Abstract
Aims
N-Desmethylclozapine and clozapine N-oxide are major metabolites of the atypical neuroleptic clozapine in humans and undergo renal excretion. The aim of this study was to investigate to what extent the elimination of these metabolites in urine contributes to the total fate of clozapine in patients and how they are handled by the kidney.
Methods
From 15 psychiatric patients on continuous clozapine monotherapy, blood and urine samples were obtained during four 2 h intervals, and clozapine and its metabolites were assayed in serum and urine by solid-phase extraction and h.p.l.c. Unbound fractions of the compounds were measured by equilibrium dialysis.
Results
The following unbound fractions in serum were found (geometric means): clozapine 5.5%, N-desmethylclozapine 9.7%, and clozapine N-oxide 24.6%. Renal clearance values calculated from unbound concentrations in serum and quantities excreted in urine were for clozapine on average 11% of the creatinine clearance, whereas those of N-desmethylclozapine and clozapine N-oxide amounted to 300 and 640%, respectively. The clearances of unbound clozapine and N-desmethylclozapine increased with increasing urine volume and decreasing pH. All renal clearance values exhibited large interindividual variations. The sum of clozapine and its metabolites in urine represented on average 14% of the dose.
Conclusions
Clozapine, N-desmethylclozapine and clozapine N-oxide are highly protein-bound in serum. Clozapine is, after glomerular filtration, largely reabsorbed in the tubule, whereas the metabolites undergo net tubular secretion. Metabolic pathways alternative or subsequent to N-demethylation and N-oxidation must make major contributions to the total fate of clozapine in patients.
Keywords: clozapine N-oxide, clozapine, N-desmethylclozapine, plasma protein binding, renal clearance, tubular secretion
Introduction
After the reintroduction of clozapine into the market and the opening of new markets for clozapine in the late 1980s, clinical and scientific interest in this atypical neuroleptic was reawakened. Its high therapeutic efficacy and the virtual absence of adverse effects on extrapyramidal motor function—a phenomenon originally detected in Central Europe around 1970 [1]—has been confirmed in numerous recent studies; moreover, clozapine has proven efficacious in some patients refractory to other neuroleptics [see 2–4 for reviews].
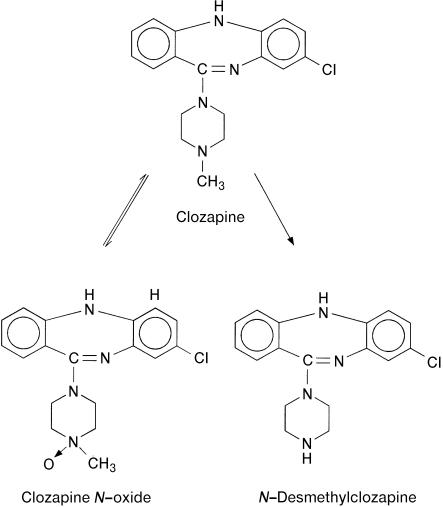
Clozapine’s clinical importance has stimulated investigation of its kinetics in patients [2], although knowledge is still incomplete, particularly with regard to the metabolic fate. When administered orally, clozapine was absorbed nearly completely [5], but mean values reported for its systemic availability were 27% and 47% only [6], apparently due to a highly variable first-pass metabolism. Elimination half-lives in patients were shorter after single doses (8 h) than after achievement of steady-state (14 h, 16 h or 17±8 h) [2, 6]. The two so-called ‘major’ metabolites, which can be measured in plasma [7–10] are N-desmethylclozapine and clozapine N-oxide (Figure 1). Their enzymatic formation in human liver microsomes or by expressed cytochrome P450 (CYP) species has been the subject of some recent investigations. N-Oxidation of clozapine was mainly catalysed by CYP3A4 [11, 12] and a flavin monooxygenase [13]. As indicated in Figure 1, the reaction is reversible in vivo [14]. Major contributions to demethylation by CYP3A4 and CYP1A2 were found in one study [11], whereas kinetic data of expressed enzymes indicated an additional important part of CYP2C19 and CYP2D6 [12]. Single-dose kinetics of clozapine in volunteers were, however, not affected by deficiencies of CYP2C19 or CYP2D6 [15]. Clozapine and the two metabolites have been quantified in urine collected from five patients and a ratio of clozapine: N-desmethylclozapine: clozapine N-oxide of 1:1:2 has been reported [16]. Recent measurements in three patients resulted in a mean ratio of 1:4:8.7 and recoveries corresponding to 1.7–13% of the dose within 12 h [17]. Apart from these data, no information is available on the quantitative importance of the two metabolites for the overall metabolic fate of clozapine in patients. Furthermore, nothing has apparently been published on the renal mechanisms that govern the excretion of clozapine and its metabolites.
Figure 1.
Structural formulas of clozapine and its metabolites and biotransformation pathways interconnecting them.
When a single clozapine dose was given to volunteers, they excreted in urine further major metabolites that had been formed by aromatic hydroxylation with or without removal of the Cl substituent [5]. Some of these had previously been identified in patient urine [18]. Nothing is known about the enzymes that catalyse their formation or on their quantitative importance in patients.
In the present investigation, clozapine, N-desmethylclozapine and clozapine N-oxide were analysed in timed serum and urine samples of 15 patients during steady-state treatment in order to elucidate the quantitative role of their renal excretion for the total fate of clozapine and their handling by the human kidney.
Methods
Materials
Clozapine, N-desmethylclozapine and clozapine N-oxide were kindly donated by Wander (Bern, Switzerland). Cartridges with 100 mg C18-silica gel (Bond Elut C18) produced by Analytichem (Harbor City, CA, USA) were purchased from ict Handelsgesellschaft (Frankfurt am Main, Germany). Thin-layer chromatography (t.l.c.) glass plates coated with nonfluorescent silica gel (Kieselgel 60) and organic solvents were obtained from Merck (Darmstadt, Germany), C18-silica gel for h.p.l.c. (Nucleosil 5 C18) from Macherey-Nagel (Düren, Germany). Blank plasma or serum and blank urine were obtained from five healthy drug-free donors recruited from laboratory staff.
Patients and study design
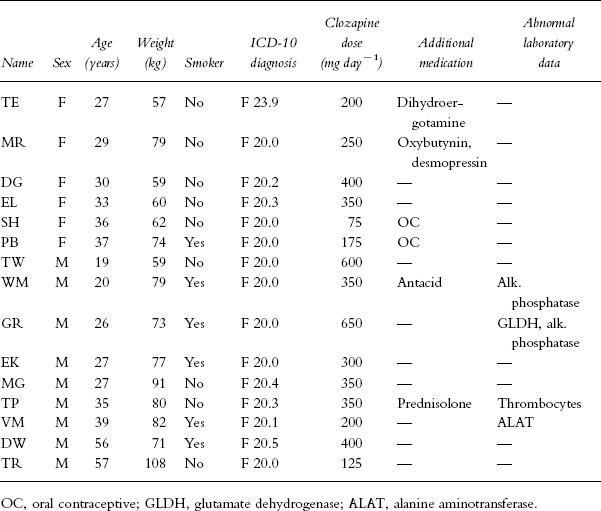
Twelve inpatients and three outpatients with an ICD diagnosis of schizophrenia (Table 1) gave their written consent for participation in the study after a half hour discussion with a psychiatrist. None of them suffered from cardiac, renal or hepatic disease. Clinical laboratory surveillance revealed elevated liver enzyme values in some patients, but in no case did the values exceed 150% of the upper limit of the reference range. Patient TP was treated with prednisolone for idiopathic thrombocytopenic purpura. In all of the patients, clozapine was the only psychotropic drug taken; the dose and dosing schedule were held constant for at least 7 days and were not changed during the investigation. Inpatients took their clozapine doses with supervision at 08.00 h, 11.45 h, 17.45 h, and 21.30 h provided that four daily doses were given. Dosing schedules were individualized according to clinical need and tolerability. Seven patients received a morning dose that corresponded to 20–33% of the daily dose (designated as the group with even distribution of doses), in three patients the morning dose was 15–18% of the daily dose and five patients took clozapine at bedtime only or with an additional dose in the afternoon. At 08.00 h, 10.5 h after the last dose and before ingestion of the morning dose, the patients were asked to empty the bladder and 18 ml blood was taken for the preparation of serum that was frozen at −20° C. After 2, 4, 6, and 8 h the patients collected urine and additional blood samples were drawn. The volume and pH of the urine were measured and aliquots were stored at −20° C. One of the authors stayed with the patients during the investigational period in order to ensure compliance with the time schedule.
Table 1.
Demographic and clinical data of patients.
Analytical methods
The procedure for solid-phase extraction of clozapine and its metabolites from serum was modified from that of Weigmann & Hiemke [8]. Cartridges with C18-silica gel were washed with 1 ml 0.5 m HCl, 1 ml acetonitrile, 2 ml methanol, and 3 ml water. Serum (1 ml) was mixed with 1 ml 1% (w/v) Na2CO3 and applied with suction. After washing with 2×0.5 ml water and 0.5 ml 50% (v/v) methanol, substances were eluted with 2×1 ml 0.1 m acetic acid in methanol. The eluate was evaporated under a stream of nitrogen at 35–40° and the residue dissolved in 0.2–1 ml of h.p.l.c. eluent, 0.1 ml of which was injected.
Separation was achieved on a 4.6×200 mm h.p.l.c. column with C18-silica gel as the stationary phase and 10 mm perchloric acid adjusted with NaOH to pH 2.5/acetonitrile (65:35, v/v) as the mobile phase, flow 1 ml min−1. Peaks were detected by their absorption at 290 nm and quantified by their heights relative to those of external standards containing 0.025–0.2 nmol (about 8–70 ng) in 0.1 ml. Retention times were 7 min for clozapine, 5.8 min for N-desmethylclozapine and 8.6 min for clozapine N-oxide. From 10 samples of blank serum spiked with concentrations of 0.05–1 μm (about 20–350 ng ml−1), the three compounds were recovered by 95±2%, 82±3% and 97±4% (means±s.d.), respectively. Standard curves constructed from the results of these recovery experiments were linear with coefficients of correlation of 0.994–0.998 and went through the origin. With each series of analyses, two control samples of blank serum were run, to one of which 1 μm clozapine and desmethylclozapine and 0.5 μm clozapine N-oxide had been added, while the other one was spiked with half these concentrations. In 12 series, the recoveries from these samples were 98±1.5% for clozapine, 83±2.5% for desmethylclozapine, and 97±1.5% for clozapine N-oxide. Values measured in patient serum were corrected by the recoveries of the control samples run in parallel. All analyses were performed in duplicate with coefficients of variation found to be 4% for clozapine, 5% for desmethylclozapine and 10% for clozapine N-oxide (n = 51). Concentrations as low as 16 ng ml−1 could be quantified without a loss of accuracy. The detection limit with a signal/noise ratio of 3–4 was 2 ng ml−1 for all three substances, and no interferences were observed in blank serum.
N-Desmethylclozapine and clozapine N-oxide were analysed in urine as described above, but samples were diluted 25 fold with water before solid-phase extraction. Standard curves constructed from six samples with final concentrations of 0.25–2 μm desmethylclozapine and clozapine N-oxide were linear and went through the origin; recoveries from these samples amounted to 92±4.5% and 91±4%, respectively. In 11 series of analyses, mean recoveries from two control samples each of which were spiked with final concentrations of 1 or 0.5 μm desmethylclozapine and 2 or 1 μm clozapine N-oxide, recoveries were 94±1% and 93±1%, respectively. Coefficients of variation in duplicate analyses amounted to 6 and 7%, respectively (n = 56).
A different extraction procedure was required for measuring clozapine in urine, because artifactual reduction of clozapine N-oxide had to be kept to a minimum. Urine (3 ml) was adjusted to pH 7.5 with 1% Na2CO3 and immediately extracted with 2 ml hexane/1-propanol (97:3, v/v) by shaking for 0.5 min and centrifuging. A measured aliquot of the organic layer was evaporated in a conical tube and the residue concentrated in the tip. For t.l.c., it was dissolved in 50 μl hexane/1-propanol of which 40 μl was applied as a spot on a 20×20 cm plate. Samples and standards containing 250–2000 ng clozapine were applied in alternating fashion and the plate was developed in 2-propanol/tert-butyl methyl ether/25% ammonia/water (12:6:0.75:0.75, v/v/v/v) to a height of 12 cm above baseline. The dried plate was scanned at 290 nm by reflectance photometry [7] and the clozapine peaks (Rf 0.75) were quantified by the trapezoidal rule. Standard curves were linear up to 750 ng clozapine. In 11 series, mean recoveries from two control samples each were 100±3%. Duplicate analyses of urine samples gave a coefficient of variation of 14% (n = 56). A control consisting of blank urine with clozapine N-oxide added in a quantity corresponding to that found in the patient sample was run with each urine sample. A mean of 0.2% of the N-oxide was found as clozapine, and this amount (corresponding on average to 16% of the clozapine originally present) was subtracted from the clozapine quantity measured.
Protein binding of clozapine and its metabolites was determined by equilibrium dialysis in 2×1 ml Teflon cells with serum adjusted to pH 7.4 by CO2 [19]. Equilibrium with the buffer compartment containing 100 mm sodium phosphate pH 7.4 and 30 mm NaCl was achieved within 4 h. The pH was checked regularly after dialysis and found to deviate from 7.4 by not more than 0.05 units. Weighed aliquots of serum and buffer were stored frozen and analysed by the procedure described above. Unbound fractions (fu) were calculated by dividing the concentrations in the buffer by those in the serum after dialysis. When five blank plasma or serum samples were spiked with 0.25–1.5 μm clozapine and desmethylclozapine and 0.125–0.75 μm clozapine N-oxide, the recoveries from the two compartments amounted to 104±6%, 83±6% and 104±4%, respectively.
In two serum samples from each patient and in all urine samples, creatinine concentrations were measured using an enzymatic test kit (Creatinin PAP, Boehringer, Mannheim, Germany).
Pharmacokinetic evaluation
Renal clearances of creatinine (CLCR) and of clozapine and its metabolites (CLR) were calculated by dividing their excretion rates measured as the quantities in urine per 2 h interval by the mean serum concentration of the respective compound (Cpl) during this interval. Division of the excreted quantities by the unbound concentrations in serum (Cpl×fu) resulted in the renal clearances of unbound clozapine and metabolites (CLRu). The ratio of CLRu and the creatinine clearance CLCR (CLRu/CLCR) served to estimate the extent of net renal secretion or reabsorption. Drug and metabolite quantities in urine as a percentage of daily dose were obtained by extrapolation from the 8 h sampling period to 24 h. In five patients, complete 24 h urine samples were available and the desmethylclozapine and clozapine N-oxide quantities measured in them proved to be on average 108% and 85%, respectively, of the values obtained by extrapolation. As a measure of the individual elimination capacity for clozapine, the drug concentration in the serum was divided by the daily dose in mg kg−1. This dose-related serum concentration (in ng ml−1 (mg kg−1)−1) is proportional to the reciprocal of the apparent oral clearance.
Patient WG had to be excluded from evaluations relating to the daily dose because of suspected noncompliance with the prescribed drug regimen. His initial serum clozapine and desmethylclozapine levels were more than 50% lower than some values measured on routine drug monitoring during the preceding weeks. Also then compliance seemed to be unreliable as reflected by large serum level fluctuations. Of the 60 urine samples, five from three patients could not be included in calculations of absolute clearance values, because sampling was considered incorrect. The criterion was a creatinine clearance that deviated by more than 50% from the mean of the other sampling intervals. The ratios CLRu/CLCR were, however, included in the evaluation.
Statistical evaluation
One-way analyses of variance served for calculations of interindividual differences and multiple regression analyses for the establishment of correlations. Original data were log transformed in order to obtain homogeneous variances. Geometric means are given in the Results section and in Table 2 coefficients of variation calculated from untransformed data, whereas in the description of methods data are arithmetic means with standard deviations. Statistical analysis was performed using JMP (SAS Institute Inc., Cary, NC, USA).
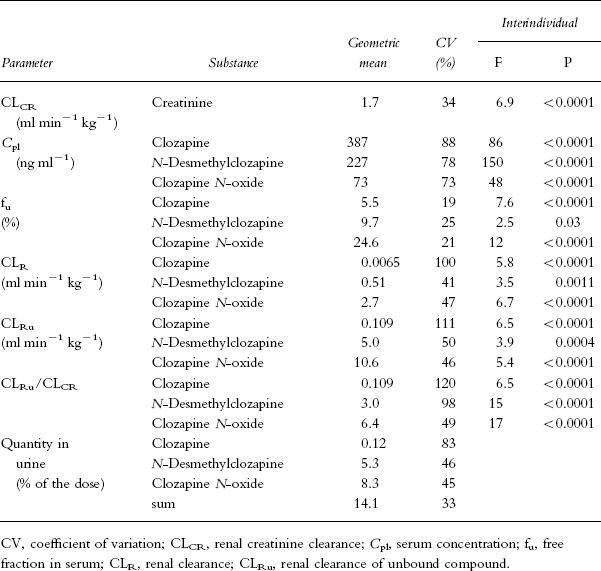
Table 2.
Renal creatinine clearance and kinetic data of clozapine and metabolites measured during four 2 h intervals in 15 psychotic patients. The degrees of freedom for variation between patients were 14, while those used for the estimation of intraindividual variation were 39 (16–19 in the case of unbound fractions).
Results
The steady-state concentrations of clozapine and its metabolites in serum varied greatly among patients (Table 2). Lowest and highest levels of clozapine relative to the daily dose differed by a factor of 6 (41–237 ng ml−1 (mg kg−1)−1). Geometric means were significantly lower in the eight evaluable male than in the six female patients (94 vs 145 ng ml−1 (mg kg−1)−1, F1,37 = 70.2, P < 0.0001). Lower mean values were also observed in the six smokers compared with eight nonsmokers (80 vs 144 ng ml−1 (mg kg−1)−1, F1,37 = 187, P < 0.0001). In addition, there was a highly significant difference in the N-desmethylclozapine/clozapine ratios in serum between smokers and nonsmokers (0.78 vs 0.48, F1,37 = 143, P < 0.0001). Relative serum clozapine levels did not differ between patients with an even distribution of doses over the day and those receiving no or a small morning dose. Clozapine N-oxide was measurable in all serum samples, though at a mean concentration of 19% only of the clozapine level. The logarithms of the mean concentrations of N-desmethylclozapine and clozapine N-oxide in the individual patients showed close linear correlations with the values for clozapine (r = 0.92 and 0.88, respectively, P < 0.001).
The free fractions of clozapine, N-desmethylclozapine and clozapine N-oxide in serum or plasma were independent of the concentrations in the range investigated. Values measured in plasma from four volunteers were in the same ranges as those in serum from patients (Table 2). Interindividual differences were generally small, though they exceeded intraindividual differences in repeated measurements (Table 2). In one patient’s serum, the free fraction of clozapine N-oxide was as low as 9.5% and in a sample taken four weeks later, it was 10.2%, whereas in all other patients and volunteers the values varied between 21 and 34%.
Measurements of compounds excreted in urine within four 2 h intervals revealed minor quantities of clozapine that (when extrapolated to 24 h) represented 0.5% of the dose or less (mean 0.12%), whereas the quantities of N-desmethylclozapine and the N-oxide corresponded on average to about 5 and 8% (Table 2); the sum of all three compounds was always below 25%. Seven patients with an even distribution of doses excreted a higher quantity of clozapine N-oxide than the seven evaluable patients who received the major doses in the evening (11.4 vs 8.1% of the dose, P < 0.05), possibly due to preferential N-oxidation during first-pass metabolism; no other difference was detected. The mean clozapine: N-desmethylclozapine: clozapine N-oxide ratios in urine were 1:43:77 in the total group and 1:44:85 in the patients with an even distribution of doses.
The mean renal clearances (CLR) of N-desmethylclozapine and clozapine N-oxide were about 80- and 400-fold, respectively, that of clozapine (Table 2). When the clearances were calculated for the unbound compounds, mean CLRu values attained by the metabolites were found to exceed the creatinine clearance by a factor of 3 in the case of N-desmethylclozapine and 6.4 in the case of clozapine N-oxide, whereas that of clozapine was one-ninth only of CLCR (Table 2). Concentrations in urine exceeded the unbound concentrations in serum by a mean factor of 7.4 for clozapine, 336 for N-desmethylclozapine and 737 for clozapine N-oxide.
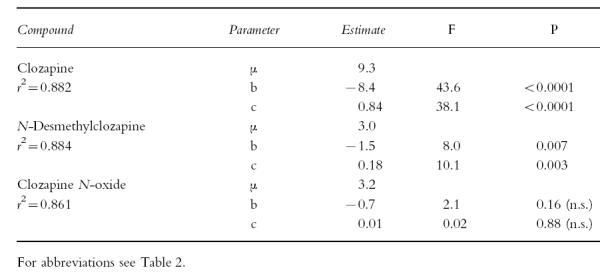
All renal clearance data exhibited large interindividual variations that by analysis of variance significantly exceeded intraindividual variation (Table 2). There was no significant contribution of sex or age to total variation (data not shown). No significant correlation existed between dose-related serum clozapine levels and renal clearance values. The clearances of the unbound compounds divided by that of creatinine (CLRu/CLCR) did not correlate with the serum concentrations of the respective compounds. When they were investigated for a relationship to urine volume and pH, significant negative regression coefficients with respect to pH and positive coefficients with respect to volume were revealed for clozapine and to a lesser extent for N-desmethylclozapine, while no significant regression coefficients were found for the N-oxide (Table 3).
Table 3.
Results of multiple regression analysis of the relationships between urinary pH and volume and renal clearances of unbound clozapine and metabolites divided by the creatinine clearance.
Estimates of parameters and significances were calculated by the formula ln (CLRu/CLCR)=μ+ai+b·ln (urine pH) + c · ln(urine volume) with ai as the patient-dependent intercept. r2 is the fraction of total variance explained by the model when individual a values are taken into account.
Discussion
The dose-related serum clozapine levels were in agreement with those found previously [20, 21], and this also applied to the relations between metabolite and parent drug concentrations [8, 9, 22]. The observation that dose-related serum clozapine levels were lower in men compared with women [23, 24] and in smokers compared with nonsmokers [23] could be confirmed as well as the finding of higher N-desmethylclozapine/clozapine ratios in smokers [25]. Since tobacco smoke induces cytochrome P450 1A2 (CYP1A2), the present data are also compatible with the close correlation between clozapine oral clearance and CYP1A2 activity determined by a caffeine test in volunteers [26].
The extent of protein binding of clozapine and its metabolites in serum is similar to that of structurally related drugs; for instance, the mean unbound fraction of the phenothiazine neuroleptic perazine was 4.6% [19], that of amitriptyline 7.8% [19] or 5.7% [27], and that of imipramine 11.5% [19], 9.4% [27] or 10.9% [28]. The somewhat larger free fraction of the secondary amine metabolite as compared with that of the tertiary amine parent compound was also analogous to the behaviour of other tricyclic psychoactive drugs [19, 27]. Regarding their N-oxide metabolites, binding to our knowledge has been measured only for amitriptylinoxide, which is 71–84% bound in plasma [29]. This is in accordance with 66–79% binding of clozapine N-oxide in 14 out of 15 patients. The reason for the much higher percentage bound in repeated samples of one female patient is unclear. This patient was being treated with a third-generation oral contraceptive (composed of desogestrel 0.15 mg and ethinylestradiol 0.03 mg) and her dose-related clozapine serum level (228 ng ml−1 (mg kg−1)−1) was at the upper end of the group investigated.
Drug concentrations in cerebrospinal fluid (CSF) that are assumed to roughly equal unbound concentrations in plasma are available for clozapine from two studies. Of nine patients with serum clozapine levels between 43 and 165 ng ml−1, seven had concentrations in CSF between 20 and 39 ng ml−1 corresponding to 26±11% of the levels in serum [30]. In 19 patients, a positive correlation was detected between clozapine levels in plasma and CSF [31]; mean values were 290 ng ml−1 in plasma and 5.6 ng ml−1 in CSF (1.9% of the plasma concentration). None of these discrepant results is in accordance with the 2.6–9.3% (mean 5.5%) free fraction of clozapine found in this study.
The low renal clearance of clozapine resulted in only 0.12% of the dose on the average being excreted unchanged in urine. This agrees with a value of 0.5% found by previous investigators [5, 32]. In contrast, Gauch & Michaelis [16] reported a clozapine: N-desmethylclozapine: clozapine N-oxide ratio of 1:1:2 in a pooled urine sample from five patients. During their work-up procedure considerable quantities of clozapine N-oxide were probably reduced to parent drug. A certain extent of reduction may also have taken place on automated analysis of clozapine and metabolites including solid-phase extraction of patient urine samples; the resulting ratio of 1:4:8.7 [8] was clearly different from the ratio 1:43:77 determined now.
Artifactual reduction of clozapine N-oxide in alkalinized plasma has been studied in detail by Lin et al. [10], and in the present study it could be shown to take place in a small percentage during solid-phase extraction of urine. Because of the enormous excess of clozapine N-oxide over clozapine in urine, values of the latter can be grossly distorted unless reduction is avoided or controlled for. Using solvent extraction at near-neutral pH, clozapine values could be corrected for the small percentage resulting from N-oxide reduction. With these precautions, a renal clearance of unbound clozapine was obtained that averaged 11% of the creatinine clearance. Since the latter is by 87% due to glomerular filtration [33], the 11% of the total renal creatinine clearance corresponds to 13% of the clearance by filtration, and thus a mean of 87% of the filtered clozapine underwent net tubular reabsorption. This fraction was decreased by a high urine flow and low pH, as can be expected for a lipophilic base.
In contrast to the parent drug, N-desmethylclozapine and clozapine N-oxide had mean renal clearances of their unbound fractions exceeding that of creatinine 3- and 6.4-fold, respectively. Thus, their renal disposition was governed by net tubular secretion. Desmethylclozapine probably also underwent reabsorption, as can be surmised from the enhancing effects of higher urine volumes and lower pH on the clearance of the unbound compound relative to creatinine clearance (Table 3). However, the balance was in favour of secretion, and, on average, at least 71% (calculated as [(3–0.87)/3]×100%) of the N-desmethylclozapine in urine must have been eliminated by this route. The excess of renal clearance over the creatinine clearance was even more marked with unbound clozapine N-oxide, possibly due to lack of reabsorption. Tubular secretion was estimated to contribute a mean 86% of renal N-oxide excretion. Net tubular secretion could also be demonstrated for amitriptylinoxide [34].
As an alternative explanation for the high renal clearances of desmethylclozapine and clozapine N-oxide, their formation from clozapine in renal tissue can be considered. Such a mechanism is, however, rather unlikely, because human kidneys have very low or nondetectable contents of cytochrome P450 [35] that would be required for metabolite formation. Even if it is assumed that the metabolites were partially formed in renal tubular cells, a difference remains between their handling and that of clozapine. Tubular secretion of organic bases involves an uphill transport by a cation/proton antiporter located at the luminal membrane [36]. Since the metabolites were concentrated in urine relative to unbound concentrations in serum more than 300-fold (desmethylclozapine) and more than 700-fold (clozapine N-oxide), it must be concluded that they are better substrates of the antiporter than the parent drug. Whether the latter is transported uphill at the luminal membrane at all, cannot be derived from the present data. The 7-fold higher concentration in urine than in plasma water can result from incomplete tubular reabsorption of filtered clozapine at a high urine flow and low urine pH. According to the relationship depicted in Table 3, a 3-fold higher clozapine concentration can be explained by the lower pH in urine (mean 6.5) than in plasma. There was no indication of saturation of tubular transport in the range of clozapine metabolite concentrations occurring in the patient group.
N-Desmethylclozapine and clozapine N-oxide usually termed the ‘major’ metabolites of clozapine were excreted in urine in quantities corresponding on average to 5.3 and 8.3%, respectively, of the dose during continuous therapy. In single-dose experiments, volunteers excreted 1.1 and 5.1% only in the form of these metabolites, while some aromatically hydroxylated derivatives represented larger percentages of the dose [5]. Nothing as yet is known on their quantitative importance in patients nor on that of the quaternary ammonium glucuronide identified in patient urine [37].
In conclusion, clozapine, N-desmethylclozapine and clozapine N-oxide are on the average protein-bound in patient serum by 95, 90 and 75%, respectively. When the free fractions are assumed to undergo glomerular filtration in the kidney, nearly 90% of the filtered clozapine must have been reabsorbed in the tubule. In contrast, the quantities excreted in urine exceeded the filtered quantities more than 3-fold for N-desmethylclozapine and about 7-fold for clozapine N-oxide, apparently due to secretion by a tubular cation/proton antiporter. Though these two metabolites are the only ones that have been detected in patient plasma, their combined quantities in urine corresponded to 14% only of the dose, such that other biotransformation products must play a major role in the overall fate of clozapine.
Acknowledgments
The authors thank Mr K. Nill for expert advice concerning h.p.l.c. technique.
References
- 1.Hippius H. The history of clozapine. Psychopharmacol. 1989;99:S3–S5. doi: 10.1007/BF00442551. [DOI] [PubMed] [Google Scholar]
- 2.Jann MW, Grimsley SR, Gray EC, Chang WH. Pharmacokinetics and pharmacodynamics of clozapine. Clin Pharmacokinet. 1993;24:161–176. doi: 10.2165/00003088-199324020-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pickar D, Hsiao JK. Clozapine treatment of schizophrenia. J Am Med Ass. 1995;274:981–983. [PubMed] [Google Scholar]
- 4.Kane JM. Drug therapy: Schizophrenia. New Engl J Med. 1996;334:34–41. doi: 10.1056/NEJM199601043340109. [DOI] [PubMed] [Google Scholar]
- 5.Dain JG, Nicoletti J, Ballard F. Biotransformation of clozapine in humans. Drug Metab Dispos. 1997;25:603–609. [PubMed] [Google Scholar]
- 6.Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16:177–187. doi: 10.1097/00004714-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Breyer U, Villumsen K. Measurement of plasma levels of tricyclic psychoactive drugs and their metabolites by UV reflectance photometry of thin layer chromatograms. Eur J Clin Pharmacol. 1976;9:457–465. doi: 10.1007/BF00606565. [DOI] [PubMed] [Google Scholar]
- 8.Weigmann H, Hiemke C. Determination of clozapine and its major metabolites in human serum using automated solid-phase extraction and subsequent isocratic high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1992;583:209–216. doi: 10.1016/0378-4347(92)80554-4. [DOI] [PubMed] [Google Scholar]
- 9.Volpicelli SA, Centorrino F, Puopolo PR, et al. Determination of clozapine, norclozapine, and clozapine-N-oxide in serum by liquid chromatography. Clin Chem. 1993;39:1656–1659. [PubMed] [Google Scholar]
- 10.Lin G, McKay G, Hubbard JW, Midha KK. Decomposition of clozapine N-oxide in the qualitative and quantitative analysis of clozapine and its metabolites. J Pharm Sci. 1994;83:1412–1417. doi: 10.1002/jps.2600831010. [DOI] [PubMed] [Google Scholar]
- 11.Eiermann B, Engel G, Johansson I, Zanger UM, Bertilsson L. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br J Clin Pharmacol. 1997;44:439–446. doi: 10.1046/j.1365-2125.1997.t01-1-00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linnet K, Olesen OV. Metabolism of clozapine by cDNA-expressed human cytochrome P450 enzymes. Drug Metab Dispos. 1997;25:1379–1382. [PubMed] [Google Scholar]
- 13.Tugnait M, Hawes EM, McKay G, Rettie AE, Haining RL, Midha KK. N-Oxygenation of clozapine by flavin-containing monooxygenase. Drug Metab Dispos. 1997;25:524–527. [PubMed] [Google Scholar]
- 14.Jann MW, Lam YWF, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn. 1994;328:243–250. [PubMed] [Google Scholar]
- 15.Dahl ML, Llerena A, Bondesson U, Lindström L, Bertilsson L. Disposition of clozapine in man: lack of association with debrisoquine and S-mephenytoin hydroxylation polymorphisms. Br J Clin Pharmacol. 1994;37:71–74. doi: 10.1111/j.1365-2125.1994.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauch R, Michaelis W. The metabolism of 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1, 4]diazepine (clozapine) in mice, dogs and human subjects. Farmaco (edizione pratica) 1971;26:667–681. [PubMed] [Google Scholar]
- 17.Weigmann H, Bierbrauer J, Härtter S, Hiemke C. Automated determination of clozapine and major metabolites in serum and urine. Ther Drug Monit. 1997;19:480–488. doi: 10.1097/00007691-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Stock B, Spiteller G, Heipertz R. Austausch aromatisch gebundenen Halogens gegen OH− und SCH3- bei der Metabolisierung des Clozapins im menschlichen Körper. Arzneim-Forsch. 1977;27:982–990. [PubMed] [Google Scholar]
- 19.Brinkschulte M, Breyer-Pfaff U. Binding of tricyclic antidepressants and perazine to human plasma. Methodology and findings in normals. Naunyn-Schmiedeberg’s Arch Pharmacol. 1979;308:1–7. doi: 10.1007/BF00499712. [DOI] [PubMed] [Google Scholar]
- 20.Centorrino F, Baldessarini RJ, Kando JC, Frankenburg FR, Volpicelli SA, Flood JG. Clozapine and metabolites: concentrations in serum and clinical findings during treatment of chronically psychotic patients. J Clin Psychopharmacol. 1994;14:119–125. [PubMed] [Google Scholar]
- 21.Centorrino F, Baldessarini RJ, Kando JC, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151:123–125. doi: 10.1176/ajp.151.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Olesen OV, Thomsen K, Jensen PN, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross-sectional study. Psychopharmacol. 1995;117:371–378. doi: 10.1007/BF02246112. [DOI] [PubMed] [Google Scholar]
- 23.Haring C, Meise U, Humpel C, Saria A, Fleischhacker WW, Hinterhuber H. Dose-related plasma levels of clozapine: influence of smoking behaviour, sex and age. Psychopharmacol. 1989;99:S38–S40. doi: 10.1007/BF00442557. [DOI] [PubMed] [Google Scholar]
- 24.Jann MW, Liu HC, Wei FC, Lin SK, Hu WH, Chang WH. Gender differences in plasma clozapine levels and its metabolites in schizophrenic patients. Hum Psychopharmacol. 1977;12:489–495. [Google Scholar]
- 25.Wetzel H, Anghelescu I, Szegedi A, et al. Pharmacokinetic interactions of clozapine with selective serotonin reuptake inhibitors: differential effects of fluvoxamine and paroxetine in a prospective study. J Clin Psychopharmacol. 1997;18:2–9. doi: 10.1097/00004714-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Bertilsson L, Carillo JA, Dahl ML, et al. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994;38:471–473. doi: 10.1111/j.1365-2125.1994.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mårtensson E, Axelsson R, Nyberg G, Svensson C. Pharmacokinetic properties of the antidepressant drugs amitriptyline, clomipramine, and imipramine: a clinical study. Curr Ther Res. 1984;36:228–238. [Google Scholar]
- 28.Kristensen CB. Imipramine serum protein binding in healthy subjects. Clin Pharmacol Ther. 1983;34:689–694. doi: 10.1038/clpt.1983.233. [DOI] [PubMed] [Google Scholar]
- 29.Midgley I, Hawkins DR, Chasseaud LF. The metabolic fate of the antidepressive agent amitriptylinoxide in man. Arzneim-Forsch. 1978;28:1911–1916. [PubMed] [Google Scholar]
- 30.Nordin C, Almé B, Bondesson U. CSF and serum concentrations of clozapine and its demethyl metabolite: a pilot study. Psychopharmacol. 1995;122:104–107. doi: 10.1007/BF02246083. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman J, Johns C, Pollack S, et al. Biochemical effects of clozapine in cerebrospinal fluid of patients with schizophrenia. In: Tamminga CA, Schulz SC, editors. Advances in Neuropsychiatry and Psychopharmacology, Schizophrenia Research. Vol. 1. New York: Raven Press; 1991. pp. 341–349. [Google Scholar]
- 32.Cheng YF, Lundberg T, Bondesson U, Lindström L, Gabrielsson J. Clinical pharmacokinetics of clozapine in chronic schizophrenic patients. Eur J Clin Pharmacol. 1988;34:445–449. doi: 10.1007/BF01046700. [DOI] [PubMed] [Google Scholar]
- 33.Luke DR, Halstenson CE, Opsahl JA, Matzke GR. Validity of creatinine clearance estimates in the assessment of renal function. Clin Pharmacol Ther. 1990;48:503–508. doi: 10.1038/clpt.1990.186. [DOI] [PubMed] [Google Scholar]
- 34.Becher B, Fischer W, Taneri Z, Scholz E, Müller WE, Breyer-Pfaff U. Urinary metabolites of amitriptylinoxide and amitriptyline in single-dose experiments and during continuous therapy. Psychopharmacol. 1992;106:303–310. doi: 10.1007/BF02245409. [DOI] [PubMed] [Google Scholar]
- 35.de Waziers I, Cugnenc PH, Yang CS, Leroux JP, Beaune PH. Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253:387–394. [PubMed] [Google Scholar]
- 36.Pritchard JB, Miller DS. Renal secretion of organic cations: a multistep process. Advanced Drug Deliv Rev. 1997;25:231–242. [Google Scholar]
- 37.Luo H, McKay G, Midha KK. Identification of clozapine N+-glucuronide in the urine of patients treated with clozapine using electrospray mass spectrometry. Biol Mass Spectrom. 1994;23:147–148. doi: 10.1002/bms.1200230305. [DOI] [PubMed] [Google Scholar]