Abstract
Aims
To review (retrospectively) the relationships between lamotrigine (LTG) dosage and plasma concentrations based on data generated in a routine therapeutic drug monitoring laboratory from a heterogeneous sample of patients with epilepsy. To distinguish patients taking concomitant anti-epileptic therapy which induced or inhibited drug metabolising enzymes, or a combination of both, together with LTG. To survey medical staff who use a routine LTG assay service with a view to establishing the utility of higher plasma LTG concentrations than those used in early clinical trials.
Methods
All patient assays for LTG received over a 12 month period (339 requests from 149 patients) were reviewed and relationships between dosage and concentration calculated and grouped according to concomitant antiepileptic drug therapy. The doctors requesting the tests were surveyed by questionnaire (n = 40 of 67 responded). They were asked for details about the patient’s seizure control, rationale used for LTG dosage adjustment and their acceptance of the proposed ‘therapeutic range’ adopted by the laboratory of 3–14 mg l−1.
Results
Linear relationships were demonstrated between LTG dosage and concentration for the 3 treatment groups (LTG plus valproic acid (VPA), LTG plus enzyme inducing antiepileptic drugs, and LTG plus VPA and inducers), however, there were significant differences between groups (P < 0.001) with a 4.4 fold difference in dosage:concentration ratios between the LTG plus VPA group and the LTG plus inducers group. The questionnaire showed that the therapeutic range was well accepted by 88% of responders, none of whom considered this higher range to be wrong.
Conclusions
Metabolic inhibition by VPA was shown to have a marked effect on LTG kinetics, suggesting either a significant LTG dosage reduction is required if plasma LTG concentrations are elevated, or alternatively, higher plasma LTG concentrations could be attained from lower dosages. The higher therapeutic range adopted by the laboratory (3–14 mg l−1) was widely accepted and increasingly applied in clinical practice in the management of patients with epilepsy.
Keywords: lamotrigine, analysis, epilepsy, anti-convulsant, therapeutic drug monitoring
Introduction
Lamotrigine (Lamictal®, Glaxo-Wellcome, BW430C, 3,5-diamino-6(2,3-dichlorophenyl)-1,2,4-triazine) is one of the newer antiepileptic drugs (AED) and is currently marketed in Australia as ‘add-on’ therapy to other AEDs. However, it is being widely used internationally as monotherapy [1]. It acts by inhibiting pre-synaptic voltage-sensitive sodium channels and excitatory neurotransmitter release (principally glutamate) and inhibits repetitive firing of action potentials characteristic of epileptic foci [2–4]. Its broad range of activity has been demonstrated in clinical trials, including placebo controlled, cross-over design, concentration controlled trials. It has been shown to have efficacy against refractory partial seizures, with or without secondary generalisation, as well as generalised tonic-clonic seizures and other generalised seizures (e.g., absence, myoclonic, tonic); it also has beneficial mood altering properties [5–8]. CNS side-effects are less marked than with either phenytoin (PHT) or carbamazepine (CBZ) [9, 10].
The kinetics of LTG are linear over the established plasma concentration range. It has bioavailability close to 100% after oral dosing, and protein binding of ∼50%. Its clearance (and hence terminal half-life) is affected by the presence or absence of concomitant enzyme inducers or inhibitors. With enzyme inhibition by valproic acid (VPA) the half-life increases from approximately 24 h to as much as 60 h, whereas with inducers (PHT, CBZ, phenobarbitone (PHB)) it is reduced to approximately 15 h [11–14]. LTG is metabolised hepatically to N-2- and N-5-glucuronides (80% and 10%, respectively) and other minor N-oxide metabolites, 70% of which are recovered in urine [15]. It has mild auto-induction of metabolism with chronic therapy [16].
A ‘therapeutic range’ for LTG is not well established or accepted as early clinical trials targeted plasma concentrations of 1–3 or 1–4 mg l−1 which did not appear to correlate well with pharmacological effects [17–20]. Even at these concentrations there have been suggestions that patients with LTG concentrations at the upper end of this range may have had better responses [21]. However, later studies suggested that these concentrations may have been too low, and showed that some patients tolerated concentrations above 10 mg l−1 with additional ‘benefits and without clinical toxicity’ [22]. In a more recently published open trial with LTG and vigabatrin (VGB), 40% of patients with ‘intractable epilepsy’ had seizure reductions of 80–100%, and mean trough LTG concentrations were 9.9 mg l−1 (range 3.4 to 19.6 mg l−1). The median plasma LTG concentration at which the monthly seizure frequency fell by at least 50% was 7.9 mg l−1 (range 2.1–15.4 mg l−1), and the median concentration at which dose-related side-effects (including ataxia, diplopia, dysarthria, headache and vomiting) appeared was 16.0 mg l−1 (range 7.9–19.4 mg l−1) [6]. These authors suggested that the LTG ‘therapeutic range is significantly higher than that previously proposed (1.5–3 mg l−1)’.
A routine assay for LTG was established in our laboratory in late 1995 and a therapeutic range of 3–14 mg l−1 adopted, based on the observations in the above study [6] and further clinical experiences of one of our group (ABB) It should be noted that an h.p.l.c. assay for LTG was available locally prior to our service, but the other laboratory continued to recognize the 1 to 3 mg l−1 range. Prior to introducing our service, successful correlation studies were undertaken with this other laboratory.
We present a retrospective review of the acceptance and utilisation of this LTG assay service, undertaken 12 months after it was introduced, by surveying all the medical staff who had requested LTG tests during this period. A particular focus of the questionnaire used was the utility and acceptance of the higher ‘therapeutic range’ quoted. These doctors were also asked about the emphasis they placed on a variety of factors influencing LTG dosage selection for their patients. In a subsequent study, we compared the distribution of 150 unselected consecutive LTG concentrations measured in our laboratory during this 12 month period, with an equivalent 150 over the following 12 months, to consider whether there was a trend toward adopting higher LTG concentrations.
Methods
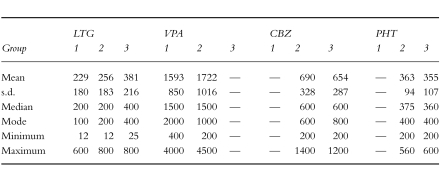
All assays performed by this laboratory over a 12 month period (October 1995 to 1996) were included in the study. This included 339 specimens drawn >6 h post-dose from 149 patients. Data were divided into three groups according to the other AED therapy each patient was prescribed. These groups were; (1) patients taking LTG plus VPA, (2) LTG plus both VPA and inducers and (3) LTG plus enzyme inducers (CBZ and/or PHT, there being none taking PHB). The mean (±s.d.), median, mode, minimum and maximum dosages of each drug for patients studied in these three groups are detailed in Table 1. The plasma LTG concentration and dosage, as well as other AED therapy were recorded. There was no LTG monotherapy group given the current regulatory status for this drug in Australia as ‘add-on’ therapy. The ratio of daily dose (mg day−1) to plasma LTG concentration (mg l−1) was calculated. The requesting doctors (n = 67) were sent a questionnaire for completion. In addition to clinical issues (considered elsewhere), questions asked included:
Table 1.
Dosing details (mg day−1) of LTG, CBZ, PHT and VPA for the individual patients included as represented in the three groups: Group 1 = LTG plus VPA; Group 2 = LTG plus VPA plus inducers (CBZ, PHT); Group 3 = LTG plus inducers (CBZ, PHT, etc) without VPA.
detail about dosage of LTG and concomitant therapy
factors affecting selection of LTG dosage (including seizure frequency, toxicity, dosage recommendations—commercial or regulatory authorities, assay result and therapeutic range reported, experience with other patients on LTG, feed-back from patient)
the usefulness and appropriateness of the ‘therapeutic range’ provided with results. Doctors were asked to select from ‘wrong’, ‘unhelpful’, ‘helpful’ or ‘very helpful’.
Where possible, rating scales were used (allowing ticking of boxes) to facilitate completion of questions and data collation.
The extension study to the above review was undertaken to determine the range of LTG concentrations measured in 150 consecutive specimens during the initial 12 month period of the service and a further 150 assayed more recently (being 2 years after introducing the service) to consider the distribution of LTG concentrations across the ‘therapeutic range’ and whether there were any shifts in the concentrations used clinically over this period.
LTG concentrations were determined by a specific h.p.l.c./u.v. technique which has been described [23]. Essentially it involves extraction of the basified plasma fraction into ethyl acetate, which is separated, dried and reconstituted in acetonitrile. Chromatographic separation was performed on a silica column with methanol/water/phospate buffer mobile-phase at detected by u.v. absorption at 280nm. The methods had within- and between-run CVs of less than 8% and performance managed through internal bi-level quality control samples assayed in parallel, and externally through an international quality assurance program (HeathControl, Cardiff, Wales) where proficiency has proved consistently acceptable.
Results
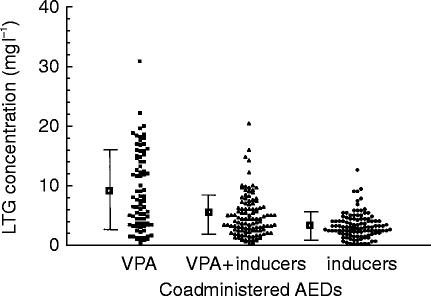
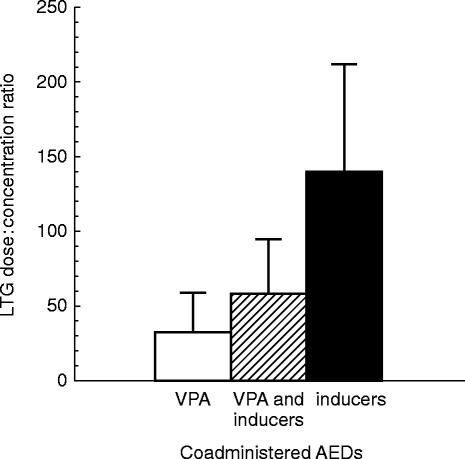
The range of LTG concentrations measured (shown in Figure 1) were, not surprisingly, highly variable but with an approximately two-fold higher mean LTG concentration in patients also taking VPA (n = 74) than those prescribed inducer(s) (CBZ and/or PHT), (n = 91). Those on combinations of LTG with VPA and inducer(s) (n = 89) had intermediate mean concentrations. However, when expressed as LTG dose to concentration ratios (shown in Figure 2), patients on inducer(s) had on average a 4.4 fold greater LTG dose:concentration ratio than those prescribed VPA without inducer(s), reflecting induction and inhibition respectively of LTG metabolism. Patients taking a combination of VPA and inducers with LTG had intermediate LTG dose:concentration ratios.
Figure 1.
Distribution of individual LTG concentrations (mg l−1) of patients included in the survey. Data have been divided according to the other AED therapy the patients were prescribed, being LTG plus VPA (n = 74), or LTG plus VPA and inducers (n = 91), LTG plus inducers (CBZ and/or PHT) (n = 89). The mean (±s.d.) has been included for comparison.
Figure 2.
Mean (±s.d.) lamotrigine dose:concentration ratios (mg day−1:mg l−1) for patients taking LTG plus VPA (open column), LTG plus VPA and inducer(s) (hatched column), or LTG plus inducer(s) (CBZ and/or PHT)(filled column).
The relationships between LTG dosage (mg day−1) and plasma LTG concentrations (mg l−1), (using repeated measures ANOVA) were linear for the three treatment groups, and were significantly different between groups (P < 0.001), as follows:
LTG plus VPA: expected LTG concentration = 0.0340 × LTG dosage(standard error of the estimate = 0.00197, approximate r2 = 0.86)
LTG plus VPA and inducers: expected LTG concentration = 0.0165 × LTG dosage(standard error of the estimate = 0.00147, approximate r2 = 0.73)
LTG plus inducers: expected LTG concentration = 0.00789 × LTG dosage(standard error of the estimate = 0.000497, approximate r2 = 0.83)
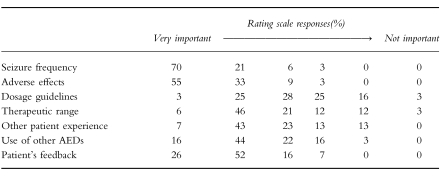
Responses to the questionnaire were received from 40 doctors (60%) relating to 95 different patients (63%). The results from the part of the questionnaire relating to the factors used by clinical staff in selecting the patient’s dosage are shown in Table 2. This shows that seizure frequency and adverse side-effects were the primary indices used to guide LTG dosage. The use of recommended LTG dosage guidelines (such as those from the manufacturer or other published sources) received a diverse response from the requesting doctors with an even distribution of doctors rating these as important or not. The therapeutic range (3-14 mg l−1) reported with the LTG assay result rated as important with 73% of doctors but clearly less than either seizure frequency or toxicity. Experiences with other patients taking LTG compared closely in importance to the therapeutic range. The use of other AEDs rated quite highly in importance, as did feedback from patients, both rating more than therapeutic range or experience with other patients on LTG, but less than seizure frequency or toxicity. Hence the lowest rating was given to recommended dosage guidelines (including those of the manufacturer, national publications, etc).
Table 2.
The ratings show the percentage of doctors (n = 40) ticking one of six boxes indicating their view of the relative importance of each modality in selecting LTG dosage for their patient(s).
In response to the specific question about the utility of the therapeutic range adopted by this laboratory (3 to 14 mg l−1), none of the doctors surveyed thought the range was ‘wrong’ and 88% found it to be ‘helpful’ or ‘very helpful’.
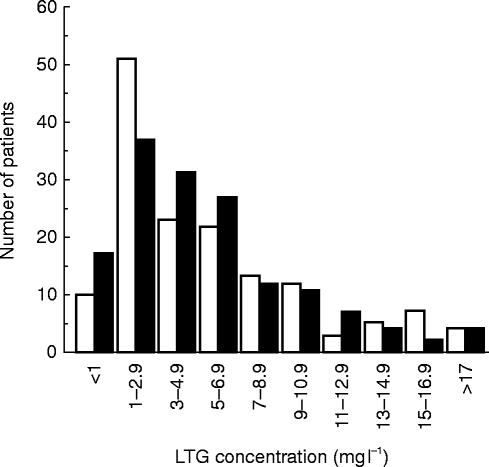
The more recent review of the frequency distribution of LTG concentrations measured over 150 consecutive assay requests (shown in Figure 3) was consistent with a ‘shift to the right’ of LTG concentrations in the second 12 month period. The results during the initial 12 months were still reflecting a marked usage (34% of the total) of LTG concentrations in the 1–3 mg l−1 range, where as at 2 years (ie., a further 12 months after the survey) there was a 27% reduction in concentrations within this range, accompanied by 35% and 22% increases in the 3–5 and 5–7 mg l−1 categories, respectively.
Figure 3.
Frequency distribution (%) of LTG concentrations measured in this laboratory during the initial 12 months period (open columns) and 12 months later (ie., at 24 months) (filled columns) following the introduction of a routine assay service. The laboratory quotes a ‘therapeutic range’ of 3–14 mg l−1 with all reports issued.
Discussion
The value of plasma LTG concentration monitoring has proved to be controversial as it has been suggested that there is a poor relationship between concentrations and pharmacological responses (either efficacious or toxic) [18–20]. As the plasma LTG concentrations used in clinical trials were typically in the 1 to 3 mg l−1 range, the possibility exists that these concentrations were too low and hence a relationship with pharmacological effects could not be demonstrated.
As noted above, clinical studies [6] showed the median plasma LTG concentration at which there was a 50% reduction in the monthly seizure frequency was 7.9 mg l−1 (range 2.1–15.4 mg l−1), and the median concentration at which dose-related side-effects appeared was 16.0 mg l−1 (range 7.9–19.4 mg l−1). Based on these data and further clinical experience suggesting the need to increase LTG dosage and concentration to achieve optimal response, we presented a higher concentration range at the time of introducing the LTG assay service in our laboratory.
The dramatic effect of enzyme inhibition (by VPA) or induction (by CBZ and/or PHT) is well established [13; 24–27]. The dose:concentration ratio (mg day−1:mg l−1) difference of 4.4 fold between LTG plus VPA, and LTG plus inducer(s) is comparable with data recently reported [28] where patients on LTG and VPA had a concentration:dose ratio (mg l−1:mg kg−1 day−1) of 3.4±2.0 compared with 0.6±2.0 for those on LTG and enzymes inducers. However, it must be observed that, while the dose:concentration ratio is very different in the three groups, there is considerable overlap, and lower LTG concentrations in patients on inducers and higher concentrations in patients on VPA, cannot be assumed without resort to assay.
LTG concentrations can be expected to rise following withdrawal from enzyme inducing AEDs. Many patients experience their best seizure control at this stage and may become seizure-free for the first time (ABB personal observation). In addition, a marked dosage reduction may be required if VPA is to be introduced to a patient’s therapy. Alternatively, the addition of VPA may be a convenient means of increasing ‘low’ plasma LTG concentrations without requiring an increase in LTG dosage and the added drug costs. This has some analogies to the competitive metabolic inhibition and ‘sparing effect’ widely used in this country for the high cost immunosuppressant drug, cyclosporin, by agents such as diltiazem [29]. The major difference in the usage of VPA as the ‘sparing agent’ with LTG is that VPA is indicated for the same clinical condition, i.e., epilepsy, and so has fewer ethical issues in relation to its use for an ‘economic’ purpose.
The survey of doctors requesting LTG assays showed that seizure frequency and adverse effects rated the most important factors in establishing LTG dosage, that many were not ‘constrained’ by published dosage guidelines, and that they did use the quoted therapeutic range as a guide. Taking these factors together, it is likely that if toxicity had proved to be dose limiting in their patients, they would not have increased the LTG dosage just to attain the advertised ‘therapeutic range’ reported by the laboratory. The fact that 88% of these same doctors then indicated that this higher therapeutic range was ‘helpful’ or ‘very helpful’ and none thought it to be ‘wrong’, suggests that we are providing a valuable guide to LTG therapy and not exposing patients to undue risk. This was further supported by the increasing trend shown (independent of the questionnaire) for a larger proportion of patients having LTG concentrations above 3 mg l−1 in the second 12 month period.
A qualification of our study is that we surveyed doctors selected on the basis of assay requests received by our laboratory. Hence we may have only surveyed those committed to the use of LTG assays, excluding those who are not. Whilst we have little knowledge of how many other medical practitioners may have been excluded, we believe that we have included the majority of consultant neurologists, and we have the only LTG assay service available in South Australia. Hence it is reasonable to conclude that few prescribers who might reasonably be setting LTG dosages were not included.
In conclusion, it would appear evident that monitoring plasma LTG concentrations is valuable given the marked and unpredictable influence concomitant AED therapy has on its pharmacokinetics, either increasing or decreasing clearance to a clinically significant extent. The therapeutic range adopted by this laboratory of 3 to 14 mg l−1 appears to have been widely accepted and applied in the management of patients with epilepsy in South Australia. Whilst we do not suggest that this particular range be universally adopted without question, and it may prove that other similar ranges (eg., 3 to 10 mg l−1) may ultimately be widely accepted, we do suggest that the lower ranges used in the earlier concentration controlled clinical trials were inadequate and additional benefit may be available from higher plasma LTG concentrations in patients for whom lower concentrations have not achieved the maximal clinical benefit. Further longitudinal studies will be required to quantify the clinical outcomes from patients maintained at these higher concentrations of LTG.
Acknowledgments
The authors thank Mr Ian Westley for performing most of the lamotrigine analyses included in this study. Aspects of this work have been presented at the 22nd International Epilepsy Congress (June 1997) and the 5th International Congress of Therapeutic Drug Monitoring and Clinical Toxicology (November 1997) and published in abstract form.
References
- 1.Hussein Z, Posner J. Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy: retrospective analysis of routine monitoring data. Br J Clin Pharmacol. 1997;43:457–465. doi: 10.1046/j.1365-2125.1997.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach M, Marden C, Miller A. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug II: neuronal studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheung H, Kamp D, Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res. 1992;13:107–112. doi: 10.1016/0920-1211(92)90065-2. [DOI] [PubMed] [Google Scholar]
- 4.Avoli M. Molecular mechanisms of antiepileptic drugs. Sci Am Sci Med. 1997;4:54–63. [Google Scholar]
- 5.Gilman J. Lamotrigine: an antiepileptic agent for the treatment of partial seizures. Annals Pharmacother. 1995;29:144–151. doi: 10.1177/106002809502900209. [DOI] [PubMed] [Google Scholar]
- 6.Schapel G, Black A, Lam E, Robinson M, Dollman W. Combination vigabatrin and lamotrigine therapy for intractable epilepsy. Seizure. 1996;5:51–56. doi: 10.1016/s1059-1311(96)80063-x. [DOI] [PubMed] [Google Scholar]
- 7.Loiseau P, Yeun A, Duche B, Menager T, Arnebes M. A random double-blind placebo-controlled cross-over add-on trial of lamotrigine in patients with treatment-resistant partial seizures. Epilepsy Res. 1990;7:136–145. doi: 10.1016/0920-1211(90)90099-h. [DOI] [PubMed] [Google Scholar]
- 8.Smith D, Baker G, Davies G, Dewey M, Chadwick D. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34:312–322. doi: 10.1111/j.1528-1157.1993.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 9.Yeun A, Chapman A. Interim report on an open multicentre lamotrigine (Lamictal) versus carbamazepine monotherpay trial in patients with epilepsy. Epilepsia. 1992;33:82. [Google Scholar]
- 10.Brodie M, Richens A, Yuen A. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet. 1995;345:476–479. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 11.Yeun W, Peck A. Lamotrigine pharmacokinetics: oral and IV infusion in man. Epilepsia. 1987;28:582–586. [Google Scholar]
- 12.Peck A. Clinical pharmacology of lamotrigine. Epilepsia. 1991;32:S9–S12. doi: 10.1111/j.1528-1157.1991.tb05883.x. [DOI] [PubMed] [Google Scholar]
- 13.Rambeck B, Specht U, Wolf P. Pharmacokinetic interactions of new antiepileptic drugs. Clin Pharmacokinet. 1996;31:309–324. doi: 10.2165/00003088-199631040-00006. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo F, Gay P, Madsen J, et al. Lamotrigine high-dose tolerability and safety in patients with epilepsy: A double-blind, placebo-controlled, eleven-week study. Epilepsia. 1996;37:857–862. doi: 10.1111/j.1528-1157.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen A, Lands G, Briemer D, et al. Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther. 1987;42:535–541. doi: 10.1038/clpt.1987.193. [DOI] [PubMed] [Google Scholar]
- 16.Richens A. Pharmacokinetics of lamotrigine. In: Richens A, editor. Clinical update on lamotrigine: a novel antiepileptic agent. Kent: Wells Medical Limited; 1992. pp. 21–27. [Google Scholar]
- 17.Fitton A, Goa K. Lamotrigine: an update of its pharmacology and therapeutic use in epilepsy. Drugs. 1995;50:691–713. doi: 10.2165/00003495-199550040-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sander J, Trevisol-Bittencourt P, Hart Y, Patsalos P, Shorvon S. The efficacy and long-term tolerability of lamotrigine in the treatment of severe epilepsy. Epilepsy Research. 1990;7:226–229. doi: 10.1016/0920-1211(90)90019-r. [DOI] [PubMed] [Google Scholar]
- 19.Pisani F, Russo M, Trio R, et al. Lamotrigine in patients with refractory epilepsy. A long-term open study. New Antiepileptic Drugs. (Suppl. 3):187–191. [PubMed] [Google Scholar]
- 20.Kilpatrick E, Forrest G, Brodie M. Concentration-effect and concentration-toxicity relations with lamotrigine: A prospective study. Epilepsia. 1996;37:534–538. doi: 10.1111/j.1528-1157.1996.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 21.Schapel G, Beran R, Vajda F, et al. Double-blind, placebo controlled, crossover study of lamotrigine in treatment resistant partial seizures. J Neurol Neurosurg Psychiatry. 1993;56:448–453. doi: 10.1136/jnnp.56.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodie M. Lamotrigine. Lancet. 1992;339:1397–1400. doi: 10.1016/0140-6736(92)91207-o. [DOI] [PubMed] [Google Scholar]
- 23.Sallustio B, Morris R. High-performance liquid chromatographic quantitation of plasma lamotrigine concentrations: Application measuring trough concentrations in patients with epilepsy. Ther Drug Monit. 1997;19:688–693. doi: 10.1097/00007691-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Brodie M. Lamotrigine versus other antiepileptic drugs: A star rating system is born. Epilepsia. 1994;35:S41, S46. doi: 10.1111/j.1528-1157.1994.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 25.Binnie C, Boas WVE, Kasteleijn-Nolste-Trenite D, et al. Acute effects of lamotrigine (BW430C) in persons with epilepsy. Epilepsia. 1986;27:248–254. doi: 10.1111/j.1528-1157.1986.tb03536.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuen A, Land G, Weatherley B, et al. Sodium valproate acutely inhibits lamotrigine metabolism. Br J Clin Pharmacol. 1992;33:511–513. doi: 10.1111/j.1365-2125.1992.tb04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vauzelle-Kervroedan F, Rey E, Cieuta C, et al. Influence of concurrent antiepileptic medication on the pharmacokinetics of lamotrigine as add-on therapy in epileptic children. Br J Clin Pharmacol. 1996;41:325–330. doi: 10.1046/j.1365-2125.1996.31610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battino D, Croci D, Granata T, Estienne M, Pisani F, Avanzini G. Lamotrigine plasma concentrations in children and adults: Influence of age and associated therapy. Ther Drug Monit. 1997;19:620–627. doi: 10.1097/00007691-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Jones T, Morris R. Survey of cyclosporin therapeutic ranges, assay methodology and use of ‘sparing agents’ in Australasian transplant centres. Ther Drug Monit. 1997;19:650–656. doi: 10.1097/00007691-199712000-00008. [DOI] [PubMed] [Google Scholar]