Abstract
Previous work has shown that the divergently transcribed Mycobacterium tuberculosis genes acr (hspX, Rv2031c) and acg (Rv2032) are induced under conditions of shallow standing culture and low oxygen and intracellularly within macrophages. We used a combination of computational and experimental methods to identify promoters for eight additional genes that are regulated in a similar manner and that comprise an acr-coregulated promoter (ACP) family. Transcriptional regulation of these ACP family members was evaluated by using a plasmid-based promoter-green fluorescent protein fusion system and flow cytometry. All promoters showed increased expression in shallow standing versus shaking cultures, in low- versus high-oxygen conditions, and intracellularly within macrophages versus extracellularly in tissue culture medium. However, there were quantitative differences in expression among promoters and among conditions for each promoter. A conserved 18-bp palindromic sequence motif was identified in all ACPs by Gibbs sampling-based computational analyses. Two such motifs overlap regions in the acr and acg promoters that were previously shown to be required for their expression. In addition, we found that 5% carbon dioxide was required for growth of Mycobacterium bovis BCG under microaerophilic (1.3% O2) culture conditions and fully prevented the growth cessation typically associated with rapid removal of oxygen. These findings are likely to be relevant to the in vivo environment and will contribute to our understanding of the pathogenesis of tuberculosis infection.
Mycobacterium tuberculosis latently infects an estimated 1.8 billion people worldwide (12). Each year, 8 million new cases of tuberculosis (TB) are reported and 2 to 3 million people die from this disease, making TB the leading cause of death from an infectious agent (38). One of the reasons for the success of M. tuberculosis as a human pathogen is thought to be its ability to persist latently in infected individuals for extended periods, often remaining dormant for decades before reemerging to cause active TB disease (32). Latently infected persons are an important public health problem because they represent a tremendous reservoir for this disease. Conventional antimicrobial agents are highly effective against tubercle bacilli only when the bacteria are actively growing (47). The resistance of dormant tubercle bacilli to conventional antimicrobial therapies further complicates treatment efforts.
The ability of M. tuberculosis to establish infection in vivo depends on a balance between the virulence of the infecting organisms and the host's capacity to mount an appropriate cell-mediated immune response against the bacilli (11). This response includes the formation of granulomas, which are lesions of differentiated macrophages and other immune cells (14). M. tuberculosis is maintained and persists within these granulomas in a poorly defined quiescent state of nonreplicating persistence. The presumed hypoxic nature of the granuloma has gained much attention as a possible signal for persistence and latency in mycobacterial infections (8, 17, 18, 45, 46). The ability of tubercle bacilli to adapt to low-oxygen growth conditions appears be important for their shift into a state of nonreplicating persistence (2, 24, 42, 43).
The Wayne model has been extensively used to study mycobacterial dormancy caused by gradual depletion of oxygen in bacterial cultures (42, 43). DNA microarrays have also been used recently to define a subset of M. tuberculosis genes induced rapidly by hypoxia (36). However, the granuloma environment is likely a complex milieu where hypoxia is only one of many signals to which these organisms must respond (4).
The response of M. tuberculosis to the intracellular environment of the macrophage is another critical aspect of its interaction with the host at many stages of infection. Several models have been developed to study the response of mycobacteria to intracellular residence within macrophages and to identify genes expressed during infection (9, 39, 41). Two-dimensional gel electrophoresis has shown differences in the protein expression profiles of intracellularly and extracellularly grown M. tuberculosis and Mycobacterium avium (22, 25, 40). Proteomic analysis has also identified several M. bovis BCG proteins that show increased expression inside macrophages (29). Genes and proteins induced during macrophage infection are likely to be important for intracellular survival and growth or subsequent stages of infection.
Expression of the 16-kDa alpha crystallin homolog protein (Acr) that is encoded by acr is increased upon oxygen limitation in vitro (6, 49) and inside macrophages (50). Previously we showed that promoters for acr and the divergently transcribed M. tuberculosis gene acg (Rv2032) are up-regulated under conditions of shallow standing culture and reduced oxygen tension and intracellularly within macrophages (33). Two-dimensional gel electrophoresis and mass spectrometry analysis of Mycobacterium bovis BCG identified several additional proteins that are present at higher levels in standing than shaking culture conditions (15).
Here, we describe eight new members of an acr-coregulated gene (ACG) family, and we further characterize the shallow standing versus shaking culture model. A conserved 18-bp palindromic sequence motif is present in all of these ACG family promoters. Copies of the conserved motif overlap two short (18- and 22-bp) regions within the acr and acg promoters that are required for expression of these divergently transcribed genes under hypoxic, intracellular, and standing growth conditions (33). In addition, we found that physiological levels of carbon dioxide prevented growth arrest of M. bovis BCG under low-oxygen conditions and that distinct morphological changes occurred in M. bovis BCG grown in shallow standing, microaerophilic cultures.
MATERIALS AND METHODS
Culture conditions.
Recombinant M. bovis BCG (Pasteur strain; Trudeau Institute), M. tuberculosis H37Rv (ATCC 25618), and M. tuberculosis H37Rv:ΔRv3133c seed lot stocks and experimental cultures were grown at 37°C in mycobacterial growth medium (Middlebrook 7H9 medium supplemented with 0.5% glycerol, 10% oleic acid-albumin-dextrose-catalase, and 0.05% Tween 80) as previously described (33). The M. tuberculosis H37Rv:Δ3133c strain was a generous gift from David R. Sherman (University of Washington, Seattle). Fresh cultures were inoculated from frozen stocks and immediately transferred to the appropriate experimental culture condition. Low-oxygen liquid cultures were grown in vented-cap tissue culture flasks (25 cm2; type 430639; Corning) in an incubator (series II; Forma Scientific, Inc.), which allowed us to control the oxygen tension by varying the flow of dry N2 gas. Vented-cap tissue culture flasks allowed for the exchange of gases between the flasks and the controlled environment of the incubator. Carbon dioxide levels were maintained by injecting CO2 gas into the incubators. Oxygen and CO2 levels within the incubators were periodically verified with an OXOR II portable oxygen analyzer (Bacharach, Inc., Pittsburgh, Pa.) and a model 2820 CO2 analyzer (Bacharach, Inc.), respectively. Low-oxygen cultures were grown under atmospheres of either 1.3% total O2 and 5% CO2 or 1.3% O2 and low CO2 (no supplemental CO2 was added to the incubator). High-oxygen cultures were grown in ambient air in 25 cm2 tissue culture flasks (Corning; type 430168) with the caps tightly sealed. The composition of ambient air is defined as 78.084% N2, 20.946% O2, 0.934% argon, and 0.033% CO2 as well as other trace elements and gases (23). High-oxygen cultures were also grown in ambient air supplemented with 5% CO2 in vented-cap tissue culture flasks. When necessary, kanamycin was used at 25 μg/ml and hygromycin was used at 50 μg/ml. Metronidazole sensitivity experiments were performed by including metronidazole (Sigma) at a final concentration of 10 μg/ml in mycobacterial growth medium.
J774.16 murine macrophage cells were maintained and passaged twice weekly in J774 medium containing Dulbecco's modified Eagle's medium (Gibco) supplemented with 20% fetal bovine serum (Gibco), 5% NCTC109 medium (Gibco), and 1% nonessential amino acids (Gibco), as described previously (27).
Mycobacterial promoter cloning.
Promoter regions from the 28 genes analyzed in this study (Table 1) were amplified by PCR from M. tuberculosis H37Rv chromosomal DNA and cloned in pGFPoriM, which carries a promoterless gfpmut2 gene as previously described (33, 34). The DNA primer sequences used for the PCR amplification of each promoter region are specified in Table 1. A hygromycin resistance cassette was cloned into each promoter-green fluorescent protein (GFP) reporter fusion construct for expression analysis in M. tuberculosis H37Rv:ΔRv3133c. Hygromycin selection was necessary because the H37Rv:ΔRv3133c targeted gene disruption was performed by inserting a kanamycin resistance cassette into the Rv3133c coding region (David R. Sherman, personal communication). The hygromycin resistance cassette was cloned from an XbaI fragment from plasmid pEN::Tnhyg into a unique NotI site in pGFPoriM.
TABLE 1.
PCR primers used to amplify M. tuberculosis H37Rv gene promoters
| Rv no. | Gene | PCR primer sequences (5′-3′)a |
|---|---|---|
| Rv0467 | aceA | F: CAATCTGTGACCGGATCCGC; R: ACATAGACAACTCCTTAACGG |
| Rv0685 | tuf | F: ACACCCGAGGACTACATGGG; R: TGGTCCCGATGTTGACGTGG |
| Rv0815c | cysA2 | F: ATTGGGTAAGCTGTCCGACG; R: CATGGGGGGAATCCTTTCGC |
| Rv1435c | F: GTCACTTGTCTCTCCTCGGC; R: GCGTCAACTCTCCTCTATTCG | |
| Rv1436 | gap | F: AGAACGGCATAGCCGTATCG; R: GTCACTTGTCTCTCCTCGGC |
| Rv1733c | F: GTAACAGGATGAGGATCTGC; R: TCATCGCTGTGAATTGCTCG | |
| Rv1734c | F: TCGACCACCAAGTTCGAGCG; R: CATGGTGATCGTGAGGTCCG | |
| Rv1737c | narK2 | F: CGCACATGATGTTGCTCCCC; R: CTCATCGACACGATCCGGGG |
| Rv1738 | F: CTCATCGACACGATCCGGGG; R: CGCACATGATGTTGCTCCCC | |
| Rv1837c | glcB | F: CGCCGTCATGGCTCACCACG; R: CATTGCTTCCTCCCTTACTGG |
| Rv1908c | katG | F: TTCGGTGCCGTGCGTTTTGC; R: GGCACAGCATTCCTTCCAGG |
| Rv1909c | furA | F: AATCAGCCTGCCGAACTCC; R: ACACTAGACAATATGACTCCC |
| Rv1996 | F: GACGTGATGTCCGCTGTCG; R: TGGCGTCCCCTCTGCATGG | |
| Rv2005c | F: ACGCTTGGATTCGGGCGTCG; R: CATCTCATTCTCCCTTCGCC | |
| Rv2006 | otsB | F: CATCTCATTCTCCCTTCGCC; R: ACGCTTGGATTCGGGCGTCG |
| Rv2026c | F: ACACCACGGTCCAGTACACG; R: ACATTTCACGCTCCTTGCGG | |
| Rv2027c | F: GAGCTGCTGGATTATCTGGC; R: CACATAGCTATGTTGACACCG | |
| Rv2031c | acr, hspX | F: GTCCGGCATGATCAACCTCC; R: GGTGGCCATTTGATGCCTCC |
| Rv2032 | acg | F: GGTGGCCATTTGATGCCTCC; R: GTCCGGCATGATCAACCTCC |
| Rv2623 | F: GGGCCATGGACTGGTCGTCG; R: ACATCGCGGTCCTCCTGTCG | |
| Rv2744c | 35kd_ag | F: CTCTGCAGCTCCGGTTGTCG; R: TCAGTTAGCTCCGCCTTCGC |
| Rv3116 | moeB2 | F: GACGAATGGATCGAAGGACG; R: ATGATTGCTCTCCTCCTGGC |
| Rv3117 | cysA3 | F: GACACAATCGGTGGATCCCG; R: CATGGCGGGAATCCTTTCGC |
| Rv3126c | F: GCAAGACTGCGTTCTTGAGC; R: GGGACCAAGGATGATGGTCC | |
| Rv3127 | F: GGGACCAAGGATGATGGTCC; R: GCAAGACTGCGTTCTTGAGC | |
| Rv3130c | F: CGGGAAATGGGTGTTCATGG; R: ATTCATGGTCAGCGCCTTCC | |
| Rv3131 | F: ATTCATGGTCAGCGCCTTCC; R: CGGGAAATGGGTGTTCATGG | |
| Rv3457c | rpoA | F: ACGCGTCCTGATCCACCAGC; R: CATGGTGTTTCTTCTTCCTTTCG |
BamHI 5′-GGATCC-3′ sites were added to the 5′ end of each primer to facilitate cloning of PCR products. F, forward; R, reverse.
Growth phase of bacterial cultures.
The growth phase for M. bovis BCG liquid cultures growing under each of the culture conditions was determined by measuring the optical densities at 600 nm and by plating CFU. Multiple flasks were inoculated for each experiment, and individual flasks were sampled for each time point. The viability of M. bovis BCG liquid cultures was assessed by plating onto 7H10 plates supplemented with oleic acid-albumin-dextrose-catalase and cycloheximide. The viability of liquid cultures was also assessed by staining the bacteria by the fluorescein diacetate-ethidium bromide method as described previously (26).
Fluorescence assays.
Flow-cytometric analysis of GFP-expressing M. bovis BCG and M. tuberculosis H37Rv bacteria was performed with the FACSCalibur system (Becton Dickinson Immunocytometry Systems) and CELLQUEST, version 3.1f, software (Becton Dickinson) as previously described (33). Recombinant M. bovis BCG cultures were grown under the appropriate culture condition prior to flow-cytometric analysis. Flow analysis of virulent M. tuberculosis H37Rv cultures was conducted after the bacteria were fixed with 4% paraformaldehyde in phosphate-buffered saline, pH 7.6, for 1 h at room temperature.
Electron microscopy.
Shaking and standing M. bovis BCG liquid cultures were grown as described above for 7 or 11 days in ambient air or at 1.3% O2-5% CO2. Samples were collected by centrifugation at 3,210 × g and washed twice with equal volumes of 0.1 M sodium cacodylate buffer containing 0.01% CaCl2 at 4°C. Samples were then fixed with 1% glutaraldehyde-1% OsO4 in 0.1 M sodium cacodylate buffer, washed, and then postfixed with 4% paraformaldehyde-1% glutaraldehyde as described previously (28). Thin sections were examined in a Zeiss 910 electron microscope operating at 80 kV; all micrographs were recorded at a magnification of ×25,000.
HPLC analysis.
Glucose, glycerol, and fermentation products were determined by high-pressure liquid chromatography (HPLC) (48) based on procedures described by Ehrlich and coworkers (13). Glucose levels were also measured with an Accu-Check blood glucose monitor (Roche Diagnostics Corporation, Indianapolis, Ind.) prior to acidification of the medium for HPLC analysis.
Sequence analysis.
Sequence motifs were identified with the Gibbs recursive sampler (http://bayesweb.wadsworth.org/gibbs/gibbs.html) by specifying a 16-bp palindromic model allowed to fragment to 24 bp, a position-specific background model, and zero to three sites per sequence. The sequences used for alignment were the intergenic regions, as defined by M. tuberculosis H37Rv genome annotation (5), corresponding to promoters that were shown to be differentially regulated. SCAN (31) was used to further search all of the intergenic regions of the M. tuberculosis H37Rv genome for additional sites described by a motif at an expectation value of ≤0.5.
Statistical analysis.
Student's t test was used to determine the significance of differences between various test results. P values <0.05 were considered significant.
RESULTS
Identification of new ACP family members.
We previously showed that expression of the M. tuberculosis promoters for the acr (hspX, Rv2031c) and acg (Rv2032) genes is coordinately up-regulated in M. bovis BCG during shallow standing culture, hypoxic, or intramacrophage conditions (33). The present study was done to identify additional acr-coregulated promoters (ACP) in M. tuberculosis. We evaluated the behavior of 27 M. tuberculosis promoters (Table 2) under ACP family-regulatory (AFR) conditions in M. bovis BCG using a plasmid-based promoter-GFP reporter system, as previously described (33). These gene candidates were chosen by a combination of computational and experimental approaches. Levels of promoter activity in recombinant BCG from shallow standing versus shaking cultures exposed to either ambient air (∼20% O2, 0.033% CO2) or low oxygen, CO2-supplemented (1.3% O2, 5% CO2) conditions were compared. Reporter activity in bacteria residing intracellularly within J774.16 macrophages was also measured. The promoterless pGFPoriM plasmid (34) and the constitutive tuf (EF-Tu, Rv0685) gene promoter-GFP plasmid, ptufGFP (33), were used as controls in all experiments.
TABLE 2.
Summary of M. tuberculosis H37Rv promoters analyzed under AFR conditions
| Rv no. | Gene | Promoter regulationa
|
Gene productb | Source of plasmid or reference | ||
|---|---|---|---|---|---|---|
| Stand vs shake | 1.3 vs 20% O2 | IC vs XC | ||||
| Rv0467 | aceA | − | − | − | Isocitrate lyase | This study |
| Rv0685 | tuf | C | C | C | EF-Tu | 33 |
| Rv0815c | cysA2 | C | C | C | Thiosulfate transferase | This study |
| Rv1435c | C | C | C | CHP | This study | |
| Rv1436 | gap | − | − | − | Glyceraldehyde-3-phosphate dehydrogenase | This study |
| Rv1733c | ↑ | ↑ | ↑ | Possible membrane protein | This study | |
| Rv1734c | − | − | − | CHP | This study | |
| Rv1737c | narK2 | ↑ | ↑ | ↑ | Nitrite extrusion protein | This study |
| Rv1738 | ↑ | ↑ | ↑ | CHP | This study | |
| Rv1837c | glcB | C | C | C | Malate synthase | This study |
| Rv1908c | katG | C | C | C | Catalase-peroxidase | This study |
| Rv1909c | furA | C | C | C | Ferric uptake regulatory protein | This study |
| Rv1996 | − | − | − | CHP | This study | |
| Rv2005c | ↑ | ↑ | ↑ | Putative ATP-binding protein | This study | |
| Rv2006 | otsB | − | − | − | Trehalose-6-phosphate phosphatase | This study |
| Rv2026c | − | − | − | CHP | This study | |
| Rv2027c | − | − | − | Unknown membrane protein | This study | |
| Rv2031c | acr, hspX | ↑ | ↑ | ↑ | 14-kDa antigen; heat shock protein | 33 |
| Rv2032 | acg | ↑ | ↑ | ↑ | Putative nitroreductase | 33 |
| Rv2623 | ↑ | ↑ | ↑ | Putative ATP-binding protein | This study | |
| Rv2744c | 35kd_ag | − | − | − | CHP | This study |
| Rv3116 | moeB2 | C | C | C | Molybdopterin metabolism protein | This study |
| Rv3117 | cysA3 | C | C | C | Thiosulfate transferase | This study |
| Rv3126c | − | − | − | HP | This study | |
| Rv3127 | ↑ | ↑ | ↑ | Putative nitroreductase | This study | |
| Rv3130c | ↑ | ↑ | ↑ | CHP | 33 | |
| Rv3131 | ↑ | ↑ | ↑ | Putative nitroreductase | 33 | |
| Rv3457c | rpoA | C | C | C | Subunit of RNA polymerase | This study |
↑, up-regulation; −, promoter was inactive; C, promoter was constitutively expressed. IC, intracellular; XC, extracellular.
Genes are annotated as described by the Pasteur Institute on TubercuList (http://genolist.pasteur.fr/TubercuList). CHP, conserved hypothetical protein; HP, hypothetical protein.
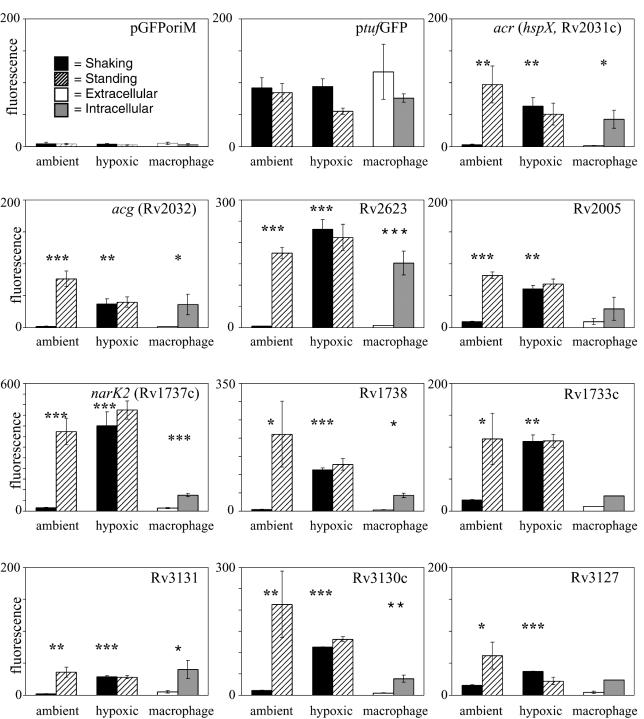
An initial set of seven ACPs (those for acr, acg, Rv3130c, Rv3131, Rv2005c, Rv2623, and Rv3127) was identified by testing promoters of genes that are closely related to either of two genes, acg and Rv2623, that previously showed strong differential expression in our model of shaking versus standing culture (15, 33) (Fig. 1). The products of Rv3131 and Rv3127 belong to the Acg classical nitroreductase functional superfamily (33), while the product of Rv2005c is a member of the Rv2623 ATP-binding protein superfamily (15). The Gibbs recursive sampler (http://bayesweb.wadsworth.org/gibbs/gibbs.html) was used to analyze the five intergenic regions that contain these seven promoters for a putative regulatory motif common to all members. A single conserved motif was identified and used to search the entire database of M. tuberculosis intergenic regions for additional ACP candidates by using SCAN (31).
FIG. 1.
Relative expression of ACP family promoters in M. bovis BCG during growth under each of the AFR culture conditions. Fluorescence readings shown are the means per bacterium (in arbitrary fluorescence units) from the flow-cytometric analysis of recombinant promoter-GFP-expressing M. bovis BCG bacteria. Recombinant bacteria were grown either shaking or standing under ambient or hypoxic conditions (1.3% O2) supplemented with 5% CO2 for 7 days. Intracellular (IC) and extracellular (XC) expression levels were analyzed after 48 h of infection in J774.16 macrophages or tissue culture media alone. Bacteria for the IC and XC experiments were pregrown for 7 days under either shaking or standing ambient culture conditions. Note the different relative fluorescence scales for different promoters. Values shown are the means of three independent experiments, and error bars denote standard deviations for each experimental condition. Asterisks above ambient culture conditions indicate that the difference in fluorescence expression between shaking and standing cultures was statistically significant; asterisks above the hypoxic shaking condition indicate that the difference in fluorescence expression between shaking ambient cultures and shaking hypoxic cultures was statistically significant; asterisks above the macrophage culture condition indicate that the difference in fluorescence expression between XC and IC grown bacteria was statistically significant (t test for independent samples; *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P < 0.001) (see Materials and Methods).
In addition to the seven ACPs already identified, several more promoter regions contained sites that matched the motif model. From these, we chose the five genes with the best statistical matches to the motif model for analysis of promoter activity. These included Rv1996, narK2 (Rv1737c), Rv1738, Rv1733c, and Rv1734c. Three of these, Rv1733c, narK2, and Rv1738 were confirmed as ACPs when tested under each of the AFR conditions (Fig. 1). The putative Rv1996 and Rv1734c promoters were not active in our GFP reporter system and could not be assessed. However, reverse transcription-PCR analysis indicated that Rv1996 mRNA is up-regulated in microaerophilic and standing conditions (G. Bai, unpublished data).
Thus, a total of at least 10 M. tuberculosis promoters responded similarly to AFR conditions (Fig. 1). Although each varied in its responsiveness to specific conditions, all but three of these promoters showed significant differences in expression (P < 0.05) in all AFR conditions. The Rv2005, Rv1733c, and Rv3127 promoters showed less-significant, but reproducible, levels of induction within macrophages than in the other AFR conditions. On this basis, we have defined the ACG family as a group of coordinately expressed genes that are up-regulated in response to at least two of the three AFR conditions and whose promoters contain at least one copy of the identified DNA motif. Rv1996 was not included in this group because it has not been tested under all AFR conditions.
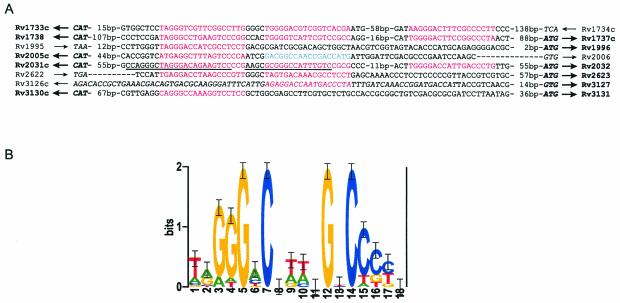
The motif identified in the original seven promoters was further refined by inclusion of the additional ACPs during reapplication of the Gibbs sampler. The resulting motif and its location in each promoter region are shown in Fig. 2A. The motif was best described with a palindromic model, although it could be identified whether or not a palindromic model was specified during Gibbs sampling. The sequence logo in Fig. 2B represents only the aligned sites and shows their palindromic nature. By allowing the Gibbs sampler to identify up to three sites per promoter with no restrictions on the spacing of these sites, we observed that many of the ACG family promoters contained more than one site, and frequently two sites that were separated by 4 bp (Fig. 2A).
FIG. 2.
The ACP family motif. (A) Promoter sequences, with sites matching the motif at a P value of <0.05 in red and an additional site identified at a P value of 0.32 in blue. Start codons, the coding region, and stop codons are italicized, and the start codons for the differentially regulated genes are in boldface. Arrows, direction of transcription of the genes; boldface arrows, genes that are differentially regulated. For our promoter studies, we amplified the sequence upstream of Rv3127 that included part of the putative Rv3126c ORF. The DNA regions that we previously showed to be necessary for regulation of acr and acg are underlined (33). Rv1996 was included in the final alignment because preliminary data suggested that its promoter may also be an ACP family member. (B) Sequence logo of the ACP family motif. The logo represents the alignment of the sites from panel A that are in red (without regard to orientation), showing the relative frequency of each base at each position of the motif (35). The y axis indicates the information content measured in bits, and error bars represent standard deviations at each position due to the limited sample size. All sequences except those between Rv1738 and Rv1737c are listed in the orientation of the published H37Rv sequence. The reverse complement of the Rv1738-Rv1737c intergenic sequence is shown so that the two ACP family motifs with the 4-bp separation in this sequence are aligned with those of the other sequences.
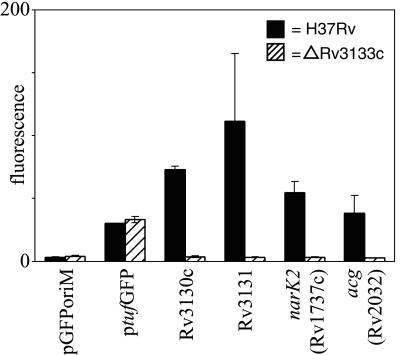
Rv3133c was recently shown to be required for the induction of the M. tuberculosis acr gene under reduced oxygen tension (36). Sequence analysis of the predicted Rv3133c protein suggests that it is a member of the LuxR family of two-component response regulators (36). We tested the expression of several of our ACP family promoter-GFP fusion constructs in M. tuberculosis H37Rv:ΔRv3133c grown under hypoxic CO2-supplemented conditions to further investigate the role of Rv3133c in the expression of other members of our ACP family (Fig. 3). Expression of all ACPs tested in M. tuberculosis H37Rv:Δ3133c was significantly reduced compared with expression in wild-type M. tuberculosis H37Rv (P < 0.05) (Fig. 3).
FIG. 3.
Comparative expression of the ACP-GFP fusion constructs in M. tuberculosis H37Rv (solid bars) and H37Rv:ΔRv3133c (hatched bars). Recombinant bacteria were grown shaking under hypoxic conditions (1.3% O2) supplemented with 5% CO2 for 7 days. Fluorescence readings shown are the means per bacterium (in arbitrary fluorescence units) from the flow-cytometric analysis of recombinant promoter-GFP-expressing M. tuberculosis H37Rv and H37Rv:ΔRv3133c bacteria. Values are the means of two or three independent experiments, and error bars denote standard deviations for each experimental condition. Expression of each of the ACP family promoters was significantly reduced (Student's t test; P < 0.05) in M. tuberculosis H37Rv:ΔRv3133c compared to expression in M. tuberculosis H37Rv.
Further characterization of the model of shaking versus standing culture and CO2 effects.
Ambient air contains only trace amounts of CO2, but CO2 levels within the human body may range from 3% in the blood (3) to >5.3% in alveolar spaces and interstitial fluids (16). Two of the AFR conditions (macrophage cultures and low O2) included CO2 supplementation, and we reasoned that M. bovis BCG might respond to differences in CO2 as well as O2 levels. Previously, we showed that supplementing ambient air with 5% CO2 did not affect regulation of the acr and acg promoters (33). However, low-O2 cultures grew poorly in the absence of supplemental CO2. We explored the effects of CO2 on ACP regulation and on M. bovis BCG growth in ambient air and under microaerophilic conditions, in shaking and standing cultures.
None of the ACP family promoter-GFP fusions was affected by 5% CO2 supplementation of ambient air except for that including the narK2 promoter. Expression from the narK2 promoter was reduced approximately 2.5-fold in ambient, 7-day (late-log-phase) standing cultures supplemented with 5% CO2 compared with that in cultures that lacked CO2 supplementation (data not shown). narK2 expression in ambient, shaking cultures was not affected by supplementation with 5% CO2.
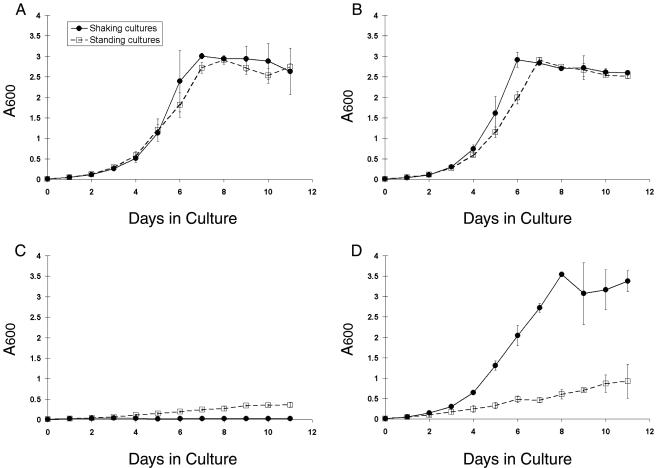
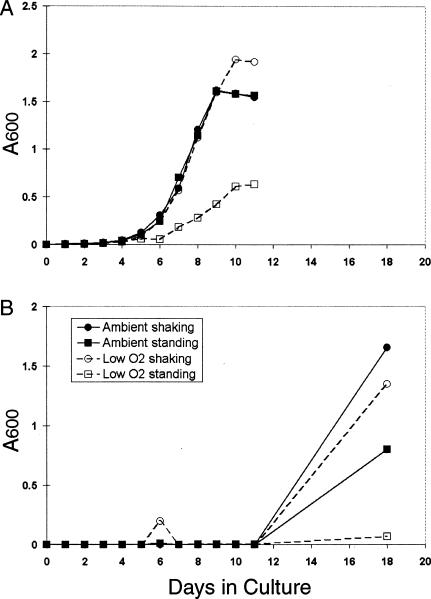
In contrast to the limited effect of CO2 supplementation on ACP expression, CO2 had significant effects on M. bovis BCG growth rates. Supplementation of ambient air with 5% CO2 increased the growth rate of shaking cultures slightly, allowing them to reach stationary phase earlier than did cultures grown under any of the other conditions examined in this study (Fig. 4B). Shaking cultures grown in microaerophilic conditions with 5% CO2 (Fig. 4D) exhibited a growth rate that was indistinguishable from that of shaking or standing cultures grown under ambient atmospheric conditions (Fig. 4A). However, shaking cultures grown in microaerophilic conditions without CO2 supplementation failed to grow during the 11 days examined (Fig. 4C). Viable bacteria could, however, be recovered from these shaking low-oxygen cultures by transferring an aliquot of the 11-day cultures into fresh mycobacterial growth medium and providing either ambient air or 5% CO2 in microaerophilic conditions (Fig. 5B). However, this resuscitation occurred only after a lag period of at least 11 additional days, during which no growth was apparent.
FIG. 4.
Effects of O2 and CO2 tension on the growth rates of M. bovis BCG in mycobacterial growth media during shallow standing or shaking conditions. Absorbance at 600 nm (A600) was measured for shaking and standing M. bovis BCG liquid cultures grown in ambient (A), 20% O2-5% CO2 (B), 1.3% O2 without CO2 supplementation (C), and 1.3% O2-5% CO2 (D) conditions. Values shown are the means of three independent experiments. Error bars denote standard deviations.
FIG. 5.
Resuscitation of microaerophilic M. bovis BCG cultures that lacked CO2 supplementation. Aliquots of 11-day standing (A) and shaking (B) cultures were subcultured (1:100) into flasks containing fresh mycobacterial growth media and grown shaking or standing under ambient or low-oxygen culture conditions supplemented with 5% CO2. Mycobacterial growth was determined by measuring the optical absorbance (A600) of individual tissue culture flasks for each time point. Data shown are from one of three representative experiments.
Standing microaerophilic M. bovis BCG cultures supplemented with 5% CO2 exhibited an arithmetic rate of growth (Fig. 4D). In the absence of CO2 supplementation, the growth rate of standing microaerophilic cultures was reduced to approximately one-half that of the CO2-supplemented standing cultures (Fig. 4C versus D). In contrast to the shaking cultures, these cultures all readily resumed typical growth rates when subcultured in the presence of either O2 or CO2 (Fig. 5A). M. bovis BCG was not sensitive to 10 μg of metronidazole/ml under any of the culture conditions tested in this study (data not shown).
HPLC analysis of M. bovis BCG carbon source utilization.
Having established CO2-mediated effects on M. bovis BCG growth rates in low-oxygen conditions, we explored a potential metabolic role for CO2. Supplementation of the medium with succinic acid or fumaric acid as Krebs cycle intermediates, Trypticase peptone as a source of amino acids, and/or yeast extract as a source of vitamins, purines, and pyrimidines all failed to restore M. bovis BCG growth under shaking or standing low-oxygen growth conditions in the absence of additional CO2. This suggests that CO2 is essential for something other than the metabolic carboxylation reactions required for tricarboxylic acid (TCA) cycle reactions and/or biosynthetic reactions not related to the TCA cycle (21).
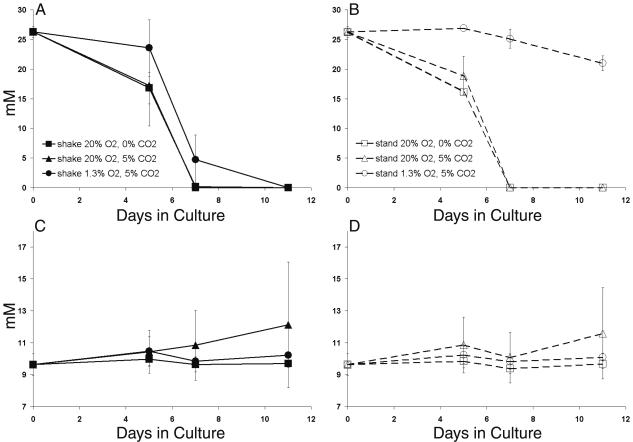
We next investigated M. bovis BCG carbon utilization in various growth conditions. The mycobacterial growth medium used in these studies provides both glycerol and glucose as carbon sources for mycobacterial growth. HPLC was used to quantitate the rate at which these two nutrients were consumed (Fig. 6). Glycerol was completely depleted from the medium by day 7 in ambient and CO2-supplemented air, but approximately 4.5 mM glycerol remained in liquid cultures after 7 days of microaerophilic growth (Fig. 6A). We noted that these microaerophilic cultures also typically reached a higher cell density than other cultures before entering stationary phase (Fig. 4D and 5A). The smaller depletion of glycerol (∼5 mM) observed with the standing low-oxygen culture (Fig. 6B) was consistent with the very slow growth rate under these conditions (Fig. 4D).
FIG. 6.
HPLC measurement of the consumption of glycerol (A and B) and glucose (C and D) by M. bovis BCG grown in shaking or standing cultures in ambient air, air supplemented with 5% CO2, or 5% CO2-supplemented microaerophilic (1.3% O2) conditions. Values shown are the means of results from three independent experiments. Error bars denote standard deviations.
Glucose was not consumed in any M. bovis BCG cultures during the course of these experiments (Fig. 6C and D). In fact, glucose levels in the bacterial growth medium appeared to increase by 11 days of growth in both shaking and standing high-oxygen cultures supplemented with 5% CO2. HPLC glucose results were verified with an Accu-Check blood glucose monitor (data not shown). No evidence of fermentation in the form of acetate or other common fermentation products was detected by HPLC analysis (48) under any of the culture conditions tested. The results indicate that regulatory changes associated with different growth conditions are not due to differences in product accumulation, i.e., glycerol is oxidized to carbon dioxide and water in all cases and glucose is never used.
Ultrastructural analysis.
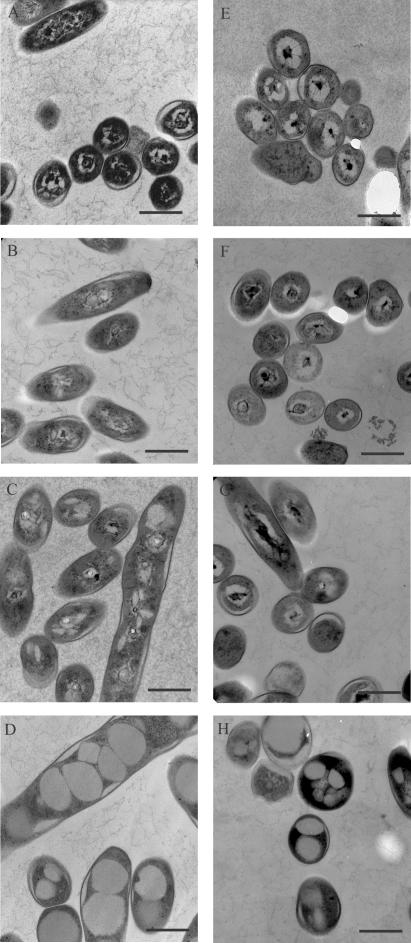
Transmission electron microscopy was used to analyze M. bovis BCG grown under various conditions (Fig. 7). Shaking and standing ambient cultures and shaking low-oxygen cultures, at both 7 (Fig. 7A to C) and 11 days (Fig. 7E to G) of growth, all had similar morphologies. At 11 days of growth, the ultrastructural morphology of the bacteria was similar to the well-defined bull's-eye-like appearance previously described for M. tuberculosis H37Rv cultures (28). In contrast, distinct morphological changes were noted in M. bovis BCG cultures grown in standing microaerophilic CO2-supplemented conditions (Fig. 7D and H). These bacteria retained good overall morphology but contained large vacuolar inclusions that were not present in the other conditions.
FIG.7.
Morphology of M. bovis BCG grown under AFR conditions. M. bovis BCG was grown for 7 (A to D) or 11 (E to H) days in ambient air in shaking cultures (A and E) or standing cultures (B and F) or in 1.3% O2-5% CO2 in shaking cultures (C and G) or standing cultures (D and H). Note the phenotypic changes, notably vacuolization, in bacteria grown in standing cultures at 1.3% O2-5% CO2 (D and H). Bars = 500 nm.
DISCUSSION
ACG promoter family.
Eight new M. tuberculosis promoters that are regulated in a manner similar to that of the previously characterized acr and acg promoters (33) were identified. Each member of this family of 10 coregulated promoters contained at least one site consistent with the conserved ACP family DNA sequence motif and was coordinately up-regulated in shallow standing cultures and in response to microaerophilic conditions. Each of these ACPs was expressed at higher levels within macrophages than in tissue culture medium.
The effects of mycobacterial culture conditions may have important implications for the interpretation of studies that examine mycobacterial gene expression within macrophages (15, 33). Prior passage within macrophages alters the behavior of M. tuberculosis H37Rv upon reentry into other macrophages or lung epithelial cells, and this is associated with de novo bacterial gene expression during the prior intramacrophage passage (1, 25, 26). Expression of 10 or more macrophage-inducible ACP family genes in shallow standing cultures prior to macrophage infection may result in bacteria that are more “primed” for intramacrophage survival and replication than bacteria from shaking cultures. At least one of these genes (acr) has previously been associated with M. tuberculosis replication within macrophages (50). The possibility that prior culture conditions may affect M. tuberculosis's interaction with macrophages during or following uptake warrants further investigation. In addition, intramacrophage gene induction is often determined relative to extracellular expression levels. Therefore, factors that independently increase a gene's expression in extracellular bacteria decrease its calculated intracellular/extracellular macrophage induction ratio, resulting in some macrophage-induced genes not being recognized as such, depending on culture conditions.
Nearly all of the ACG family members identified in this study have unknown or putative functions, emphasizing that there is still much to be learned about the role of this family of genes in M. tuberculosis biology. Aside from acr, only narK2, which is located immediately upstream of the dormancy-inducible narX gene in M. tuberculosis (19), has been characterized at the functional level. Both acr and narK2 have been previously associated with M. tuberculosis latency, and the microaerophilic induction of narK2 is consistent with the findings of others (18, 36). In Escherichia coli, NarK is involved in the extrusion of nitrite produced during nitrate respiration (7) and its expression is strongly induced by oxygen limitation (20). Reduction of nitrate to nitrite in M. tuberculosis cultures undergoing hypoxic shiftdown is greatly increased over reduction in aerobically growing cultures (44). This suggests a critical role for the NarK2 nitrite extrusion protein during the microaerophilic conditions that may be encountered within granulomas.
The products of ACP family genes acg, Rv3127, and Rv3131 belong to a superfamily of putative classical nitroreductases (33). These proteins may be involved in detoxification of nitrogen-containing by-products generated within macrophages or granulomas during infection. The products of two others, Rv2623 and Rv2005c, are members of a superfamily of putative ATP-binding proteins in M. tuberculosis (15). Both Rv2623 and Rv2005c were also rapidly induced by hypoxia in a previous study (36). Expression of the Rv2623 protein is greatly increased in 5-day-old standing versus shaking mycobacterial cultures (15), in the Wayne dormancy model (2), intracellularly within the cultured macrophage cell line THP-1 (29), and in murine lungs concurrent with induction of Th1-mediated immunity (37). Little is known about the functions of these putative ATP-binding proteins in mycobacteria, although a global regulatory role has been proposed for the product of Rv2623 based on what is known about its homologues in other organisms (15). The remaining three genes, Rv1738, Rv3130c, and Rv1733c, have putative open reading frames (ORFs) of unknown function (5).
Promoter structure and regulation.
All ACPs contained one to three sites consistent with the ACP family DNA motif, and in three cases these sites were shared by two divergently transcribed ACPs (promoters for acr and acg, narK2 and Rv1738, and Rv3130c and Rv3131). In a fourth case, Rv2005c responded to the AFR conditions but the promoter of the divergently transcribed ORF of Rv2006, a putative trehalose-phosphatase gene, did not respond. Rv3127 and Rv3126c are also predicted to be divergently transcribed genes (5), although only 24 bp separates their putative start codons. Rv3127 responded to the AFR conditions, but Rv3126c was not active in any conditions. The Rv3127 annotation is strongly supported by the gene's similarity to other putative classical nitroreductase gene family members (33). The DNA region upstream of Rv3127 contained one copy of the ACP family DNA motif, which was located within the putative Rv3126c ORF. Sherman and coworkers observed similar induction levels of Rv3126c and Rv3127 mRNA under hypoxic conditions (36). We think it likely that the actual Rv3126c start site differs from the annotated site (5) and that the Rv3126c upstream DNA fragment that we tested did not contain all of the necessary promoter and regulatory sequences. The regions upstream of the remaining two ACP family members (those for Rv1733c and Rv2623) did not contain divergently transcribed promoters.
All of the ACP family members were also rapidly induced at the transcriptional level by hypoxia in a previous study (36). Computational analysis using the Gibbs recursive sampler and SCAN suggests that an additional 11 promoters of these hypoxia-induced genes from the previous study (Rv0079, Rv0569, Rv0574c, Rv1813c, ctpF [Rv1997], fdxA [Rv2007c], Rv2626c, Rv2627c, Rv2628, Rv2629, and Rv3134c) have the ACP family sequence motif (L. A. McCue, unpublished data). Future studies will determine whether these additional hypoxia-induced genes are also regulated by other AFR conditions.
Gibbs sampling results revealed that the ACP motif is significantly conserved relative to background (i.e., those regions of the promoters not included in the motifs) in the set of eight intergenic regions shown in Fig. 2A, yielding a maximum a posteriori probability value of 41.85. In fact, our background composition model is position specific and therefore well suited for the analysis of a genome that is high in G+C content overall (65.5%) but that has significant variation in this percentage in some regions (5). Many of the ACPs contained at least two sequences that matched the ACP family motif with high confidence (P < 0.05), and frequently the spacing between these sites in a promoter was 4 bp. It remains to be determined whether this conserved spacing or the number of motif copies is important for regulation. Only one site in the Rv2005c promoter was identified at this level of confidence; however a second site 4 bp from the first was predicted with lower confidence (P = 0.32).
The palindromic nature of the ACP motif is consistent with a role in gene regulation, as the majority of characterized prokaryotic transcription factors interact with DNA as homodimers and recognize palindromic sequences. A previous study by us (33) also supports a regulatory role for the ACP family motif. Specifically, expression of both the acr and acg promoters was inactivated by short deletions (18 or 22 bp) of a sequence that contained copies of the ACP family motif. Sherman and coworkers previously showed that the putative response regulator Rv3133c is required for expression of acr in hypoxic conditions (36). Our finding that Rv3133c is needed for expression of several additional ACP family members under low oxygen tension suggests that it may be a general regulator of all the ACG family promoters. Other recent work indicates that Rv3133c is required for M. bovis BCG survival in the low-oxygen latency model (2). It remains to be tested whether Rv3133c directly binds the ACP family motif to positively regulate the expression of these genes and whether the ACGs are required for hypoxia-induced dormancy survival.
Similarity in the behaviors of the ACG family promoters in standing cultures, under reduced oxygen tension, and within macrophages suggests that there may be common regulatory mechanisms that respond to key aspects of these microenvironments. It is also likely that at least some of the ACPs are regulated at multiple levels, as has been shown for that of narK2 (18). Strong induction of all ACPs in response to microaerophilic conditions indicates that oxygen limitation is an important environmental signal for the corresponding family of genes. Previously, we speculated that localized oxygen depletion may occur in the immediate vicinity of settled bacteria in our shallow standing, ambient atmosphere culture model (15, 33). This is supported by the insensitivity of the hypoxic cultures to shaking versus standing conditions (Fig. 1). However, the differing levels of expression observed for each promoter in response to different conditions suggest that the environmental signals in standing cultures and within macrophages are more complex than simply low oxygen. It is also likely that there are multiple levels of regulatory control of these genes. The shallow standing culture model and hypoxic culture conditions will be useful for defining some of these additional factors.
Effects of atmospheric conditions on mycobacterial growth, metabolism, and gene expression.
It has been suggested that low oxygen tension within tubercle lesions plays an important role in the disappearance or dormancy of the tubercle bacillus (10, 46). However, the degree of anaerobiosis within these caseous lesions remains uncertain, and low oxygen tension may only be one of the characteristics of the tubercle involved in triggering mycobacterial dormancy.
Importantly, our studies indicate that CO2 greatly affects the growth rate of M. bovis BCG under microaerophilic conditions. Others have noted enhanced growth of mycobacteria in the presence of CO2 (30), but the present study is the first report of the ability of CO2 to sustain mycobacterial growth under microaerophilic conditions. This observation may have physiological relevance to human infection as well as important implications for the interpretation of in vitro data on oxygen limitation in mycobacterial latency models. Pathogenic mycobacteria encounter various concentrations of CO2 depending upon their local environment. Bacteria in ambient air outside of the host encounter very low levels of CO2 (23), while alveolar air, blood, and interstitial fluids contain ∼5 to 6% CO2 (16).
The Wayne model of mycobacterial persistence suggests that the transition to a low-O2 environment is the primary signal for entry into a state of nonreplicative growth (46). We propose that limiting CO2 also contributes to mycobacterial growth inhibition in low-O2 conditions. This may have important implications for dormancy models of mycobacterial latency as well as for understanding the adaptation and long-term survival of mycobacteria within granulomas. For example, comparatively CO2-rich hypoxic conditions within macrophages may facilitate the adaptation of tubercle bacilli to reduced levels of both CO2 and O2 within a granuloma.
Viable bacteria could be recovered from the nonreplicating microaerophilic M. bovis BCG cultures (Fig. 4C) by providing either O2 or CO2, although recovery from slowly replicating standing cultures was more rapid and quantities recovered were larger (Fig. 5). The relationship of these nonreplicating bacteria to the nonreplicating persistent bacteria described in the Wayne model warrants further study. The Wayne model of nonreplicating persistence involving slow stirring achieves a level of anaerobic survival designated NRP-2 by slowly reducing the dissolved O2 below 0.06% (43). In contrast, the insensitivity of M. bovis BCG to metronidazole under all of the culture conditions we studied suggests that our low-oxygen cultures are microaerophilic and do not reach the fully anaerobic stage.
Ultrastructural analysis.
The cause of the morphological changes observed in M. bovis BCG cultures grown standing in CO2-supplemented microaerophilic conditions is not clear (Fig. 7D and H). The bacteria were similar in appearance to previously described M. tuberculosis H37Ra grown intracellularly within J774.16 macrophages for 5 days (28). It was suggested that these vacuoles were lipid inclusions caused by increased synthesis of lipids, with the excess lipid being blocked from transport out of the cell (28). Another possibility is that the inclusions function as lipid or carbohydrate storage vacuoles necessary for the adaptation of these organisms for survival under the microaerophilic conditions found in the standing cultures. Further characterization of these vacuoles is necessary to determine their composition. The extensive cell wall thickening that was previously observed in M. bovis BCG cultured under anaerobic conditions for 6 months (6) was not observed under our culture conditions.
This report defines an M. tuberculosis ACP gene family whose expression is similarly up-regulated in standing cultures, under low oxygen tension, and intracellularly within macrophages. We identified a conserved 18-bp palindromic sequence motif in all ACP family members and propose that this motif serves as a regulatory locus, possibly through Rv3133c, that controls the expression of ACPs under AFR conditions. Further characterization of this regulatory pathway will contribute to our understanding of M. tuberculosis biology and its response to environmental signals. Our observation that CO2 plays a critical role in M. bovis BCG growth under microaerophilic conditions also has important implications for the interpretation of hypoxia-induced models of mycobacterial dormancy and possibly human infections.
Acknowledgments
We thank David R. Sherman for providing the M. tuberculosis H37Rv:ΔRv3133c strain and Keith Derbyshire for the plasmid pEN::Tnhyg. We also thank Chris McIntosh, James Mittler, and Shalini Varma for technical support with this project and Terry Miller and Yvonne Kress for helpful discussions. We acknowledge support from the Wadsworth Center's Computational Molecular Biology and Statistics, Molecular Genetics, Flow Cytometry, and Electron Microscopy core facilities.
This work was supported in part by National Institutes of Health grant AI4565801 (K.A.M.) and the Potts Foundation (K.A.M.). L.A.M. was supported by NIH grant HG01257 and Department of Energy grant DE-FG02-01ER63204.
Editor: V. J. DiRita
REFERENCES
- 1.Bermudez, L. E., F. J. Sangari, P. Kolonoski, M. Petrofsky, and J. Goodman. 2002. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect. Immun. 70:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boon, C., R. Li, R. Qi, and T. Dick. 2001. Proteins of Mycobacterium bovis BCG induced in the Wayne dormancy model. J. Bacteriol. 183:2672-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burtis, C., and E. Ashwood (ed.). 1994. Tietz textbook of clinical chemistry, 2nd ed. W. B. Saunders Company, Philadelphia, Pa.
- 4.Chan, K., T. Knaak, L. Satkamp, O. Humbert, S. Falkow, and L. Ramakrishnan. 2002. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proc. Natl. Acad. Sci. USA 99:3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham, A. F., and C. L. Spreadbury. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J. Bacteriol. 180:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMoss, J. A., and P. Y. Hsu. 1991. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J. Bacteriol. 173:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick, T., B. H. Lee, and B. Murugasu-Oei. 1998. Oxygen depletion induced dormancy in Mycobacterium smegmatis. FEMS Microbiol. Lett. 163:159-164. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau, E., P. Fontan, R. Manganelli, S. Soares-Appel, and I. Smith. 2002. Mycobacterium tuberculosis genes induced during infection of human macrophages. Infect. Immun. 70:2787-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubos, R. J. 1953. Effect of the composition of the gaseous and aqueous environments on the survival of tubercle bacilli in vitro. J. Exp. Med. 97:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlap, N. E., and D. E. Briles. 1993. Immunology of tuberculosis. Med. Clin. N. Am. 77:1235-1251. [DOI] [PubMed] [Google Scholar]
- 12.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich, G. G., D. F. Goerlitz, J. H. Bourell, G. V. Eisen, and E. M. Godsy. 1981. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl. Environ. Microbiol. 42:878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton, M. J., and M. W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florczyk, M. A., L. A. McCue, R. F. Stack, C. R. Hauer, and K. A. McDonough. 2001. Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins. Infect. Immun. 69:5777-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyton, A. C. 1991. Textbook of medical physiology, 8th ed. W. B. Saunders Company, Philadelphia, Pa.
- 17.Hu, Y. M., P. D. Butcher, K. Sole, D. A. Mitchison, and A. R. Coates. 1998. Protein synthesis is shutdown in dormant Mycobacterium tuberculosis and is reversed by oxygen or heat shock. FEMS Microbiol. Lett. 158:139-145. [DOI] [PubMed] [Google Scholar]
- 18.Hutter, B., and T. Dick. 2000. Analysis of the dormancy-inducible narK2 promoter in Mycobacterium bovis BCG. FEMS Microbiol. Lett. 188:141-146. [DOI] [PubMed] [Google Scholar]
- 19.Hutter, B., and T. Dick. 1999. Up-regulation of narX, encoding a putative ′fused nitrate reductase' in anaerobic dormant Mycobacterium bovis BCG. FEMS Microbiol. Lett. 178:63-69. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnikow, T., I. Schroder, and R. P. Gunsalus. 1992. Regulation of narK gene expression in Escherichia coli in response to anaerobiosis, nitrate, iron, and molybdenum. J. Bacteriol. 174:7104-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozliak, E. I., J. A. Fuchs, M. B. Guilloton, and P. M. Anderson. 1995. Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J. Bacteriol. 177:3213-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B. Y., and M. A. Horwitz. 1995. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J. Clin. Investig. 96:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lide, D. R. (ed.). 1997. CRC handbook of chemistry and physics. CRC Press, Cleveland, Ohio.
- 24.Lim, A., M. Eleuterio, B. Hutter, B. Murugasu-Oei, and T. Dick. 1999. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J. Bacteriol. 181:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonough, K. A., M. A. Florczyk, and Y. Kress. 2000. Intracellular passage within macrophages affects the trafficking of virulent tubercle bacilli upon reinfection of other macrophages in a serum-dependent manner. Tuber. Lung Dis. 80:259-271. [DOI] [PubMed] [Google Scholar]
- 26.McDonough, K. A., and Y. Kress. 1995. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect. Immun. 63:4802-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonough, K. A., Y. Kress, and B. R. Bloom. 1993. The interaction of Mycobacterium tuberculosis with macrophages: a study of phagolysosome fusion. Infect. Agents Dis. 2:232-235. [PubMed] [Google Scholar]
- 28.McDonough, K. A., Y. Kress, and B. R. Bloom. 1993. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61:2763-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monahan, I., J. Betts, D. Banerjee, and P. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147:459-471. [DOI] [PubMed] [Google Scholar]
- 30.Nayebi, M. 1970. The effect of mechanical agitation and CO2 on the growth of the BCG, H37Ra and RlRv strains of Mycobacterium tuberculosis. J. Med. Lab. Technol. 27:218-221. [PubMed] [Google Scholar]
- 31.Neuwald, A. F., J. S. Liu, and C. E. Lawrence. 1995. Gibbs motif sampling: detection of bacterial outer membrane protein repeats. Protein Sci. 4:1618-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrish, N. M., J. D. Dick, and W. R. Bishai. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107-112. [DOI] [PubMed] [Google Scholar]
- 33.Purkayastha, A., L. A. McCue, and K. A. McDonough. 2002. Identification of a Mycobacterium tuberculosis putative classical nitroreductase gene whose expression is coregulated with that of the acr gene within macrophages, in standing versus shaking cultures, and under low oxygen conditions. Infect. Immun. 70:1518-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowland, B., A. Purkayastha, C. Monserrat, Y. Casart, H. Takiff, and K. A. McDonough. 1999. Fluorescence-based detection of lacZ reporter gene expression in intact and viable bacteria including Mycobacterium species. FEMS Microbiol. Lett. 179:317-325. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, T. D., and R. M. Stephens. 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 18:6097-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small, P. M., and P. I. Fujiwara. 2001. Management of tuberculosis in the United States. N. Engl. J. Med. 345:189-200. [DOI] [PubMed] [Google Scholar]
- 39.Smith, I., O. Dussurget, G. M. Rodriguez, J. Timm, M. Gomez, J. Dubnau, B. Gold, and R. Manganelli. 1998. Extra and intracellular expression of Mycobacterium tuberculosis genes. Tuber. Lung Dis. 79:91-97. [DOI] [PubMed] [Google Scholar]
- 40.Sturgill-Koszycki, S., P. L. Haddix, and D. G. Russell. 1997. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 18:2558-2565. [DOI] [PubMed] [Google Scholar]
- 41.Triccas, J. A., W. J. Britton, and B. Gicquel. 2001. Isolation of strong expression signals of Mycobacterium tuberculosis. Microbiology 147:1253-1258. [DOI] [PubMed] [Google Scholar]
- 42.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13:908-914. [DOI] [PubMed] [Google Scholar]
- 43.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wayne, L. G., and L. G. Hayes. 1998. Nitrate reduction as a marker for hypoxic shiftdown of Mycobacterium tuberculosis. Tuber. Lung Dis. 79:127-132. [DOI] [PubMed] [Google Scholar]
- 45.Wayne, L. G., and K. Y. Lin. 1982. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 37:1042-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 47.Wayne, L. G., and H. A. Sramek. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolin, M., and T. Miller. 1993. Bacterial strains from human feces that reduce CO2 to acetic acid. Appl. Environ. Microbiol. 59:3551-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan, Y., D. D. Crane, and C. E. Barry III. 1996. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J. Bacteriol. 178:4484-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry III. 1998. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]