Abstract
Aims
Little is known about the frequency with which suspected adverse drug reactions are seen by general practitioners after the prescription of newly marketed drugs. We investigated age and sex specific incidence rates of suspected adverse drug reactions recorded by general practitioners in England after the prescription of selected newly marketed drugs.
Methods
Information was collected from 48 national cohort studies of newly marketed drugs studied by prescription-event monitoring. Questionnaires were sent to prescribers asking for details of events and suspected adverse drug reactions recorded in the patients’ notes occurring after the drugs were prescribed.
Results
During the 48 cohort studies, a total of 513 608 patients were investigated, of which 221 781 (43.2%) were males and 285 862 (55.7%) were females. The overall incidence of suspected adverse drug reactions in males was 12.9 per 10 000 patient-months of exposure (95% confidence limits 12.3 to 13.5), and in females was 20.6 per 10 000 patient-months of exposure (95% confidence limits 19.9 to 21.3). The overall age-standardised relative risk of an adverse drug reaction in females compared with males was 1.6 (1.5–1.7). This sex difference was significant in all age groups above 19 years of age, and was relatively consistent across all age groups (χ2 test for heterogeneity = 9.2, P = 0.3). The highest rate of recording in males was in the 50–59 year age group, and in females was in the 30–39 year age group.
Conclusions
In general practice in England, suspected adverse drug reactions to newly marketed drugs are recorded more often in adults aged between 30 and 59 years of age and are 60% more common in women than in men. The sex difference occurs in all age groups over 19 years of age.
Keywords: prescription-event monitoring, adverse drug reactions, general practice, age, gender, post-marketing surveillance
Introduction
An adverse drug reaction is any undesirable effect of a drug beyond its anticipated therapeutic effects occurring during clinical use. In contrast, an adverse drug event is an untoward occurrence after exposure to a drug that is not necessarily caused by the drug [1]. It is estimated that side effects occur in approximately 5% of patients taking a drug [2]. However, little is known about the frequency with which adverse drug reactions to newly marketed medicines are experienced by patients or are reported to doctors. Previous analysis of suspected adverse drug reactions reported on yellow-cards to the Committee of Safety of Medicines in the UK, suggests that side effects are age-related, with reporting rates substantially higher in the elderly [3]. It is estimated that about 3% of all admissions to geriatric units in the U.K. are due to adverse drug effects, and that in a further 8% of admissions, an adverse drug reaction is a contributory cause [4]. Earlier studies suggest that the frequency of adverse drug reactions is significantly higher in females than males [5, 6], and that females present more commonly with gastro-intestinal and cutaneous allergic reactions. It has been estimated that 83% of adverse drug reactions in males and 93% in females are due to dose related effects [6].
It is unclear whether this age and sex related increase is due to greater drug consumption or an increased vulnerability to drug toxicity with advancing age and female sex. A study of the relationship between spontaneous reports of adverse drug reactions reported to the Committee on Safety of Medicines, and age and sex specific estimates of general practice prescription volumes, has previously been published [7]. This study suggested that the numbers of reported adverse drug reactions per million prescriptions was highest between the ages of 35 and 59 years, and was higher for females than males over the age of 15 years. When rates of serious adverse drug reactions were examined, there was little difference between males and females. Rates of adverse drug reactions to a first prescription were highest in patients aged over 70 years of age.
Our aim was to investigate the age and sex specific incidence rates of suspected adverse drug reactions recorded by general practitioners in England following the prescription of selected newly marketed drugs. We also examined the overall age-related pattern of recording of clinical events (any untoward occurrences after exposure to a drug, not necessarily caused by the drug), in order to see whether this pattern differed from the adverse drug reaction recording patterns.
Methods
We analysed event rates and rates of suspected adverse drug reactions recorded by general practitioners during 48 cohort studies of selected long-term use, newly marketed drugs, performed by prescription-event monitoring between 1982 and 1997 [8]. A detailed description of the methodology of prescription-event monitoring has recently been published elsewhere, and an overview is provided here [8]. Prescription-event monitoring studies are observational cohort studies performed on selected drugs in general practice, soon after the drug is launched on the UK market. Drugs are usually selected for monitoring on the basis that they are intended for widespread long-term use in general practice.
Prescription data are obtained in the following way. After a drug is prescribed by a general practitioner in England, the patient has the prescription dispensed by a pharmacist. The pharmacist sends the prescription to the central Prescription Pricing Authority, which covers the whole of England, for reimbursement purposes. During a prescription-event monitoring study, the Prescription Pricing Authority provides, in confidence, details of every dispensed prescription for the selected study drug prescribed by general practitioners in England soon after the drug’s launch. The aim is to continue collecting prescription data on all patients in England who have been prescribed the study drug until a cohort size of between 10 000 and 15 000 patients has been achieved. At the time of the present analysis 48 individual cohort studies performed between 1982 and 1997 had been completed for long-term use drugs, and were available for inclusion. These 48 studies do not include the short-term use drugs studied by prescription-event monitoring, such as anti-infective agents.
At the time of each individual cohort study event data had been obtained in the following way. Questionnaires, known as ‘green forms’, were sent to prescribers asking for details and dates of all events recorded in the patients’ notes occurring after the drugs were prescribed, whether or not the events were thought to be related to the study drug. The general practitioners were also asked to note any events that were suspected adverse drug reactions, and whether or not any events were reported to the Committee on Safety of Medicines or the manufacturer. Doctors were also asked to record demographic details, indication for the drug, exposure dates, and reasons for stopping drugs. The questionnaires were sent out about 6 months after the first prescription of the study drug in the majority of the cohorts, and after about 1 year for seventeen of the 48 drugs.
Definitions
The definitions used in this study are as follows:
1 Events: The definition of an event used in this analysis was that provided on the green form questionnaires. This was ‘any new diagnosis, any reason for referral to a consultant or admission to hospital, any unexpected deterioration (or improvement) in a concurrent illness, any suspected adverse drug reaction, or any other complaint which was considered of sufficient importance to enter in the patients’ notes’.
2 Suspected adverse drug reactions: In the present analysis, our definition of suspected adverse drug reactions was those events that the prescribing doctors indicated were suspected adverse drug reactions on the green forms. All suspected adverse drug reactions recorded on the green form were included in the analysis, whether or not they had also been reported to the Committee on Safety of Medicines or the manufacturer.
Coding
Data obtained from the green forms were entered onto computer. The events were coded using a specially developed dictionary, designed to deal with every-day terms used by general practitioners, and organised in a system organ classification. Coding was performed by trained clerks. There was a daily quality assurance procedure supervised by a senior research fellow, and a weekly coding meeting supervised by medical staff.
Analysis
For each age band and sex we determined the total number of suspected adverse drug reactions recorded during the 48 studies and divided this total by the age and sex specific total duration of drug exposure. This provided overall age and sex specific incidence rates of suspected adverse drug reactions. Age specific event rates were also calculated in the same way, but the huge volume of event data generated for the 48 studies precluded an analysis of overall age-sex specific event rates using our current software. The relative risk and 95% confidence limits of having an adverse drug reaction recorded by general practitioners for females compared with males (reference group) in each of the age groups was calculated. We also calculated the relative risk and 95% confidence limits of having an event recorded by general practitioners in each age group using the 0–19 year age band as the reference group. Calculation of rates, relative risks and 95% confidence limits were performed using Stata Statistical Software: Release 5.0 [9].
Results
During 47 of the 48 cohort studies, a total of 959 898 patients were identified from prescription data. Of these, a total of 58 778 (6.1%) patients were not available for inclusion in the study, mainly because doctors were unable to complete the green forms, for example if the patient had moved. In these 47 studies, the total number of patients for whom green forms were returned to the Drug Safety Research Unit was 506 116. This gave an overall mean response rate for 47 of the 48 studies of 56.2% (standard deviation, s.d., 7.1; range: 42.0%–68.4%). The first prescription-event monitoring study was conducted for flunitrazepam in 1982. We are unable to accurately determine the total number of patients identified for inclusion in this study and therefore cannot calculate a response rate for flunitrazepam. In the flunitrazepam study, green forms were returned for 7492 patients. Therefore the total number of patients included in the present analysis was 513 608.
There were 221 781 (43.2%) males and 285 862 (55.7%) females. The sex of 5965 (1.2%) patients was unknown. The mean cohort size was 10 700 (s.d.: 3629) patients. The overall mean age of males was 54.6 (s.d.: 17.0) years, and for females was 55.5 (s.d.: 17.4) years (Table 1).
Table 1.
Characteristics of the cohorts used in the present analysis.

The overall number of adverse drug reactions recorded by age and sex is shown in Figure 1. The overall incidence of suspected adverse drug reactions was 16.9 per 10 000 patient months of drug exposure (95% confidence limits: 16.5 to 17.4). The incidence of suspected adverse drug reactions in males was 12.9 per 10 000 patient months of drug exposure (95% confidence limits: 12.3 to 13.5). The overall incidence of suspected adverse drug reactions in females was 20.6 per 10 000 patient months of drug exposure (95% confidence limits 19.9 to 21.3). The highest rate of recording in males was in the 50–59 year age group (14.8; 13.4 to 16.4). The highest rate of recording in females was in the 30–39 year age group (24.9; 22.3 to 27.6).
Figure 1.

Age and sex specific incidence rates of suspected adverse drug reactions (ADRs) ▪ female, ▴ male.
In males recording rates rose from 9.5 per 10 000 patient-months of exposure in the 0–19 year age group to over 14 per 10 000 patient months of exposure between the ages of 20 and 59. In males aged 60 to 79 years recording rates were over 12 per 10 000 patient months of exposure, and the rate was 11.8 in over 80 year olds. In females recording rates rose from 10.2 per 10 000 patient months of exposure in the 0–19 year age group, to 21.6 in the 20–29 year age group, and over 24 in women aged 30–49 years. Recording rates then fell slightly to between 20 and 21 in women between 50 and 79 years of age. Over the age of 80 years, recording rates in women were nearly 18 per 10 000 patient months of exposure.
The relative risks of a suspected adverse drug reaction in females compared with males (reference group) by age group is given in Table 2. Over the age of 19 years, females were 43% to 69% more likely to have a suspected adverse drug reaction recorded by the general practitioner. The overall relative risk was 1.6 (95% confidence limits: 1.5–1.7), and there was no evidence of heterogeneity of risk (χ2 test for heterogeneity = 9.2, P = 0.3).
Table 2.
Relative risk of an adverse drug reaction in females compared with males (reference).

Figure 2 shows the age-specific event rates recorded on the green forms. The overall event rate was 1381.2 per 10 000 patient-months of treatment (95% confidence limits: 1377.0 to 1385.4), which was 82 times greater than the overall rate of suspected adverse drug reactions. The rate of events per 10 000 patient-months of treatment increased between the 0–19 year age group (rate: 1100.6; 95% confidence limits: 1081.7 to 1120.0) and the 20–29 year age group (1294.1; 1277.2 to 1311.3). The rate then plateaued until the 70–79 year age group, when the rate increased to 1608.3 per 10 000 patient-months of treatment (1596.9 to 1619.7). The event rate was highest in the over 80 year old age group at 1898.0 per 10 000 patient-months of treatment (1877.3 to 1919.8).
Figure 2.
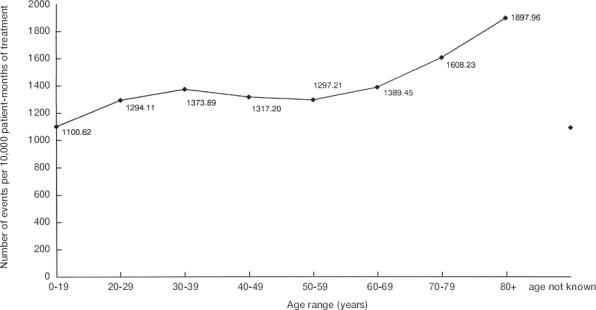
Age specific event rates.
Table 3 displays the relative risks of having a reported clinical event by age band using the 0–19 year age range as the comparator group. The relative risk of an event was highest in the 70–79 year age group (relative risk: 1.46; 95% confidence limits: 1.43–1.49) and the 80 years and over age group (1.72; 1.69–1.76).
Table 3.
Relative risk of an event by age (0–19 year old age group is reference).

Discussion
The main advantage of our study was that we were able to calculate incidence rates of recorded suspected adverse drug reactions as a function of patient-months of drug exposure. In addition, the data refer to newly marketed drugs over a range of chemical types and clinical indications. The setting was in general practice rather than in hospitals, where much previous research has been based [10]. We found that the number of suspected adverse drug reactions to a first prescription for a newly marketed drug was significantly higher in females compared with males in all age groups above 19 years of age, and the size of the increased risk was relatively consistent across the age-bands (overall relative risk: 1.6; 1.5–1.7). Our results are supported by a previous study which found a significant association between female sex and frequency of adverse drug reactions [6]. It should be noted that the rates reported in this paper were rates of all suspected adverse drug reactions recorded by general practitioners. Some suspected adverse reactions may have been falsely attributed to the drug, and in some cases other drugs may have been given concurrently. Between the ages of 15 and 64 years, consulting rates for women exceed that for men in general practice [11]. This may be one of the reasons for the increased recording of suspected adverse drug reactions in females in our study. A pharmacological explanation for the increase in adverse drug reaction rates in females may be lower body size and weight in females with consequent changes in apparent volume of distribution [12].
The elderly are widely believed to be predisposed to adverse drug reactions [10], due to increased drug consumption, polypharmacy, and the effects of ageing on distribution, metabolism and patterns of drug usage. We found that rates of recording of clinical events increased with age, particularly in people aged 70 years and over. Apart from children under 4 years of age, who are much less likely to receive new drugs, these results are in broad agreement with overall consulting rates previously reported from general practice [11]. In contrast to the event data, adverse drug reaction recording rates declined in the eighth and ninth decades. The highest rate of adverse drug reactions in males was in the 50–69 year age group and in females was in the 30–49 year age group. The pattern of these results is very similar to the demographic pattern of suspected adverse drug reactions reported to the Committee on Safety of Medicines [7]. Other studies have shown no clear linear association between increasing patient age and rates of suspected adverse drug reactions [6, 10, 13–15].
Limitations
The mean response rate was 56% overall, and ranged from 42%-68% for individual studies. This could cause a selection bias, because the experiences of patients on whom information was returned to the Drug Safety Research Unit may be different to the experiences of those patients on whom the general practitioners did not return a green form. Responding doctors are also likely to be different to non-responders. A study of the characteristics of responders and non-responders during prescription-event monitoring studies found that high prescribers of newly marketed drugs were significantly less likely to respond than less frequent users of new drugs [16]. This has an important effect on response rates because 10% of doctors, account for 42% of prescriptions, but their response rate is only 44%. High prescribers of new drugs may not be able to adequately monitor their patients [16], and may be ‘poorer’ quality prescribers [17]. If their patients had a higher rate of adverse events, including more suspected adverse drug reactions, this would result in a relative under-estimate of the true suspected adverse drug reaction rates. On the other hand, it could be argued that general practitioners would be more likely to return green forms if a patient had experienced a suspected adverse drug reaction, thus resulting in an overestimate of the true rates. A recent prescription-event monitoring study found that general practitioners were as consistent in their recording of events unlikely to be related to the drug (such as upper respiratory tract infection) as they were in their recording of possible drug-related events [18], suggesting that differential response by type of event is minimal.
There are three sources of recording bias, which may have resulted in an underestimate of the incidence rates of suspected adverse drug reactions that we found. Firstly, doctors may not have noted all suspected adverse drug reactions on our questionnaire. Secondly, doctors may not have completed our questionnaire, if they had already submitted a yellow-card to the Committee on Safety of Medicines. Finally, doctors may have recorded events but did not tell us that they were suspected adverse drug reactions.
There may have been differential bias in recording suspected adverse drug reactions for various age-sex groups [5]. For example, adverse events in the elderly may be attributed to underlying diseases or ageing rather than an adverse drug effect. The effect of this bias cannot be measured in this study. However, once a doctor suspects a drug reaction, there appears to be no reason for a differential recording bias on the green form [5]. In addition, the elderly may not report adverse drug reactions to their general practitioners as often as younger patients, for example because of difficulties attending surgery due to poor mobility.
We have not corrected for severity of underlying disease or age related differences in dosing regimens. Furthermore, we cannot discount age or sex related differences in the types of patients being treated with new drugs. It is possible that patients with more severe disease will experience more adverse drug reactions. Therefore potential demographic differences in severity of disease may explain the age and sex related differences in rates that we found. Certain drugs were more likely to be prescribed for females (for example, the selective serotonin reuptake inhibitors and sumatriptan), and others for males (for example, finasteride). If the adverse drug reaction profiles were significantly different, this may have affected the overall sex difference in rates that we found. However, given the large overall size of the aggregated cohort (513 608 patients), the roughly equal sex distribution overall (221 781 males and 285 862 females), and the wide range of drugs, drug classes and indications, we feel that it is unlikely that differences for certain drugs would have made a significant impact on the aggregated results.
Conclusion
In general practice in England, suspected adverse drug reactions to newly marketed drugs are recorded more often in adults aged between 30 and 59 years of age and are 60% more common in women than in men. The sex difference occurs in all age groups over 19 years of age, and is relatively consistent across age bands. After the age of 59 years, rates of recording of suspected adverse drug reactions plateau until the age of 80 years, and then decrease slightly in the very elderly. In contrast, rates of recording of all clinical events increased with age, particularly in people aged 70 years and over. Our results are supported by previous research which has found a significant relationship between female sex and adverse drug reactions [14, 15].
Acknowledgments
We are very grateful to the general practitioners in England who supported the prescription-event monitoring studies. We thank the Prescription Pricing Authority, the Family Health Services Authorities of England, and the Office for National Statistics, for their important participation in this program.
References
- 1.Asscher AW, Parr GD, Whitmarsh VB. Towards the safer use of medicines. Br Med J. 1995;311:1003–1005. doi: 10.1136/bmj.311.7011.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jick H. Adverse drug reactions. In: Maonde RF, editor. Topics in Clinical Pharmacology and Therapeutics. New York: Springer-Verlag; 1986. pp. 397–404. [Google Scholar]
- 3.Mann RD. Safety monitoring: methodology of the past and future. Pharmacoepidemiology and Drug Safety. 1992;2:215–224. [Google Scholar]
- 4.Overstall PW. Ageing and disease. In: Souhami RL, Moxham J, editors. Text book of Medicine. Edinburgh, London, Melbourne and New York: Churchill Livingstone; 1990. pp. 161–181. [Google Scholar]
- 5.Simpson JM, Bateman DN, Rawlins MD. Using the adverse reactions register to study the effects of age and sex on adverse drug reactions . Statistical Medicine. 1987;6:863–867. doi: 10.1002/sim.4780060716. [DOI] [PubMed] [Google Scholar]
- 6.Domecq C, Naranjo CA, Ruiz I, Busto U. Sex-related variations in the frequency and characteristics of adverse drug reactions . Int J Clin Pharmacol Ther Toxicol. 1980;18:362–366. [PubMed] [Google Scholar]
- 7.Mann RD, Rawlins MD, Fletcher P, Wood SM. Age and spontaneous reporting of adverse reactions in the United Kingdom. Pharmacoepidemiology and Drug Safety. 1992;1:19–23. [Google Scholar]
- 8.Mann RD, Wilton LV, Pearce GL, Mackay FJ, Dunn NR. Prescription-Event Monitoring (PEM) in 1996—a method of non-interventional observational cohort pharmacovigilance. Pharmacoepidemiology and Drug Safety. 1997;3:S5–S11. doi: 10.1002/(SICI)1099-1557(199710)6:3+<S5::AID-PDS272>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.StataCorp. Stata Statistical Software: Release 5.0. College Station, TX: Stata Corporation; 1997. [Google Scholar]
- 10.Klein LE, German PS, Levine DM. Adverse drug reactions among the elderly: a reassessment. J Am Geriatr Soc. 1981;29:525–530. doi: 10.1111/j.1532-5415.1981.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 11.Office of Population Censuses and Surveys. Consulting rates of patients in general practice-results. Morbidity Statistics from General Practice. 1995. pp. 24–62. Fourth National Study 1991–1992. London: HMSO.
- 12.Laurence DR, Bennett PN. Clinical Pharmacology. 6. Edinburgh, London and New York: Churchill Livingstone; 1987. Unwanted effects of drugs: adverse reactions; pp. 153–175. [Google Scholar]
- 13.Schneider JK, Mion LC, Frengley JD. Adverse drug reactions in an elderly outpatient population. Am J Hosp Pharm. 1992;49:90–96. [PubMed] [Google Scholar]
- 14.Gurwitz JH, Avorn J. Old age—is it a risk for adverse drug reactions? Agents Actions Suppl. 1990;29:13–25. doi: 10.1007/978-3-0348-7292-8_3. [DOI] [PubMed] [Google Scholar]
- 15.Hoigne R, Lawson DH, Weber E. Risk factors for adverse drug reactions—epidemiological approaches. Eur J Clin Pharmacol. 1990;39:321–325. doi: 10.1007/BF00315403. [DOI] [PubMed] [Google Scholar]
- 16.Inman W, Pearce G. Prescriber profile and post-marketing surveillance. Lancet. 1993;342:658–61. doi: 10.1016/0140-6736(93)91763-c. [DOI] [PubMed] [Google Scholar]
- 17.Majeed A, Evans N, Head P. What can PACT tell us about prescribing in general practice? Br Med J. 1997;315:1515–1519. doi: 10.1136/bmj.315.7121.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilton L, Freemantle S, Martin RM, Mann RD. Is the incidence of upper respiratory tract infection independent of drug treatment in large cohort studies of longer term use drugs? Pharmacoepidemiology and Drug Safety. 1998;7:54–55. doi: 10.1002/(sici)1099-1557(199808)7:1+<s4::aid-pds352>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]


