Scholar]
- 22.Tsunoda K, Aabe K, Hagino T, et al. Hypotensive effect of losartan, a non peptide angiotensin II receptor antagonist in essential hypertension. Am J Hypertens. 1993;6:28–32. doi: 10.1093/ajh/6.1.28. [DOI] [PubMed] [Google Scholar]
- 23.Grossman E, Peleg E, Carrol J, Shamiss A, Rosenthal T. Haemodynamic and humoral effects of the angiotensin II antagonist losartan in essential hypertension. Am J Hypertens. 1994;7:1041–1044. doi: 10.1093/ajh/7.12.1041. [DOI] [PubMed] [Google Scholar]
- 24.Rett K, Wicklmay R, Dietze GJ. Metabolic effects of kinins: historical and recent developments. J Cardiovasc Pharmacol. 1990;15:S57–S59. [PubMed] [Google Scholar]
- 25.Gohlke P, Mattfeldt T, Mall G, Lamberty V, Unger T. Chronic low dose converting enzyme inhibitor treatment induces cardiac capillary proliferation. Hypertension. 1991;18 400(abstract) [Google Scholar]


