Abstract
Our previous animal studies showed that maternal Porphyromonas gingivalis infection in a subcutaneous chamber is associated with hepatic and uterine translocation, as well as systemic induction of maternal inflammatory responses, both of which were associated with fetal growth restriction (FGR). However, P. gingivalis-challenged dams had fetuses with either FGR (2 standard deviations below mean weight of nonchallenged dams) or normal weight. Therefore, the objective of this study was to determine whether maternal infection with P. gingivalis compromises normal fetal development via direct placental invasion and induction of fetus-specific placental immune responses characterized by a proinflammatory Th1-type cytokine profile. P. gingivalis-specific DNA was detected in placentas and fetuses of FGR and normal littermates from P. gingivalis-infected dams. Th1- and Th2-type cytokine mRNA as well as tumor necrosis factor alpha and transforming growth factor β2 mRNA were examined in placental tissue by using reverse transcription-PCR to determine Th1/Th2 ratios. For eight litters containing both normal-weight and FGR fetuses, P. gingivalis DNA was detected only in the placentas of FGR fetuses. All fetuses and all amniotic fluid samples from infected and control dams were negative for P. gingivalis DNA. mRNA levels of gamma interferon and interleukin-2 (IL-2) were significantly increased in placentas of FGR fetuses, while expression of IL-10 was significantly decreased in the same group. These data indicate that, in P. gingivalis-challenged dams, within each litter there is placenta-specific translocation of P. gingivalis that results in growth restriction of the targeted fetus, which is associated with a shift in the placental Th1/Th2 cytokine balance.
Periodontitis is a chronic infectious disease, and the primary etiological agents are gram-negative, anaerobic bacteria that occupy the tooth-associated biofilm in the subgingival plaque. Among them, Porphyromonas gingivalis is thought to be a major periodontal pathogen (1, 2). Human studies in patients with periodontitis and animal studies have suggested P. gingivalis may be an important component in the underlying association in linking periodontitis to preterm birth (6). Patients with periodontal diseases show that P. gingivalis-specific sensitized T cells are present in the peripheral blood, indicating systemic exposure to P. gingivalis (7, 22). Our previous animal studies showed that maternal P. gingivalis infection in a subcutaneous chamber is associated with hepatic and uterine translocation, and this dissemination is correlated with fetuses that exhibit fetal growth restriction (FGR) (15).
A successful pregnancy is thought to be characterized by a dominant Th2-type immunity with predominance of interleukin-4 (IL-4) and IL-10 expression over tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IL-2 in the placenta (28). Th2-type cytokines are associated with humoral immunity, which is known to favor implantation, induce and maintain allograft tolerance, and control harmful maternal responses (13). Th1-type cytokines induce several cytotoxic and inflammatory reactions which are known to be potentially deleterious for the conceptus by modulating the integrity of the placental membrane and uterine tone (20, 21, 29, 30, 32). For example, TNF-α is believed to induce macrophage matrix metalloproteinases to weaken the placental membrane and release prostaglandin E2, which induces uterine constriction (4, 33). The administration of TNF-α and IFN-γ to pregnant mice increases fetal abortion and fetal abnormalities such as reduction of fetal weight and retardation of eye development (34). Thus, infectious challenges that trigger abnormal pregnancies may be mediated by changes in the placental Th1/Th2 balance. In addition, transforming growth factor β2 (TGF-β2) is a potent immunosuppressive factor and can help to reduce activation of NK cells by potentially abortive events, such as intrauterine exposure to lipopolysaccharide or to interferons. It has been reported that TGF-β2 production is defective in women with repeated miscarriages (14).
Our previous studies (17, 24-26) and recent work in a murine model (15) showed that P. gingivalis infection can result in hepatic and uterine dissemination, triggering growth restriction of selected fetuses. Since not all fetuses within litters were affected, we hypothesized that translocation of P. gingivalis or its components into the placenta may induce local immune responses that impair placental function, thereby mediating FGR. We further hypothesized that P. gingivalis-associated immune responses would be characterized by a shift of placental antiinflammatory Th2-type immunity to proinflammatory Th1-type immunity and, consequently, induce the occurrence of pregnancy complications, such as FGR and fetal death.
MATERIALS AND METHODS
Animal experiments.
A mouse subcutaneous chamber model was used to establish a localized infection with P. gingivalis strain A7436. Animal husbandry and bacterial preparation for inoculation are described in detail in Lin et al. (15). Briefly, mice were implanted with stainless steel chambers and immunized with 109 CFU of heat-killed P. gingivalis. Female mice were bred with the same-strain male mice and challenged with 107 CFU of live P. gingivalis on gestation day (GD) 7.5, while the control group received broth. On GD 16.5, animals were sacrificed and pregnancy outcomes were assessed. FGR was defined as fetuses with weights 2 standard deviations (SD) smaller (i.e., less than 0.46 g) than normal fetal weight (NFW; 0.56 ± 0.05 g). Amniotic fluid was collected from each fetal-placental unit and pooled per pregnant animal. Placentas and fetuses were collected, washed with sterile saline solution, and stored at −80°C for future use.
mRNA expression of cytokines in placenta.
mRNA expression of cytokines was analyzed from all placentas from FGR fetuses and their littermates and from selected placentas (numbers 1, 3, 5, 7, etc.) from the control group and the P. gingivalis-challenged group with all normal-weight fetuses. Total RNA from each placenta was extracted using Tri reagent (Sigma, St. Louis, Mo.) according to the manufacturer's instructions. Briefly, placental samples were cut into half, and one half was homogenized and digested in 500 μl of Tri reagent for 5 min at room temperature to ensure complete dissociation of nucleoprotein complexes. After adding 100 μl of chloroform with vigorous shaking, samples were centrifuged at 12,000 × g for 15 min at 4°C, which separated the mixture into three phases. The aqueous phase containing RNA was collected and mixed with 250 μl of isopropanol. After centrifugation, the supernatant was removed and the RNA pellet was washed with 75% ethanol. After drying the pellet for 10 min with air, 10 μl of DNase-RNase-free water was added to dissolve the RNA pellet. The concentration of each RNA sample was adjusted to 1 μg/μl. cDNA was synthesized using a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech). Briefly, 1 μg of total RNA was reverse transcribed in a total volume of 15 μl using cloned FPL CpureMurine reverse transcriptase, and first-strand cDNA was synthesized using NotI-d(T)18 bifunctional primer according to standard procedures. Specific primers were used for PCR to obtain double-stranded DNA (amplicon) of IFN-γ (8), IL-2 (11), IL-12 (12), IL-4 (23), IL-10 (19), TNF-α (10), TGF-β2 (31), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, a housekeeping gene) for each sample.
Following PCR amplification, amplicons (10 μl) were separated by 2% Tris-acetate (1× TAE) agarose gel electrophoresis and stained with 0.5 μg of SYBR Gold (Molecular Probes, Eugene, Oreg.)/ml. We used LumiAnalyst combined with Image Analysis software version 3.1 (Boehringer Mannheim GmbH, Mannheim, Germany) to measure the relative intensities of bands corresponding to specific amplicons. Relative levels of mRNA were expressed by calculating the ratio of each band's intensity (i.e., specific DNA of each sample) normalized to the intensity of the GAPDH signal.
P. gingivalis detection.
All placentas from FGR fetuses and their littermates were analyzed for P. gingivalis DNA. Two placentas selected from control dams and selected placentas (numbers 1, 3, 5, 7, etc.) from P. gingivalis-challenged dams with all normal-weight fetuses were analyzed. DNA from placental tissue of NFW and FGR fetuses or whole fetuses was extracted for the detection of P. gingivalis as described elsewhere (15). Each placenta or fetus was scored as positive or negative for the presence of P. gingivalis DNA detected by PCR. Positive controls included P. gingivalis alone or tissues spiked with exogenous P. gingivalis. The detection limit for P. gingivalis alone was 10 CFU/PCR, and the detection limit for the P. gingivalis-spiked tissues was 100 CFU/PCR (data not shown). Six representative amniotic fluid samples from dams with FGR were cultured (as described previously in reference 15) to determine whether live bacteria were present.
Statistical analysis.
To determine whether fetal exposure to P. gingivalis, as determined by P. gingivalis DNA in placental samples, was correlated with FGR, exact logistic regression models were used to control for clustering of fetuses within dams. Similarly, the effects of P. gingivalis on mean fetal weight were adjusted for litter-dam clustering using mixed models with the dam as a random stratification factor, with a P value of less than 0.05 as statistically significant. In addition, statistically significant differences in placental cytokine mRNA expression were determined by conditional logistic regression with exact inference for small sample size to control for clustering of mouse pups within dams by treating the dam as a factor in a stratified model (16, 27). Relative cytokine expression for individual cytokines and for Th1 and Th2 groups of cytokines was compared separately for placentas of fetuses in challenged versus control dams and for growth-restricted fetuses versus normal-weight fetuses among challenged dams using mixed models with the dam as a random stratification factor. Results are presented as mean values ± the standard error of the mean for the different groups of dams. Data were analyzed using Statistical Analysis Software (SAS Institute, Cary, N.C.).
A Z score transformation was completed to normalize Th1 or Th2 profiles and to determine relative shifts in the overall Th1/Th2 ratio. Individual cytokine values for each placenta within each dam were transformed into Z scores (equation 1). Individual Z scores for each individual cytokine were averaged for the placentas to arrive at a cytokine-specific Z score for each dam (equation 2). Z scores for Th1- and Th2-type cytokines were computed for dams by averaging the Z scores of Th1 (IFN-γ and IL-2 plus IL-12) (equation 3) and the Z scores of Th2 (IL-4 plus IL-10) (equation 4).
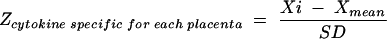 |
(1) |
where Xi is the relative intensity of a specific cytokine mRNA over GAPDH intensity in an individual placenta, Xmean is the mean relative intensity of a specific cytokine mRNA in all placental samples, and SD is the standard deviation of the mean of relative intensity of a specific cytokine mRNA in all placental samples.
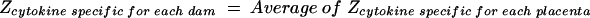 |
(2) |
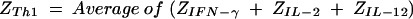 |
(3) |
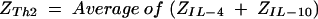 |
(4) |
RESULTS
Exposure of mice to P. gingivalis during pregnancy resulted in two possible outcomes for the litters. Either all fetuses were normal weight (NFW), or one or more fetuses in the litter were low weight (FGR). Because abortifacient or teratogenic agents seldom have 100% penetrance, a litter with both normal and low-weight fetuses provides an opportunity to examine differences at the placental and fetal levels. These groups help distinguish effects mediated through maternal mechanisms or placental and fetal mechanisms. Consequently, placental results were divided into four groups: group I, placentas from control dams (36 placentas from 8 dams); group II, placentas from P. gingivalis-challenged dams with all normal-weight fetuses (32 placentas from 12 dams); group III, placentas of normal-weight fetuses from P. gingivalis-challenged dams with both normal- and low-weight fetuses within the litter (18 placentas from 5 dams); and group IV, placentas of low-weight fetuses from P. gingivalis-challenged dams with one or more low-weight fetuses within the litter (30 placentas from 8 dams).
Detection of placental P. gingivalis by PCR.
In the broth-challenged control group (group I), all placental samples examined (two per mouse) were negative for P. gingivalis DNA (data not shown). In the 20 P. gingivalis-challenged animals, eight dams had one or more FGR fetuses. Overall, there was a total of 107 fetuses scored as P. gingivalis positive or negative and as FGR present or absent (Table 1). For the 31 placentas from FGR fetuses, 39% had positive PCR signals for P. gingivalis and 61% were P. gingivalis negative (Table 1). All of the 76 NFW fetuses had placentas that were also P. gingivalis negative.
TABLE 1.
Average fetal weight and PCR detection of P. gingivalis DNA in placentas from NFW and FGR fetuses among P. gingivalis-challenged dams
| Fetal growth | Presence of PCR signal for P. gingivalis DNA | No. of fetuses | Mean fetal wt ± SD (g) |
|---|---|---|---|
| FGRa | Yes | 12 | 0.27 ± 0.09c |
| No | 19 | 0.39 ± 0.04d | |
| NFWb | Yes | 0 | |
| No | 76 | 0.55 ± 0.03 |
FGR, fetal growth restriction.
NFW, normal fetal weight.
Significantly different from FGR P. gingivalis negative and NFW (P < 0.001; mixed models with the dam as a random stratification factor).
Significantly different from NFW (P < 0.001; mixed models with the dam as a random stratification factor).
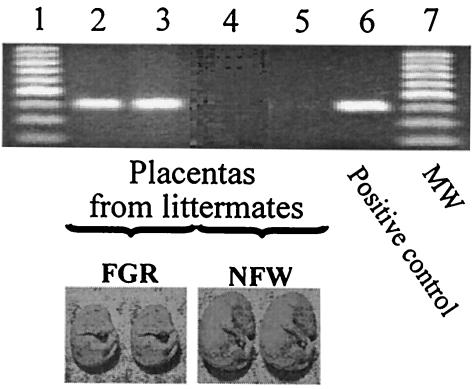
The mean fetal weight of the FGR P. gingivalis-positive group was significantly lower than that of the FGR P. gingivalis-negative and NFW groups (Table 1). In addition, the fetal weight of the FGR P. gingivalis-negative group was also significantly less than that of the NFW fetuses (Table 1). As an example, Fig. 1 shows the P. gingivalis distribution in placental tissues of a representative P. gingivalis-challenged dam. PCR signals from placental tissue taken from littermates (two NFW, two FGR) are shown. Note that no P. gingivalis amplicon signal was present for the two NFW fetuses, but signal was present for both FGR fetuses. The association between P. gingivalis signal in placenta and FGR was statistically significant at a P level of <0.001 (Table 1). In contrast, there was no detectable P. gingivalis PCR signal in FGR or NFW fetuses, nor was there live P. gingivalis detected in the anaerobic culture of amniotic fluid from either FGR or NFW fetuses (data not shown).
FIG. 1.
PCR detection of P. gingivalis DNA in placentas from NFW and FGR littermates. P. gingivalis DNA PCR signals were detected in placentas from two FGR fetuses, but not in placentas from their two normal-weight littermates (NFW). Representatives of NFW and FGR fetuses are shown below.
Placental mRNA expression of Th1- and Th2-type cytokines.
The relative expressions of selected genes (Th1 cytokines IFN-γ, IL-2, and IL-12; Th2 cytokines IL-4 and IL-10; a proinflammatory cytokine, TNF-α; and an immunosuppressive cytokine, TGF-β2) in placenta are shown in Table 2. When cytokine mRNA expression in placentas from control dams (group I) was compared with that in placentas from all challenged dams collectively (groups II, III, and IV), IFN-γ was increased from 1.8 to 5.7% (P = 0.0031) and IL-2 was increased from 0.2 to 3.5% (P = 0.0007). In contrast, mRNA expression for IL-4 was decreased from 17.1 to 7.1% (P = 0.0022) and TGF-β2 decreased from 59.8 to 18.5% (P = 0.0064). These data are consistent with our hypothesis that live P. gingivalis infection is associated with increases in inflammatory Th1-type cytokines (IFN-γ and IL-2) and decreases in antiinflammatory Th2-type (IL-4) and immunosuppressive (TGF-β2) cytokines. To further examine the relationship between changes in Th1- and Th2-type cytokines after challenge with P. gingivalis, the challenged group was subdivided into groups II, III, and IV based on fetal weights (Table 2). When cytokine mRNA expression in placentas of FGR fetuses (group IV) was compared with that in their littermates (group III), all Th1-type cytokines examined (IFN-γ, IL-2, and IL-12) increased significantly (P < 0.05), while IL-10 decreased significantly (P = 0.05). When cytokine mRNA expression in placentas of FGR fetuses (group IV) was compared with that in the control group (group I), Th1-type cytokines (IFN-γ and IL-2) were significantly increased while Th2-type cytokines (IL-4 and IL-10) were significantly decreased (P < 0.05).
TABLE 2.
Relative intensity of cytokine mRNA expression in placentas of control and P. gingivalis-challenged groupsa
| Cytokine | Relative mRNA expression in group (no. of placentas analyzed)
|
|||
|---|---|---|---|---|
| I (36) | II (32) | III (18) | IV (30) | |
| Th1 | ||||
| IFN-γ | 1.8 ± 1.1 | 3.7 ± 1.1 | 3.4 ± 0.9 | 11.5 ± 1.4b,c |
| IL-2 | 0.2 ± 0.8 | 2.3 ± 0.6 | 2.8 ± 0.7 | 5.2 ± 1.0b,c |
| IL-12 | 19.1 ± 4.6 | 14.5 ± 5.3 | 13.2 ± 3.9 | 30.9 ± 5.3c |
| Th2 | ||||
| IL-4 | 17.1 ± 2.6 | 8.4 ± 3.1 | 8.9 ± 1.9 | 4.7 ± 2.7b |
| IL-10 | 18.3 ± 5.9 | 27.7 ± 5.8 | 20.5 ± 5.6 | 7.5 ± 7.0b,c |
| TNF-α | 13.9 ± 5.1 | 10.6 ± 6.8 | 18.9 ± 4.8 | 16.5 ± 6.3 |
| TGF-β2 | 59.8 ± 10.8 | 24.6 ± 9.1 | 19.3 ± 8.9 | 14.9 ± 9.2b |
Relative intensity was calculated as the specific cytokine/GAPDH ratio (represented by percentage) conditioning on dam. Group I, placentas from 8 control dams; group II, placentas from 12 P. gingivalis-challenged dams with all normal-weight fetuses; group III, placentas of normal-weight fetuses from 5 P. gingivalis-challenged dams with both normal- and low-weight fetuses in litter; group IV, placentas of low-weight fetuses from 8 P. gingivalis-challenged dams with one or more low-weight fetuses.
P < 0.05 compared with group I (statistical test).
P < 0.05 compared with group III (statistical test).
Changes in the Th1/Th2 ratio after P. gingivalis challenge in placentas of low-weight fetuses.
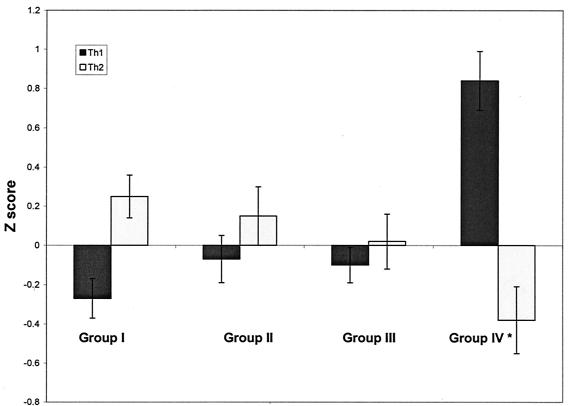
The average Z scores of Th1- or Th2-type cytokines were used to demonstrate the relative balance in the placental Th1 and Th2 immunity after P. gingivalis challenge (Fig. 2). In the control group, Th2 was dominant in the placenta (Fig. 2, group I). However, following P. gingivalis challenge, Th2-type cytokines were lower while Th1-type cytokines were higher, with a loss in Th2 dominance (Fig. 2, groups II and III). Although normal-weight fetuses in the challenged group maintained a similar placental Th1/Th2 profile as those in the control group, growth-restricted fetuses demonstrated an inversion in the cytokine ratio, with a shift to a predominant Th1 cytokine profile (Fig. 2, group IV).
FIG. 2.
Average Z scores of mRNA expression of Th1- or Th2-type cytokines in placentas from different treatment and fetal-weight groups. Group I, placentas from fetuses from control dams; group II, placentas from P. gingivalis-challenged dams with all normal-weight fetuses; groups III and IV, placentas from NFW fetuses (group III) or FGR fetuses (group IV) from P. gingivalis-challenged dams with at least one FGR fetus in their litters.
DISCUSSION
These data show that maternal P. gingivalis challenge can result in P. gingivalis translocation to placental tissue and induction of FGR, while nonaffected littermates show no evidence of P. gingivalis signals in their corresponding placentas. However, P. gingivalis signal was not detected in 61% of placentas from FGR fetuses. The absence of a PCR signal may be a false-negative finding due to lack of sensitivity, which may be overcome in future studies using nested PCR. Alternatively, the effect on fetal growth may not require placental translocation, as there are effects on circulatory levels of maternal cytokines following P. gingivalis challenge that may impair fetal growth, such as an increase in maternal serum TNF-α levels (15).
The placenta is a rich source of a variety of cytokines of both maternal and fetal origins which have an essential role in maintaining pregnancy and inducing parturition in humans and animals. Studies investigating ascending vaginal-cervical infection and adverse pregnancy outcomes have associated changes in placental cytokine levels with premature rupture of membranes and preterm labor (3). Complicated pregnancy outcomes (e.g., FGR, abortion, and preeclampsia) are associated with increased placental proinflammatory Th1-type cytokines and/or diminished expression of antiinflammatory Th2-type cytokines (20, 21, 30). For example, TNF-α stimulates the programmed death of human primary villous trophoblast cells, and IFN-γ augments TNF-mediated killing of trophoblasts (35). In addition, the induction of major histocompatibility complex class I and II molecules on the surface of trophoblast cells by IFN-γ presents fetal antigens to maternal T cells and strengthens cell-mediated immune responses, leading to fetal rejection (36). Antiinflammatory cytokines (e.g., IL-4 and IL-10) produced by the placenta have been suggested to play a vital role in the survival to term of the fetal allograft by counteracting deleterious inflammatory cytokines (e.g., TNF-α and IFN-γ), which would ordinarily be expected to protect an individual from tissue with foreign antigens (5, 9, 18, 29).
P. gingivalis infection in this model appears to induce a placental shift in Th1 and Th2 cytokine expression (Table 2). P. gingivalis challenge that resulted in FGR was associated with a 6.4-, 26-, and 1.6-fold increase in placental IFN-γ, IL-2, and IL-12, respectively, as well as a 3.6- and 2.5-fold decrease in IL-4 and IL-10, respectively, compared to the control group. These findings parallel the observed shifts in Th1 and Th2 associated with vaginal infection.
Previous work with this model (15) demonstrated that P. gingivalis challenge induces maternal immune responses, as shown by an elevation in the serum proinflammatory cytokine TNF-α and a decrease in the antiinflammatory cytokine IL-10. These systemic changes have been associated with FGR. Our findings here, suggesting that the translocation of P. gingivalis into the placenta was associated with the incidence of FGR, were also consistent with the changes of pro- and antiinflammatory cytokine mRNA expression in the placentas. These observations indicate that the disseminated organism can induce placental inflammation. However, we found no evidence that P. gingivalis was present in either the fetus or the amniotic fluid samples obtained from P. gingivalis-challenged dams by PCR or culture methods (data not shown). Furthermore, when amniotic fluid was analyzed using enzyme-linked immunoassays for the cytokines listed in Table 2, amniotic fluid showed no detectable changes in cytokine concentrations (data not shown). Therefore, our data suggest that placental immune and inflammatory responses are correlated with consequent abnormal pregnancy outcomes and may result from placental invasion by P. gingivalis and/or its virulence factors, or from the maternal inflammatory responses to P. gingivalis challenge.
Our findings are consistent with the concept that P. gingivalis infection suppresses expression of protective Th2 cytokines as well as the immunosuppressive cytokine TGF-β2. When P. gingivalis also results in FGR, there is a specific enhancement of Th1 cytokine profiles not seen in the placentas from normal-weight fetuses, even in littermates of FGR fetuses. These data suggest that FGR occurs only when there is a dual effect of Th2 suppression and Th1 enhancement.
In conclusion, in the mouse chamber model, localized P. gingivalis infection can disseminate to the placenta and is associated with a shift in placental Th2-type humoral immunity to Th1-type cellular immunity and also FGR or fetal death (15). After P. gingivalis challenge, several changes occur in both maternal and fetal tissues that are correlated with FGR fetuses. Table 3 summarizes the changes reported from our previous work (15) as well as the present study. These results support the hypothesis that proinflammatory Th1 cytokine dominance during pregnancy is correlated with adverse pregnancy outcomes. The data suggest that as long as antiinflammatory Th2-type cytokines maintain a balance with proinflammatory Th1-type cytokines in the placenta, the fetus is normal weight even in litters containing FGR littermates.
TABLE 3.
Summary of major changes occurring in dams, fetuses, and placentas following P. gingivalis challenge of pregnant mice
| Maternal groupa | Maternal serum cytokine levels | Maternal serum IgG levels | Fetal weight | Placental changes |
|---|---|---|---|---|
| Pg− NFW | Normal | Normal | All normal weight (group I) | Th2 dominance |
| Pg+ NFW | Normal | Normal | All normal weight (group II) | Trend for lower Th2 and higher Th1 responses |
| Pg+ FGR | Significant TNF-α increase and significant IL-10 decrease | Significant increase | Normal weight (group III) | Trend for lower Th2 and higher Th1 responses |
| Significant TNF-α increase and significant IL-10 decrease | Significant increase | Growth restricted (group IV) | Th1 dominance |
See the text for descriptions of maternal groups. Pg−, P. gingivalis negative; Pg+, P. gingivalis positive.
Acknowledgments
We thank John Preisser for his statistical consulting work and Sandra Elmore for her technical assistance in the project.
This work was supported by National Institute of Dental and Craniofacial Research grant RO-1 DE 12453.
Editor: J. N. Weiser
REFERENCES
- 1.Anonymous. 1996. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann. Periodontol. 1:926-932. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1998. Periodontal diseases: pathogenesis and microbial factors. J. Am. Dent. Assoc. 129(Suppl.):58S-62S. [DOI] [PubMed] [Google Scholar]
- 3.Bowen, J. M., L. Chamley, J. A. Keelan, and M. D. Mitchell. 2002. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta 23:257-273. [DOI] [PubMed] [Google Scholar]
- 4.Chaouat, G., E. Menu, B. Mognetti, M. Moussa, and D. G. Lappree. 2000. Cytokines and human reproduction, p. 499-512. In J.-P. R. Constantin and A. Bona (ed.), Cytokines and cytokine receptors: physiology and pathological disorders. Harwood Academic Publishers, New York, N.Y.
- 5.Clark, D. A., and K. Croitoru. 2001. TH1/TH2, 3 imbalance due to cytokine-producing NK, γδ T and NK-γδ T cells in murine pregnancy decidua in success or failure of pregnancy. Am. J. Reprod. Immunol. 45:257-265. [DOI] [PubMed] [Google Scholar]
- 6.Dasanayake, A. P., D. Boyd, P. N. Madianos, S. Offenbacher, and E. Hills. 2001. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J. Periodontol. 72:1491-1497. [DOI] [PubMed] [Google Scholar]
- 7.Gemmell, E. 1999. The proportion of interleukin-4, interferon-gamma and interleukin-10-positive cells in Porphyromonas gingivalis-specific T-cell lines established from P. gingivalis-positive subjects. Oral Microbiol. Immunol. 14:267-274. [DOI] [PubMed] [Google Scholar]
- 8.Gray, P. W., and D. V. Goeddel. 1983. Cloning and expression of murine immune interferon cDNA. Proc. Natl. Acad. Sci. USA 80:5842-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, H. N., K. H. Chao, H. F. Chen, S. U. Chen, M. Y. Wu, and Y. S. Yang. 2001. Distribution of Th1 and Th2 cell populations in human peripheral and decidual T cells from normal and anembryonic pregnancies. Fertil. Steril. 76:797-803. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, S., M. Naito, C. Kaizu, K. Kuwata, G. Hasegawa, N. Mukaida, and L. D. Shultz. 2000. Lipopolysaccharide-induced cytokine and receptor expression and neutrophil infiltration in the liver of osteopetrosis (op/op) mutant mice. Liver 20:465-474. [DOI] [PubMed] [Google Scholar]
- 11.Kashima, N., C. Nishi-Takaoka, T. Fujita, S. Taki, G. Yamada, J. Hamuro, and T. Taniguchi. 1985. Unique structure of murine interleukin-2 as deduced from cloned cDNAs. Nature 313:402-404. [DOI] [PubMed] [Google Scholar]
- 12.Kita, M., Y. Liu, and J. Imanishi. 1996. Role of cytokines in the murine model of viral myocarditis induced by encephalomyocarditis virus. Eur. Cytokine Netw. 7:499. [Google Scholar]
- 13.Krishnan, L. 1996. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-γ response and increased production of T helper 2 cytokines. J. Immunol. 156:644-652. [PubMed] [Google Scholar]
- 14.Leah, R. G., G. Vince, K. C. Flanders, S. Mcintyre, M. Michel, J. Mowbray, J. Underwood, J. D. Baird, and D. A. Clark. 1995. Uterine TGF beta 2 in human normal and pathologic pregnancies, p. 115-122. In G. Chaounat, A. Hazent, and R. Grydman (ed.), Early embryo development, uterus preparation and role of cytokine in implantation and labour. Foundation Marcel Merieux, Lyon, France.
- 15.Lin, D. M., M. A. Smith, C. Champagne, J. Elter, J. Beck, and S. Offenbacher. 2003. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect. Immun. 71:5156-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luta, G., G. G. Koch, W. E. Cascio, and W. T. Smith. 1998. An application of methods for clustered binary responses to a cardiovascular study with small sample size. J. Biopharm. Stat. 8:87-102. [DOI] [PubMed] [Google Scholar]
- 17.Madianos, P. N., S. Lieff, A. P. Murtha, K. A. Boggess, R. L. Auten, Jr., J. D. Beck, and S. Offenbacher. 2001. Maternal periodontitis and prematurity. Part II: maternal infection and fetal exposure. Ann. Periodontol. 6:175-182. [DOI] [PubMed] [Google Scholar]
- 18.Makhseed, M., R. Raghupathy, F. Azizieh, A. Omu, E. Al-Shamali, and L. Ashkanani. 2001. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod. 16:2219-2226. [DOI] [PubMed] [Google Scholar]
- 19.Moore, K. W., P. Vieira, D. F. Fiorentino, M. L. Trounstine, T. A. Khan, and T. R. Mosmann. 1990. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 248:1230-1234. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann, T. R. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima, T. 1999. T-cell antigen specificity in humans following stimulation with Porphyromonas gingivalis. Arch. Oral Biol. 44:1045-1053. [DOI] [PubMed] [Google Scholar]
- 23.Noma, Y., P. Sideras, T. Naito, S. Bergstedt-Lindquist, C. Azuma, E. Severinson, T. Tanabe, T. Kinashi, F. Matsuda, Y. Yaoita, and T Honjo. 1986. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature 319:640-646. [DOI] [PubMed] [Google Scholar]
- 24.Offenbacher, S., S. Lieff, K. A. Boggess, A. P. Murtha, P. N. Madianos, C. M. E. Champagne, R. G. McKaig, H. L. Jared, S. M. Mauriello, R. L. Auten, Jr., W. N. P. Herbert, and J. D. Beck. 2001. Maternal periodontitis and prematurity. Part I: obsteric outcome of prematurity and growth restriction. Ann. Periodontol. 6:166-174. [DOI] [PubMed] [Google Scholar]
- 25.Offenbacher, S., H. L. Jared, P. G. O'Reilly, S. R. Wells, G. E. Salvi, H. P. Lawrence, S. S. Socransky, and J. D. Beck. 1998. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann. Periodontol. 3:233-250. [DOI] [PubMed] [Google Scholar]
- 26.Offenbacher, S., V. Katz, G. Fertik, J. Collins, D. Boyd, G. Maynor, R. McKaig, and J. Beck. 1996. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 67:1103-1113. [DOI] [PubMed] [Google Scholar]
- 27.Preisser, J. S., and G. G. Koch. 1997. Categorical data analysis in public health. Annu. Rev. Public Health 18:51-82. [DOI] [PubMed] [Google Scholar]
- 28.Raghupathy R. 2001. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin. Immunol. 13:219-227. [DOI] [PubMed] [Google Scholar]
- 29.Raghupathy, R. 1997. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18:478-482. [DOI] [PubMed] [Google Scholar]
- 30.Romagnani, S. 1994. Human TH1 and TH2 subsets: “eppur si muove”! Eur. Cytokine Netw. 5:7-12. [PubMed] [Google Scholar]
- 31.Shaddy, R. E., Y. L. Zhang, and W. L. White. 1996. Murine and pediatric myocardial growth factor mRNA expression using reverse transcription-polymerase chain reaction. Biochem. Mol. Med. 57:10-13. [DOI] [PubMed] [Google Scholar]
- 32.Shiraishi, H. 1996. Murine experimental abortion by IL-2 administration is caused by activation of cytotoxic T lymphocytes and placental apoptosis. J. Clin. Lab. Immunol. 48:93-108. [PubMed] [Google Scholar]
- 33.So, T., A. Ito, T. Sato, Y. Mori, and S. Hirakawa. 1992. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol. Reprod. 46:772-778. [DOI] [PubMed] [Google Scholar]
- 34.Vassiliadis, S. 1994. Interferon-induced class II expression at the spongiotrophoblastic zone of the murine placenta is linked to fetal rejection and developmental abnormalities. Haematologia 26:29-37. [DOI] [PubMed] [Google Scholar]
- 35.Yui, J. 1994. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta 15:819-835. [DOI] [PubMed] [Google Scholar]
- 36.Wood G. W. 1997. Cytokines in reproduction: molecular mechanisms of fetal allograft survival, p. 213-237. Landes Bioscience, Georgetown, Tex.