Abstract
Epidemiological studies have shown a potential association between maternal periodontitis and pregnancy complications. We used a pregnant murine model to study the effect of infection with the periodontal pathogen Porphyromonas gingivalis on pregnancy outcomes. Female BALB/c mice were inoculated with heat-killed P. gingivalis (109 CFU) in a subcutaneous chamber and mated 2 weeks later. At gestation day (GD) 7.5, mice were challenged with live P. gingivalis (107 CFU) (n = 20) or broth (control; n = 8) and sacrificed at GD 16.5. Fetal growth restriction (FGR, <0.46 g) was defined as fetuses with weights 2 standard deviations (SD) smaller than controls (0.56 ± 0.05 g [mean ± SD]). Among the 20 challenged mice, 8 had both normal-weight (0.51 ± 0.11 g) and FGR (0.34 ± 0.1 g) fetuses within the same litter. All other challenged dams had normal-weight fetuses (0.57 ± 0.04 g). Maternal liver, uterus, and spleen samples were examined for P. gingivalis DNA using a PCR technique. Of the eight challenged mice with FGR fetuses, three had PCR signals for P. gingivalis in liver and uterus, but not in the spleen. Liver, uterus, and spleen were negative for P. gingivalis DNA among all other challenged and control mice. In serum of dams with FGR fetuses, tumor necrosis factor alpha levels were elevated significantly, while interluekin-10 levels were significantly reduced compared to levels in dams with normal fetuses. P. gingivalis-specific serum immunoglobulin G levels were significantly elevated in dams with FGR fetuses compared to dams without any FGR fetuses. These data demonstrate that P. gingivalis-induced murine FGR is associated with systemic dissemination of the organism and activated maternal immune and inflammatory responses.
Fetal growth restriction (FGR) refers to insufficient fetal growth in fetal weight or height for gestational age, and it is usually defined as less than 2 standard deviations (SD) below the mean for gestational age (8). FGR is correlated with preterm low birth weight, perinatal mortality, and neonatal morbidity (29), and it is associated with diseases in adulthood such as noninsulin diabetes mellitus (42). An infant's chances of survival increase rapidly with increasing birth weight (29). In 1998, 65% of all infant deaths occurred among the 7.6% of infants born with low birth weight (LBW) (<2,500 g), and 51% of all infant deaths occurred in the 1.5% of infants born with very low birth weight (<1,500 g). The percentage of infants classified as LBW did not change during the early 1980s but gradually increased until 1998, and it has remained unchanged through 2000 (29). Preventive procedures have not been successful in decreasing the incidence of LBW, primarily because the etiology of most FGR births is unknown.
The effects of microbial infections on pregnancy outcomes have been observed in all mammalian species, including humans, and have been extensively studied in several animal models (6, 30). Other studies suggest that some adverse pregnancy outcomes are associated with subclinical infections such as periodontitis. Previous human cross-sectional and case-control investigations (14, 32, 33, 35, 36) and animal experiments (10, 11) all have shown a potential association between chronic periodontitis and reduced fetal weight.
Periodontal disease is a common chronic local inflammatory process characterized by bacterial challenge and release of various toxic products (extracellular vesicles and lipopolysaccharides [LPS]) by periodontal pathogens and by host immune responses comprised of a cellular infiltration of neutrophils, macrophages, and lymphocytes and the release of cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6. The primary etiologic factor for periodontal diseases is bacteria that accumulate in the gingival sulcus. Porphyromonas gingivalis, a gram-negative, black-pigmented anaerobic rod bacterium, is thought to be one major periodontal pathogen and the primary etiological agent in the destructive forms of periodontal disease (2, 3). P. gingivalis displays pathogenic properties not only in periodontal diseases (13) but also in such systemic diseases as cardiovascular diseases and adverse pregnancy outcomes (9, 26, 34, 36). These findings indicate that periodontal pathogens may play a role in the development and progression of systemic pathology.
An animal model is needed in order to investigate the association between local infection and fetal growth and to better understand the host-pathogen interactions. In addition, since there is a low frequency (<6%) of chromosomal abnormalities in rodent embryos (15), laboratory mice can be a useful model to study the mechanisms of human abnormal pregnancy outcomes (16). A mouse subcutaneous chamber model was developed by Arko to study Neisseria infection (5), and this model was adapted by Genco et al. (23, 24) to model a localized chronic infection with P. gingivalis. Offenbacher and coworkers used this model to study the effects of P. gingivalis infection on pregnancy outcomes in golden hamsters (10, 11). Immunization of mice (24) or hamsters (11) with heat-killed P. gingivalis induced a primary immune response. The sensitization to P. gingivalis permitted the establishment of a chronic low-grade infection following a subsequent secondary live P. gingivalis challenge. This chronic infection model more closely mimics the chronic infection with periodontal pathogens observed in human patients. Using this model adapted to hamsters, Offenbacher and coworkers found that maternal exposure to P. gingivalis A7436 could induce deleterious effects on the fetus (10, 11).
Based on these studies, we hypothesize that P. gingivalis and/or its components can disseminate from a local infectious site through the circulatory system and into remote organs (e.g., liver and uterus), induce both systemic and placental inflammatory responses, and result in abnormal pregnancy outcomes. In this study, we examined the organism's dissemination following localized infection and the induction of maternal immune and inflammatory responses in pregnant mice.
MATERIALS AND METHODS
Bacterial strain and preparation of bacterial suspensions.
P. gingivalis strain A7436 was originally isolated from a patient with refractory periodontitis, and the stock bacteria were stored in Wilkins Chalgren anaerobic broth medium (WC broth; DSMZ, Braunschweig, Germany) containing 10% skim milk at −80°C. Bacteria were cultivated in WC broth at 37°C in an anaerobic chamber (Coy Laboratory Products Inc., Ann Arbor, Mich.) with 5% H2, 10% CO2, and 85% N2. Bacterial suspensions were prepared from primary cultures at their log phase of growth. Bacterial concentration was evaluated by spectrophotometry (Cecil Instruments Ltd., Cambridge, United Kingdom), with a measured optical density at 600 nm of 1 corresponding to 109 bacteria/ml, and adjusted to the desired treatment concentration by dilution with broth.
Animal husbandry.
Female BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were obtained at 6 to 8 weeks of age and maintained under standardized conditions of 12-h light-dark cycle (0700 to 1900 light), constant temperature of 25°C, and regular mouse chow and water ad libitum. All procedures were in accordance with animal welfare guidelines and were approved by the University of North Carolina at Chapel Hill (UNC-CH) Animal Welfare Committee and the Institutional Animal Care and Use Committee.
Subcutaneous chamber implantation.
Chambers were constructed from a cylindrical coil spring made of 4-mm-diameter surgical stainless steel wire, cut into 10-mm lengths. One chamber was implanted subcutaneously in the dorsolumbar region of 7- to 9-week-old female mice. At least 2 weeks were allowed for complete wound healing and chamber wrapping with connective tissue before intrachamber inoculation (24).
Chronic localized infections with P. gingivalis strain A7436 of pregnant female BALB/c mice.
The treatment groups and injection schedules are illustrated in Fig. 1. All female mice were immunized by intrachamber injection of 0.1 ml of suspension containing 109 CFU of heat-killed P. gingivalis A7436. Two weeks later, female mice were mated with male mice for one night. The next morning, all female mice were removed and examined for the presence of a vaginal plug. If a plug was found, that day was recorded as gestation day (GD) 0.5. On GD 7.5, pregnant mice were randomly assigned into the control group (n = 8), which received an intrachamber injection of 0.1 ml of WC broth, or the P. gingivalis-challenged group (n = 20), which received an intrachamber injection of 0.1 ml of broth containing 107 CFU of live P. gingivalis A7436.
FIG. 1.
Experimental timeline. HK = heat killed; W = week; P.g. = P. gingivalis.
Sacrifice, evaluation of pregnancy outcomes, and tissue sampling.
The female pregnant mice were sacrificed by CO2 asphyxiation on GD 16.5. The resorption sites and viable fetuses were counted and recorded for each mouse (Table 1). Each fetus was removed from its chorioamniotic sac and weighed to the nearest microgram. Maternal liver, spleen, and uterus were collected and washed with sterile saline solution. Maternal blood was drawn by cardiac puncture, and serum was collected. Amniotic fluid was collected from each fetal-placental unit and pooled per pregnant mouse. All samples were stored at −80°C until analysis.
TABLE 1.
Pregnancy outcomes in P. gingivalis-challenged pregnant mice compared to control (nonchallenged) mice
| Exptl group (n) | No. of litters with FGR fetuses/total no. of litters | Meana fetal wt ± SD (g) | No. of litters with resorptions/ total no. of litters |
|---|---|---|---|
| Control (8) | 0/8 (0%) | 0.56 ± 0.05 | 4/8 (50%) |
| P. gingivalis- challenged (20) | 8/20 (40%) | 0.51 ± 0.05b | 17/20 (85%)c |
Calculated mean for all fetuses from all litters of each group of mice.
Significantly lower than control group; P < 0.05 (Student's t test).
Not significantly different from the control group; P = 0.06 (Student's t test).
P. gingivalis microbial culture from tissue samples.
Samples of chamber fluid were collected on GD 16.5 when mice were sacrificed. A few drops of fluid samples were placed in tubes containing 10 ml of prereduced anaerobic WC broth and cultured anaerobically for 3 days at 37°C. Growth was recorded at that time, with positive growth manifesting as broth cloudiness. Two drops of growth-positive broth was further streaked for isolation onto anaerobic reduced agar. After 3 days of anaerobic culture at 37°C, aspect of colonies was observed and compared to P. gingivalis-specific colonies for confirmation of organism.
P. gingivalis 16S rRNA gene detection by PCR.
Tissue samples weighing approximately 20 mg for liver and 50 mg for other tissues were treated for DNA extraction using a GeneChoice DNA Tissue Mini kit (PGC Scientific Corporation, Frederick, Md.). Briefly, 400 μl of lysis buffer and 40 μl of protease K were added to each sample and incubated at 52°C in a thermomixer with continuous shaking overnight. Nonlysed materials were removed by centrifugation, and lysates were mixed with DNA binding buffer. DNA was collected by a series of washes through a spin column and a final elution. The quality and quantity of extracted DNA were determined spectrophotometrically. One microgram of DNA was used as template for each PCR. Two specific primers for the P. gingivalis 16S rRNA gene were synthesized at the UNC Nucleic Acid Core Facility. Their sequences are as follows: (i) 5′-AGGCAGCTTGCCATACTGCG-3′ and (ii) 5′-ACTGTTAGCAACTACCGATGT-3′ (40). PCR was performed using a HotStarTaq DNA polymerase kit (Qiagen, Valencia, Calif.) following the manufacturer's instructions. A DNA thermal cycler (GeneAmp PCR System 9700; PE Biosystems, Foster City, Calif.) was used for amplification, with an initial denaturation step at 95°C for 15 min followed by 36 cycles of denaturation at 95°C for 30 s, primer annealing at 60°C for 1 min, and extension at 72°C for 1 min, and then a final extension step at 72°C for 10 min (40). The amplified material was subjected to electrophoresis at 90 mV on a 2% Tris-acetate (1× TAE) agarose gel, using a 100-bp DNA ladder (Promega Corporation, Madison Wis.) as a size marker. The gel was stained with SYBR Gold (Molecular Probes, Eugene, Oreg.), and a photograph was taken under UV light. The presence of P. gingivalis was determined by the existence of an amplicon of the expected size of 404 bp. Positive controls included P. gingivalis alone or tissues spiked with exogenous P. gingivalis. The detection limit for P. gingivalis alone was 10 CFU/PCR, and for the P. gingivalis-spiked tissue it was 100 CFU/PCR (data not shown).
Measurement of serum levels of TNF-α, IFN-γ, IL-2, IL-4, IL-6, and IL-10 by ELISA.
TNF-α, gamma interferon (IFN-γ), IL-2, IL-4, IL-6, and IL-10 concentrations in maternal serum were quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kits (all from R&D Systems, except IL-6 from Biosource Inc.) following the manufacturers' protocols. Briefly, serum samples were added to 96-well ELISA plates that were coated with 1 μg of anti-mouse cytokine monoclonal antibodies/ml. A secondary biotinylated antibody specific for the cytokine of interest was added as the detecting antibody, followed by a streptavidin-horseradish peroxidase conjugate (enzyme). The substrate used to bind enzyme and produce color was tetramethylbenzidine. The reaction was stopped by the addition of 2 N hydrochloric acid, and the optical density was determined using a Vmax microplate reader (Molecular Devices, Palo Alto, Calif.) at 450 nm against a standard curve based on known concentrations of the murine recombinant cytokine.
Maternal serum IgG estimation using checkerboard immunoblotting.
Maternal serum immunoglobulin G (IgG) was analyzed following the procedure of Sakellari et al. (39) as modified by Madianos et al. (32). Briefly, P. gingivalis antigens were prepared by disrupting the concentration-adjusted bacterial colonies in phosphate-buffered saline with a microultrasonic cell disrupter for 10 s. A sheet of 15- by 15-cm ECL nitrocellulose membrane (Amersham Life Science, Arlington Heights, Ill.) was soaked back to back in distilled H2O for 5 min and in phosphate-buffered saline for 10 min before loading into an Immunetics miniblotter (Cambridge, Mass.). Next, 135 μl of vortexed bacterial suspension or protein A from Staphylococcus aureus was deposited in parallel lanes on the membrane using an immunoblotter device and incubated overnight at 4°C. Mouse serum samples diluted to 1:500, as well as standard serial dilutions of mouse IgG (500, 1,000, and 2,000 ng/ml), were loaded in lanes perpendicular to the antigen lanes and incubated at room temperature for 1 h. After washing to remove nonbound antibody, the membrane was incubated with goat anti-mouse IgG peroxidase-conjugated antibody (Fab fragment) diluted 1:30,000 for 1 h. Positive reactions were detected by enhanced chemiluminescence, using Western blotting detection reagents and an imager workstation. A LumiAnalyst combined with Image Analysis software version 3.1 (Boehringer Mannheim GmbH, Mannheim, Germany) was used to measure the Boehringer light units of each dot. Antibody concentrations were estimated by comparing the signal intensity of unknown samples (i.e., mouse sera) to that generated by the known concentrations of mouse IgG captured by protein A on the same membrane.
Statistical analyses.
The association between the categorical pregnancy outcome data (number of resorptions and/or FGR fetuses) and P. gingivalis dissemination (PCR detection of DNA in maternal tissue samples) was tested by using Fisher's exact test. Statistical testing of mean fetal weight and numbers of fetal resorptions per mouse were performed using Student's t test or exact logic regression, while the cytokine and immunoglobulin concentrations in maternal sera were compared between groups using one-way analysis of variance (ANOVA). P values of <0.05 were considered statistically significant, assuming two-tailed distributions. The fetal weights are presented as mean values ± the SD of the mean, and cytokine and immunoglobulin concentrations are presented as mean values ± the standard error (SE) of the mean.
RESULTS
Monitoring of maternal mice following P. gingivalis challenge.
No chamber sloughing or ulceration and no secondary lesions were found in any of the animals, which is consistent with previous studies (23). There were no significant differences in maternal weight gain at the time of sacrifice between broth- and P. gingivalis-challenged groups (P = 0.8).
Fetal weight at GD 16.5.
The recorded fetal weights at the time of sacrifice (GD 16.5) are summarized in Table 1. Fetuses with weights 2 SD smaller than normal fetal weight (NFW; i.e., less than 0.46 g) were defined as FGR. Of 20 P. gingivalis-challenged mice, 8 had both low-weight (FGR) fetuses (0.34 ± 0.08 g) and normal-weight (NFW) fetuses (0.54 ± 0.02 g) within their litter. The remaining P. gingivalis-challenged mice had all normal-weight fetuses within their litter (Tables 1 and 2). In addition, there was a 70% increase in fetal resorptions in the P. gingivalis-challenged group (Table 1). Overall, P. gingivalis challenge resulted in a decrease in mean litter weight among survivors that was statistically significant (P < 0.05) and an increase in fetal resorptions which approached statistical significance (P = 0.06).
TABLE 2.
Association of maternal serum cytokine levels with FGR
| Exptl group (n) | Meana fetal wt ± SD (g) (mean no. of fetuses/litter) | Mean maternal serum cytokine level ± SE (pg/ml)
|
||
|---|---|---|---|---|
| TNF-αb | IL-10b | IL-6b | ||
| Control, no FGR fetuses (8) | 0.56 ± 0.05 (7) | 2.91 ± 2.01 | 3.37 ± 1.2 | 18.8 ± 11.0 |
| P. gingivalis-challenged, no FGR fetuses (12) | 0.57 ± 0.06 (8) | 6.64 ± 2.64 | 2.53 ± 0.78 | 40.9 ± 17.5 |
| P. gingivalis-challenged, with FGR fetuses (8) | 0.43 ± 0.04c (7) | 27.50 ± 8.16d,e | 0.09 ± 0.09d | 57.5 ± 28.4 |
Calculated mean and pooled estimate of SD for all fetuses from all litters of each group of mice.
Detection sensitivities of ELISA kits are 3 pg/ml for IL-6, 4 pg/ml for IL-10, and 5 pg/ml for TNF-α, based upon 2 SD above the detection limit.
Significantly different from the control group; P < 0.05 (exact logistic regression).
Significantly different from the control group; P < 0.05 (ANOVA).
Significantly different from the P. gingivalis-challenged group with no FGR fetuses; P < 0.05 (ANOVA).
P. gingivalis culture from chamber fluid samples.
Previous studies demonstrated that immunization of mice with heat-killed P. gingivalis prior to infection with live bacteria allows for the colonization of live bacteria within the chamber and their protection from host clearance (23). P. gingivalis was cultured from the chamber fluid samples collected from all challenged mice, showing that live bacteria survived in the chamber environment (data not shown). Chamber fluid samples collected from control mice did not show any bacterial growth.
P. gingivalis DNA detection in liver and uterus samples.
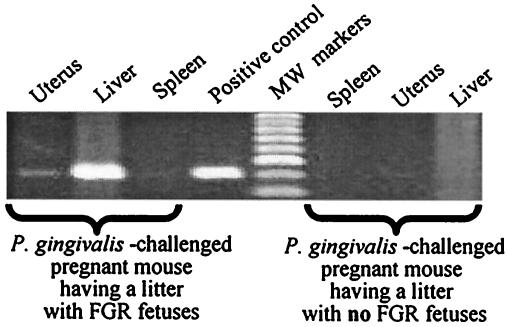
Using PCR, samples were examined for P. gingivalis DNA in order to determine the potential pathway of P. gingivalis dissemination from the locally infected site into remote tissues. No P. gingivalis PCR signals were detected in the liver, spleen, or uterus of control mice. Three of eight P. gingivalis-challenged mice with FGR fetuses in their litters had strong PCR signals for P. gingivalis in the liver and uterus, but not in the spleen (Fig. 2). For the other 12 test mice with normal-weight fetuses, no P. gingivalis PCR signal was found in maternal liver, spleen, or uterus. Statistical analysis showed an association between P. gingivalis DNA detection and fetal growth restriction (P < 0.05).
FIG. 2.
PCR detection of P. gingivalis DNA in tissues from P. gingivalis-challenged pregnant mice having litters with and without FGR fetuses. P. gingivalis-specific DNA can be detected in the maternal liver and uterus of a representative P. gingivalis-challenged mouse with FGR fetuses, but not in the samples of a representative P. gingivalis-challenged mouse with all normal-weight fetuses.
Maternal immune responses after P. gingivalis challenge.
To evaluate the systemic responses following localized infection, both cytokine levels and P. gingivalis-specific antibody levels were analyzed in maternal serum. Results from sera were averaged and compared between three groups of mice based on P. gingivalis challenge and fetal weight. That is, Pg-NFW represents the control group challenged with broth alone and having normal-weight fetuses, Pg+NFW represents the group of dams challenged with P. gingivalis and having all normal-weight fetuses, and Pg+FGR represents the group of dams challenged with P. gingivalis and having at least one low-weight fetus in the litter.
Maternal serum levels of TNF-α, IL-6, and IL-10 were compared among the three groups (Table 2). The concentration of TNF-α was increased in maternal serum from mice infected with P. gingivalis, with a significant increase in dams with FGR fetuses (i.e., Pg+FGR) compared to levels in the mice having normal fetuses (i.e., Pg-NFW and Pg+NFW) (P < 0.05). There was a statistically significant decrease in serum IL-10 in maternal mice with FGR fetuses (0.09 pg/ml [Pg+FGR]) compared with the control mice (3.37 pg/ml [Pg-NFW]) (P < 0.05). Although measured concentrations from samples from all groups were below the company's published detection limit (4 pg/ml), five out of six dams with low-weight fetuses had no detectable amounts of IL-10 compared to the control group, who had seven out of seven dams with detectable levels (data not shown). The IL-6 level in maternal serum showed a tendency to be higher in P. gingivalis-challenged mice, but it was not statistically significant (P = 0.3) (Table 2). Other cytokines assayed (IL-2, IL-4, and IFN-γ) did not show statistically significant differences between these groups (data not shown).
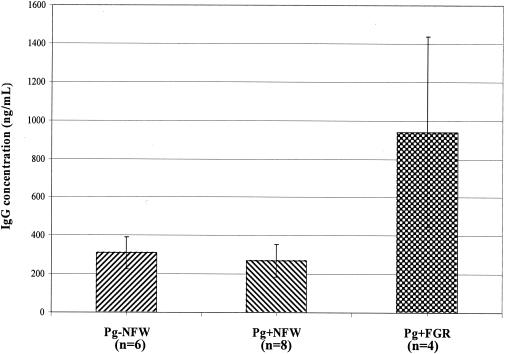
Using checkerboard immunoblotting, serum levels of IgG specific to P. gingivalis were analyzed and used as a marker of maternal immune response. There was a significant elevation of IgG concentration in the group of P. gingivalis-challenged mice with FGR fetuses compared with those of the prechallenge sera, the control group, and the P. gingivalis-challenged group with normal-weight fetuses (Fig. 3). However, serum was not available from all animals in each group, leading to a small sample size. The P. gingivalis-specific IgG levels in amniotic fluid samples also showed a statistically significant increase in the group of challenged mice with FGR fetuses, compared with the control group and the P. gingivalis-challenged group with all normal-weight fetuses (Fig. 4). IgG levels in maternal serum and in amniotic fluid probably reflect maternal response to infection, based upon reports that murine fetuses cannot produce IgG before birth (4, 20).
FIG. 3.
P. gingivalis-specific IgG concentration in maternal serum of immunized mice pre- and postchallenge. There is a significant elevation of IgG concentration in the group of P. gingivalis-challenged mice with FGR (Pg+FGR) compared with the pre-live P. gingivalis-challenge sera, broth-challenged group (Pg-NFW), and P. gingivalis-challenged group with NFW fetuses (Pg+NFW).
FIG. 4.
P. gingivalis-specific IgG concentration in amniotic fluid. There is an increase of IgG in the group of P. gingivalis-challenged mice with FGR (Pg+FGR) compared with the broth-challenged group (Pg-NFW) and P. gingivalis-challenged group with NFW fetuses (Pg+NFW).
DISCUSSION
This study, using a subcutaneous chamber model with P. gingivalis infection to investigate the association between infection with a periodontal pathogen and abnormal pregnancy, demonstrated that 40% of P. gingivalis-challenged mice had low-weight fetuses. Furthermore, P. gingivalis challenge resulted in a 70% increase in fetal resorption rate compared with broth. These abnormal pregnancy outcomes resulting from a localized infection are associated with bacterial dissemination (P. gingivalis DNA detection in maternal liver and uterus) and maternal immune responses (elevated P. gingivalis-specific IgG levels in serum and amniotic fluid) and inflammatory responses (elevated serum TNF-α and decreased serum IL-10 levels).
Previous studies showed that immunization and subsequent challenge with P. gingivalis into subcutaneous chambers implanted in mice augmented inflammatory responses (Th1-dominant response) with increasing levels of TNF-α and IFN-γ in chamber exudates 7 days postchallenge (27, 28). It is possible that local inflammatory responses assist in bacterial dissemination from the local site of infection (e.g., a subcutaneous chamber) to remote sites. In our study, live P. gingivalis A7436 persisted within the subcutaneous chamber, based upon positive culture of chamber fluid collected at time of sacrifice (data not shown). It is interesting that there were no P. gingivalis PCR signals detected in the spleen samples, suggesting that the liver is preferentially involved in clearance, rather than the spleen. This is consistent with a direct hematogenous route of dissemination and hepatic clearance. In P. gingivalis-challenged animals without evidence of FGR, there was no hepatic, uterine, or spleen P. gingivalis signal. In total, these PCR data are consistent with the concept that P. gingivalis dissemination to the liver and uterus is necessary for FGR.
Although PCR is a rapid and sensitive technique for the detection of bacterial DNA sequences, limitations of the technique should be considered in the interpretation of findings. For example, the PCR technique cannot determine if intact bacteria are present, or whether such cells are viable or functional, and only demonstrates the presence of bacterial DNA. In our study, bacterial DNA was not always detected in the liver or placenta from abnormal pregnancies. Several possibilities could explain why P. gingivalis was detected in some affected dams but not others. It could be that P. gingivalis genomic DNA was degraded, that the levels of the organism were below the PCR detection limit, or that the biopsy samples did not contain disseminated bacteria. Alternatively, the effect of P. gingivalis on FGR may be mediated by bacterial products such as LPS or by host mediators such as TNF-α, rather than direct dissemination in some challenged animals.
The maternal humoral and cytokine responses with and without P. gingivalis challenge demonstrated increases in IgG specific to P. gingivalis and TNF-α. Previous studies conducted in our laboratory had already shown a significant increase of serum IL-6 in nonpregnant mice for at least 5 days after localized P. gingivalis challenge (31). The present findings demonstrate that localized infection with P. gingivalis in the subcutaneous chamber can induce systemic responses among pregnant animals as well. The serum level of specific IgG to P. gingivalis was elevated in P. gingivalis-challenged dams with FGR fetuses. The serum TNF-α level in P. gingivalis-challenged mice with FGR fetuses also showed a significant increase compared to the broth group and the P. gingivalis-challenged group with normal-weight fetuses. Thus, the maternal TNF-α level appears to be highly associated with FGR. In our previous studies of LPS challenge, immunization did not afford protection for the fetus, rather it enhanced the effects on growth restriction (10, 11). This is probably related to activation or sensitization of the immune responses and subsequent intensification of the inflammatory process. The IL-6 level in serum of the P. gingivalis-challenged groups appeared to increase compared to the broth group, although it was not statistically significant. In contrast, levels of the antiinflammatory cytokine IL-10 decreased significantly in dams with FGR fetuses compared with levels in the broth group. The increase of TNF-α and the decrease of IL-10 suggest that localized infections with P. gingivalis induce systemic inflammatory responses.
Different mechanisms have been proposed to explain P. gingivalis-induced systemic responses. Intrachamber P. gingivalis immunization may induce the production of Th1 cells specific for P. gingivalis locally and systemically (21, 22), and a low-dose subsequent challenge with live P. gingivalis could activate these Th1 cells to secrete higher levels of proinflammatory cytokines (e.g., TNF-α) and suppress the production of antiinflammatory cytokines (e.g., IL-10). It is also possible that the activated maternal innate immune system (such as monocyte/macrophage activation) secretes TNF-α and IL-6 following infection or LPS stimulation (38, 41).
Inflammatory responses that eliminate pathogens and protect mothers from bacterial infection are also capable of inducing the release of toxic factors and compromising fetal growth. Most of the abortifacient and embryolethal effects of endotoxin are mediated by TNF-α, as evidenced by the effects of TNF-α inhibitors such as pentoxifylline and anti-TNF-α-antibody, which prevent endotoxin-induced lethality and abortion (7, 25). In our study, the increase of TNF-α and decrease of IL-10 in maternal serum seem to be directly related to FGR. Increases in serum TNF-α and suppressed IL-10 in pregnant women have also been implicated in the pathogenesis of FGR, premature rupture of membranes (37), preterm labor (1), and the endothelial dysfunction of preeclampsia (12).
Levels of antibodies against periodontal pathogens in serum are useful markers for periodontal infection (17) as in many other common viral and bacterial infections (18, 19). Our data suggest that the elevated level of specific IgG to P. gingivalis in maternal serum and amniotic fluid was associated with FGR and P. gingivalis detection in maternal liver and uterus. This specific humoral immune response may be enhanced by either greater bacterial dissemination or an activated maternal cellular immune response resulting in greater IgG response, or both. Regardless, it would appear that increased IgG not only reflects bacterial exposure but is also associated with FGR and resorption in P. gingivalis-challenged dams. However, the exact role of IgG could not be determined in our study.
In summary, localized infection with P. gingivalis is capable of mediating FGR and resorption in mice via systemic dissemination of the microorganism and activation of the maternal inflammatory response as well as an induction of a specific maternal IgG response.
Acknowledgments
We thank Christine Downey, Russ Levy, Sandra Elmore, and Jermaine Fuller for their technical assistance in the project.
This work was supported by National Institute of Dental and Craniofacial Research grant RO-1 DE 12453.
Editor: J. N. Weiser
REFERENCES
- 1.Amory, J. H., J. Hitti, R. Lawler, and D. A. Eschenbach. 2001. Increased tumor necrosis factor-alpha production after lipopolysaccharide stimulation of whole blood in patients with previous preterm delivery complicated by intra-amniotic infection or inflammation. Am. J. Obstet. Gynecol. 185:1064-1067. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1996. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann. Periodontol. 1:926-932. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1998. Periodontal diseases: pathogenesis and microbial factors. J. Am. Dent. Assoc. 129(Suppl.):58S-62S. [DOI] [PubMed]
- 4.Appleby, P., and D. Catty. 1983. Transmission of immunoglobulin to foetal and neonatal mice. J. Reprod. Immunol. 5:203-213. [DOI] [PubMed] [Google Scholar]
- 5.Arko, R. J. 1989. Animal models for pathogenic Neisseria species. Clin. Microbiol. Rev. 2:S56-S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, S., J. C. Pons, A. Richard, M. Chiesa, J. Bouyer, and E. Papiernik. 1991. Vaginal infections, cervical ripening and preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 38:103-108. [DOI] [PubMed] [Google Scholar]
- 7.Chaouat, G., M. A. Assal, J. Martal, R. Raghupath, J. Elliot, T. Mosmann, and T. G. Wegmann. 1995. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-τ. J. Immunol. 154:4261-4268. [PubMed] [Google Scholar]
- 8.Chatelain, P. 2000. Children born with intra-uterine growth retardation (IUGR) or small for gestational age (SGA): long term growth and metabolic consequences. Endocr. Regul. 34:33-36. [PubMed] [Google Scholar]
- 9.Chiu, B. 1999. Multiple infections in carotid atherosclerotic plaques. Am. Heart J. 138:S534-S536. [DOI] [PubMed] [Google Scholar]
- 10.Collins, J. G., M. A. Smith, R. R. Arnold, and S. Offenbacher. 1994. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect. Immun. 62:4652-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, J. G., H. W. Windley III, R. R. Arnold, and S. Offenbacher. 1994. Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect. Immun. 62:4356-4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad, K. P., T. M. Miles, and D. F. Benyo. 1998. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 40:102-111. [DOI] [PubMed] [Google Scholar]
- 13.Cutler, C. W., J. R. Kalmar, and C. A. Genco. 1995. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 3:45-51. [DOI] [PubMed] [Google Scholar]
- 14.Dasanayake, A. P., D. Boyd, P. N. Madianos, S. Offenbacher, and E. Hills. 2001. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J. Periodontol. 72:1491-1497. [DOI] [PubMed] [Google Scholar]
- 15.de Boer, P., F. A. van der Hoeven, E. M. Wolters, and J. A. Mattheij. 1991. Embryo loss, blastomere development and chromosome constitution after human chorionic gonadotropin-induced ovulation in mice and rats with regular cycles. Gynecol. Obstet. Investig. 32:200-205. [DOI] [PubMed] [Google Scholar]
- 16.Dudley, D. J., C. L. Chen, D. W. Branch, E. Hammond, and M. D. Mitchell. 1993. A murine model of preterm labor: inflammatory mediators regulate the production of prostaglandin E2 and interleukin-6 by murine decidua. Biol. Reprod. 48:33-39. [DOI] [PubMed] [Google Scholar]
- 17.Ebersole, J. L. 1990. Systemic humoral immune responses in periodontal disease. Crit. Rev. Oral Biol. Med. 1:283-331. [DOI] [PubMed] [Google Scholar]
- 18.Ekman, M. R., M. Leinonen, H. Syrjala, E. Linnanmaki, P. Kujala, and P. Saikku. 1993. Evaluation of serological methods in the diagnosis of Chlamydia pneumoniae pneumonia during an epidemic in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 12:756-760. [DOI] [PubMed] [Google Scholar]
- 19.Elkins, C., K. Yi, B. Olsen, C. Thomas, K. Thomas, and S. Morse. 2000. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J. Clin. Microbiol. 38:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahey, J. L., and W. F. Barth. 1965. The immunoglobulins of mice. IV. Serum immunoglobulin changes following birth. Proc. Soc. Exp. Biol. Med. 118:596. [DOI] [PubMed] [Google Scholar]
- 21.Gemmell, E., T. A. Winning, P. S. Bird, and G. J. Seymour. 1998. Cytokine profiles of lesional and splenic T cells in Porphyromonas gingivalis infection in a murine model. J. Periodontol. 69:1131-1138. [DOI] [PubMed] [Google Scholar]
- 22.Gemmell, E., D. A. Grieco, M. P. Cullinan, B. Westerman, and G. J. Seymour. 1999. The proportion of interleukin-4, interferon-gamma and interleukin-10-positive cells in Porphyromonas gingivalis-specific T-cell lines established from P. gingivalis-positive subjects. Oral Microbiol. Immunol. 14:267-274. [DOI] [PubMed] [Google Scholar]
- 23.Genco, C. A., D. R. Kapczynski, C. W. Cutler, R. J. Arko, and R. R. Arnold. 1992. Influence of immunization on Porphyromonas gingivalis colonization and invasion in the mouse chamber model. Infect. Immun. 60:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genco, C. A., C. W. Cutler, D. Kapczynski, K. Maloney, and R. R. Arnold. 1991. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect. Immun. 59:1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gendron, R. L., F. P. Nestel, W. S. Lapp, and M. G. Baines. 1990. Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumor necrosis factor-alpha. J. Reprod. Fertil. 90:395-402. [DOI] [PubMed] [Google Scholar]
- 26.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 27.Houri-Haddad, Y., W. A. Soskolne, and L. Shapira. 2001. Immunization to Porphyromonas gingivalis enhances the local pro-inflammatory response to subcutaneous bacterial challenge. J. Clin. Periodontol. 28:476-482. [DOI] [PubMed] [Google Scholar]
- 28.Houri-Haddad, Y., W. A. Soskolne, A. Halabi, V. Barak, and L. Shapira. 2000. Repeat bacterial challenge in a subcutaneous chamber model results in augmented tumor necrosis factor-alpha and interferon-gamma response, and suppression of interleukin-10. Immunology 99:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyert, D. L., M. A. Freedman, D. M. Strobino, and B. Guyer. 2001. Annual summary of vital statistics—2000. Pediatrics 108:1241-1255. [DOI] [PubMed] [Google Scholar]
- 30.Kohmura, Y., T. Kiridae, F. Kirikae, M. Nakano, and I. Sato. 2000. Lipopolysaccharide (LPS)-induced intra-uterine fetal death (IUFD) in mice is principally due to maternal cause but not fetal sensitivity to LPS. Microbiol. Immunol. 44:897-904. [DOI] [PubMed] [Google Scholar]
- 31.Lin, D. M., G. Sera, P. N. Madianos, C. M. Champagne, and S. Offenbacher. 2001. Effect of P. gingivalis A7436 infection on endocrine and immune system of female BALB/c mice. J. Dent. Res. 80:125. [Google Scholar]
- 32.Madianos, P. N., S. Lieff, A. P. Murtha, K. A. Boggess, R. L. Auten, Jr., J. D. Beck, and S. Offenbacher. 2001. Maternal periodontitis and prematurity. Part II: maternal infection and fetal exposure. Ann. Periodontol. 6:175-182. [DOI] [PubMed] [Google Scholar]
- 33.Offenbacher, S., S. Lieff, K. A. Boggess, A. P. Murtha, P. N. Madianos, C. M. E. Champagne, R. G. McKaig, H. L. Jared, S. M. Mauriello, R. L. Auten, Jr., W. N. P. Herbert, and J. D. Beck. 2001. Maternal periodontitis and prematurity. Part I: obsteric outcome of prematurity and growth restriction. Ann. Periodontol. 6:166-174. [DOI] [PubMed] [Google Scholar]
- 34.Offenbacher, S., P. N. Madianos, C. M. Champagne, J. H. Southerland, D. W. Paquette, R. C. Williams, G. Slade, and J. D. Beck. 1999. Periodontitis-atherosclerosis syndrome: an expanded model of pathogenesis. J. Periodontal Res. 34:346-352. [DOI] [PubMed] [Google Scholar]
- 35.Offenbacher, S., H. L. Jared, P. G. O'Reilly, S. R. Wells, G. E. Salvi, H. P. Lawrence, S. S. Socransky, and J. D. Beck. 1998. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann. Periodontol. 3:233-250. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 37.Poniedzialek-Czajkowska, E. 2000. Zwiazek stezenia IL-6 i TNF-alfa z liczba ciaz i porodow u pacjentek z przedwczesnym peknieciem pecherza plodowego. Ginekologia Polska 71:752-757. [PubMed] [Google Scholar]
- 38.Sacks, G., I. Sargent, and C. Redman. 1999. An innate view of human pregnancy. Immunol. Today 20:114-118. [DOI] [PubMed] [Google Scholar]
- 39.Sakellari, D., S. S. Socransky, S. Dibart, C. Eftimiadi, and M. A. Taubman. 1997. Estimation of serum antibody to subgingival species using checkerboard immunoblotting. Oral Microbiol. Immunol. 12:303-310. [DOI] [PubMed] [Google Scholar]
- 40.Slots, J., A. Ashimoto, M. J. Flynn, G. Li, and C. Chen. 1995. Detection of putative periodontal pathogens in subgingival specimens by 16S ribosomal DNA amplification with the polymerase chain reaction. Clin. Infect. Dis. 20(Suppl. 2):S304-S307. [DOI] [PubMed] [Google Scholar]
- 41.Vizi, E. S., J. Szelenyi, Z. S. Selmeczy, Z. Papp, Z. H. Nemeth, and G. Hasko. 2001. Enhanced tumor necrosis factor-alpha-specific and decreased interleukin-10-specific immune responses to LPS during the third trimester of pregnancy in mice. J. Endocrinol. 171:355-361. [DOI] [PubMed] [Google Scholar]
- 42.Woods, K. A., C. Camacho-Hubner, M. O. Savage, and A. J. Clark. 1996. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 335:1363-1367. [DOI] [PubMed] [Google Scholar]