Abstract
Aims
The present study assessed the influence of urinary flow rate and urine pH on the renal excretion of the NMDA-receptor antagonist memantine.
Methods
In a randomized, open, four-period cross-over trial, 12 healthy male volunteers received 10 mg memantine daily for 43 days. After reaching steady state conditions the volunteers were allocated to four different regimens to alter urine pH and urinary flow, which were each separated by a 1 week period while the study medication continued (A: acidification of urine pH, low urinary flow; B: acidification of urine pH, high urinary flow; C: alkalinization of urine pH, low urinary flow; D: alkalinization of urine pH, high urinary flow).
Results
The renal clearance of memantine (CLR) in regimen A and B was 7–10 fold higher in comparison with regimen C and D (P < 0.05). There were small but statistically significant differences of CLR between the two regimens with acidic urine pH (A: median: 210.2 ml min−1 vs B: median: 218.7 ml min−1) and between the two regimens with alkaline urine pH (C: median: 19.4 ml min−1 vs D: median: 30.5 ml min−1). The amount of memantine excreted into the urine within one regimen (Ae0–24 h) was 5.7–7.4 fold higher in regimens A and B than C and D (P < 0.05). Differences of the AUC(0,24 h) and Cmax/AUC(0,24 h) were significant (P < 0.05) between each of the regimens with acidic urine pH (A, B) and regimens (C, D) with alkaline urine pH (A vs C, A vs D, B vs C, B vs D) but not between regimens A vs B or C vs D.
Conclusions
The present study demonstrated a considerable effect of urine pH, whereas no clinically relevant change of the renal excretion of memantine with urinary flow could be detected. As the renal excretion of memantine may have an impact on therapeutic efficacy changes of dietary habits that may alter urine pH should be avoided during treatment with memantine.
Keywords: healthy volunteers, memantine, pharmacokinetics, renal elimination, urinary flow, urine pH
Introduction
Memantine (1-amino-3,5-dimethyladamantane; Akatinol Memantine), a derivate of amantadine (1-amino-adamantane) is used in Europe mainly in the treatment of dementia [1–3].
The analysis of the safety data of several controlled clinical studies indicates a plasma concentration dependence and time dependence of neurological adverse effects such as confusion, agitation, insomnia, dizziness, headaches and akathisia [4]. Hence, physiological factors which influence the plasma concentration of memantine should be evaluated.
Memantine is a weak base with a pKa of 10.27 and it is predominantly excreted unchanged via the kidneys [5]. Urine pH has been shown to be a major determinant for the excretion of alkaline drugs like memantine, other examples are, e.g. flecainide (pKa = 9.3) [6] or methoxyphenamine (pKa = 10.45) [7]. Another determinant for renally excreted drugs is urine flow. Thus, gross changes of urine pH and/or urinary flow may lead to either toxic effects or ineffective treatment.
Therefore, the aim of this study was to evaluate the influences of urine pH and urinary flow on the elimination of memantine. As patients usually receive a long-term treatment with memantine, influences of urine pH and urinary flow on memantine kinetics were assessed under multiple dose conditions.
Methods
Subjects
Twelve healthy male volunteers (age, range: 22–31 years; body weight, range 61–95 kg) gave their written informed consent to participate in the study. Their health status was checked by medical history, physical examination, blood chemistry, urine analysis, and ECG. The study protocol was approved by the Ethics Committee of the Medical Faculty of the University of Göttingen.
Study design
The study refers to an open, controlled, randomized and mono-centre study, conducted as a four-period crossover design with multiple dosing in 12 male healthy volunteers according to Good Clinical Practice (GCP). The subjects received daily medication of one tablet containing 10 mg memantine hydrochloride (8.37 mg memantine free base, Akatinol Memantine) at 08.00 h for 43 days. Starting on day 21 under steady state conditions, urine pH and urinary flow of the subjects were altered according to the following regimens:
A:—acidified urine pH (pH 5) with reduced urinary flow (50 ml h−1)B:—acidified urine pH (pH 5) with increased urinary flow (175 ml h−1)C:—alkalinized urine pH (pH 8) with reduced urinary flow (50 ml h−1)D:—alkalinized urine pH (pH 8) with increased urinary flow (175 ml h−1)
The sequences of the regimens were randomly allocated to the subjects and were separated by a 1 week period of continued regular intake of memantine. To alkalinize the urine the volunteers received doses of 4 g sodium bicarbonate (food grade, Merck, Germany). The alkalinising treatment was started at 14.00 h on the prestudy day and lasted until 22.00 h on the study day. Doses were administered in 4 h intervals. At every time of intake the volunteers received water, a total volume of 600 ml during regimen C (reduced urinary flow) and a total volume of 6000 ml during regimen D (increased urinary flow).
To acidify the urine the volunteers received doses of 1 g ammonium chloride (food grade, Merck, Germany) every 3 h until 23.00 h on the study day starting at 14.00 h on the prestudy day. At every time of intake the volunteers received water, a total volume of 600 ml during regimen A (reduced urinary flow) and a total volume of 6000 ml during regimen B (increased urinary flow).
The volunteers entered the research unit at 14.00 h on the prestudy day and remained there until 20.00 h on the following study day. At 08.00 h on the study day they took the study medication (10 mg memantine). Blood samples for plasma concentration measurements of memantine were drawn before intake of the medication and 2, 4, 6, 8, 10, 12, 14 and 24 h thereafter. Additional trough levels were taken on day 1, 10 and 15 for control of steady state conditions and for calculation of memantine excretion under conditions of unchanged urine pH and urinary flow (U). Urine collection periods for measurement of memantine concentrations were 0–2 h, 2–4 h, 4–6 h, 6–8 h, 8–10 h, 10–12 h, 12–14 h, 14–24 h. Volume and pH of the urine samples were recorded and three aliquots were taken. Plasma and urine samples were stored at −20° C until analysis.
Analytical methods
Memantine in plasma and urine samples was determined with a validated assay method which employed gas chromatography with mass selective detection (Güntner, Merz+Co., unpublished) and was conducted according Good Laboratory Practice (GLP). In brief, 0.5 ml plasma or urine was treated at 70° C for 30 min after addition of 0.5 ml hydrochloric acid (2n). After cooling, the mixture was made alkaline by addition of 0.25 ml sodium hydroxide solution (32% w/v). Subsequently the analytes were extracted into 1 ml n-hexane for 30 min. The organic layer was then transferred into a reaction vial containing 15 μl N-methyl-bis-trifluoro acetamide. The sample volume was finally reduced to 150 μl at 70° C. Typically, 3 μl of sample were injected in splitless mode into the GC apparatus. The injection port was kept at 250° C. The separation was carried out on a HP1 methyl silicone fused silica capillary (25 m, 0.2 mm i.d.) with helium as the carrier gas. The column temperature was increased from 50° C to 250° C over 6.75 min in three steps. The interface to the mass selective detector was kept at 280° C. The TFA (tri-fluoroacetic acid)-derivatives of memantine (275±2 amu) and amantadine (247±2 amu) as the internal standard were monitored.
Calibration samples were prepared from drug free plasma and urine, respectively. The concentration signal relationship was linear in the range from 8.4 to 267 ng ml−1 for plasma and 0.08–16 μg ml−1 for urine. The interassay variability was below 2.5% for the plasma samples and below 1.5% for urine samples.
Pharmacokinetic analysis
Primary investigational parameters were renal clearance (CLR) and total plasma clearance (CLT) of memantine as well as the amount of memantine excreted into urine within each regimen A–D (Ae0–24 h). For the computation of the individual values of CLR and CLT a compartmental pharmacokinetic model (first-order absorption) was developed using the pharmacokinetic modelling program NONMEM [8]. Pharmacokinetic modelling was employed because under steady state conditions and with non-constant clearance, standard pharmacokinetic formulae are not valid. The clearances were estimated taking into account the individual plasma and urine concentrations of memantine and the variables urine pH and urinary flow. Calculations were done for regimens A, B, C and D and for unchanged conditions of urine pH and urinary flow (U) using the plasma concentrations obtained on study day 1, 10 and 15. Extra-renal clearance was assumed to be independent of urine pH and urinary flow. Figure 1 shows the pharmacokinetic model. The following differential equation was used to calculate the rate constants for renal (kR) and extra-renal (kXR) excretion, where kR can be described as a function of urine pH and urinary flow [kR = F (urine pH, urinary flow)]. C is memantine plasma concentration, t is time, kA is the rate constant for absorption and A is dose fraction of memantine resorbed divided by central volume of distribution. CLR and CLXR are the product of the volume of distribution with kR and kXR, respectively. CLT is the sum of both clearances.
Figure 1.
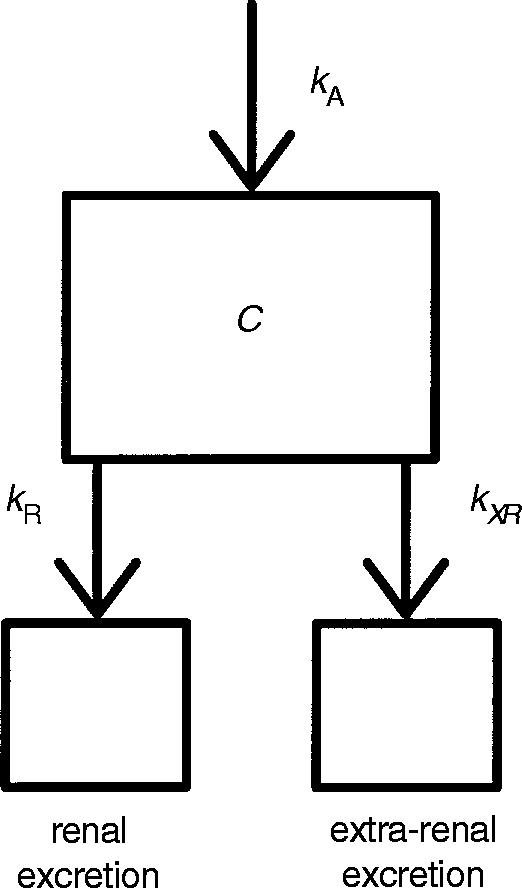
Compartmental pharmacokinetic model of memantine excretion. kA is the rate constant for memantine absorption, kR the rate constant for renal excretion and kXR the rate constant for extra-renal excretion. CLR and CLXR are the product of the volume of distribution with kR and kXR, respectively.
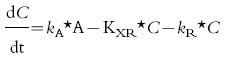 |
Secondary parameters were peak concentration (Cmax), time to reach peak concentration (tmax), taken from the original data and the area under the memantine plasma concentration time curve of each regimen from A–D (AUC(0,24h)). AUC was calculated using the linear trapezoidal rule.
Plots of ΔU/Δt (amount excreted during one sampling period) vs plasma concentration at the midpoint of the sampling period were done in order to determine effects of memantine plasma concentration on renal clearance.
Statistical analysis
A descriptive statistical analysis of the primary investigational parameters CLT, CLR and Ae0–24 h as well as the secondary parameters AUC(0,24h), Cmax, Cmax/AUC(0,24h) and tmax of the different regimens A, B, C and D was done by calculating median, 25% quartile and 75% quartile.
The data are expressed as median, 25% quartile and 75% quartile. Further statistical analysis were done using the Friedman repeated measures anova (analysis of variance) on ranks for comparisons of multiple groups. Post-tests for comparisons between single groups were carried out using the Wilcoxon’s signed rank test with α-adjustment according Bonferroni-Holm. P < 0.05 was considered statistically significant.
Results
Raw data
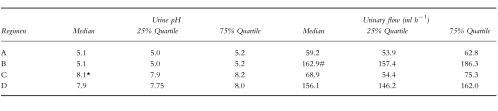
The achieved urinary flow rates and urine pH of the regimens A–D are shown in Table 1.
Table 1.
Urinary flow and urine pH during the respective regimens (median, 25% quartile, 75% quartile; urinary flow: (#P < 0.05, regimen B vs D; urine pH: *P < 0.05, regimen C vs D); Regimen A: acidic urine pH, low urinary flow; B: acidic urine pH, high urinary flow; C: alkaline urine pH, low urinary flow; D: alkaline urine pH, high urinary flow.
Steady state concentrations were effectively reached after 21 days, as proven by memantine trough plasma levels on days 1, 10 and 15 after start of the medication (data not shown).
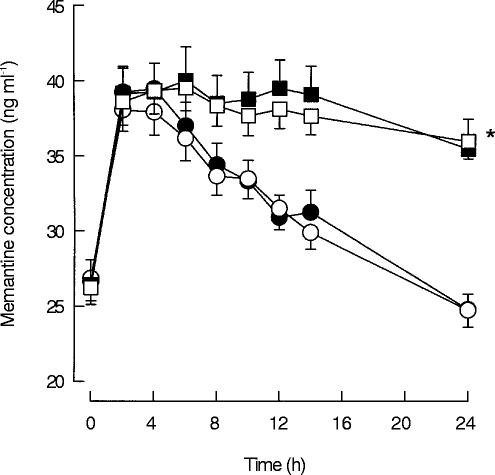
In Figure 2 the plasma concentration–time curves of memantine under the different treatment regimens are shown. Trough levels of memantine during all regimens were similar (regimen A: median: 27.0 ng ml−1, 25% quartile: 22.2 ng ml−1, 75% quartile: 30.6 ng ml−1; regimen B: median: 26.6 ng ml−1, 25% quartile: 22.9 ng ml−1, 75% quartile: 29.9 ng ml−1; regimen C: median: 26.4 ng ml−1, 25% quartile: 24.2 ng ml−1, 75% quartile: 28.8 ng ml−1; regimen D: median: 25.9 ng ml−1, 25% quartile: 24.4 ng ml−1, 75% quartile: 29.0 ng ml−1), but concentrations at the end of the 24 h dosing interval of the regimens were statistically significant higher (P < 0.05) in the regimens with alkaline urine pH in comparison with the regimens with acidic urine pH, whereas differences between regimens A vs B or regimens C vs D could not be detected (regimen A: median: 24.5 ng ml−1, 25% quartile: 23.0 ng ml−1, 75% quartile: 26.3 ng ml−1; regimen B: median: 24.0 ng ml−1, 25% quartile: 21.5 ng ml−1, 75% quartile: 26.8 ng ml−1; regimen C: median: 35.6 ng ml−1, 25% quartile: 30.2 ng ml−1, 75% quartile: 39.2 ng ml−1; regimen D: median: 36.4 ng ml−1, 25% quartile: 32.8 ng ml−1, 75% quartile: 38.7 ng ml−1).
Figure 2.
Memantine plasma concentration-time course for the respective treatment regimens A–D (mean±s.e. mean). (•) Regimen A: Acidic urine pH, low urinary flow; (○) Regimen B: Acidic urine pH, high urinary flow; (▪) Regimen C: Alkaline urine pH, low urinary flow; (□) Regimen D: Alkaline urine pH, high urinary flow. 24 h after the intake of memantine, statistically significant differences (*P < 0.05) were detected between regimens with acidic and alkaline urine pH (A vs C, A vs D, B vs C, B vs D).
Primary parameters
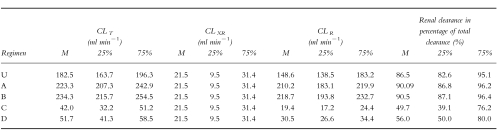
CLR and CLT for unchanged conditions of urine pH and urinary flow and for the regimens A–D are given in Table 2.
Table 2.
Total (CLT), extra-renal (CLXR) and renal clearance (CLR) of memantine during the respective regimens (A–D) and under conditions of unchanged urinary flow and urine pH (U). Extra-renal clearance was assumed to be independent of urine pH and urinary flow therefore estimated values are identical for the respective regimens (median = M, 25% quartile = 25%, 75% quartile = 75%). Regimen A: acidic urine pH, low urinary flow; B: acidic urine pH, high urinary flow; C: alkaline urine pH, low urinary flow; D: alkaline urine pH, high urinary flow. U = conditions of unchanged urine pH and urinary flow. For statistical comparisons see results section.
Statistically significant differences of CLR and CLT (P < 0.05) were found between all regimens (A–D). CLR and CLT were statistically significantly different (P < 0.05) between regimens A–D and conditions of unchanged urine pH and urinary flow (U). Mean CLR in the regimens with an acidic urine pH (A and B) was 7–10 fold higher in comparison to the regimens with alkaline urine pH (C and D). CLR between the regimens with an acidic urine pH (A vs B) and between the regimens with an alkaline urine pH (C vs D) was slightly different (Table 2).
Under the condition of unchanged urine pH and urinary flow, CLR reached ≈75% of CLR in regimens with acidic urine conditions. In the regimens with an acidic urine pH (A, B) the percentage of CLR of CLT is ≈90%, similar to the proportion under conditions of unchanged urine pH and urinary flow (U). In the regimens with an alkaline urine pH (C, D) only 50% of total clearance are due to CLR (Table 2). The difference of CLR between regimens with high (A, C) and low urinary flow (B, D) is ≈ 9 ml min−1 regardless of acidic or alkaline urine conditions. Under acidic urine conditions this is 4% of renal clearance but under alkaline conditions this same difference amounts to 30–45%.
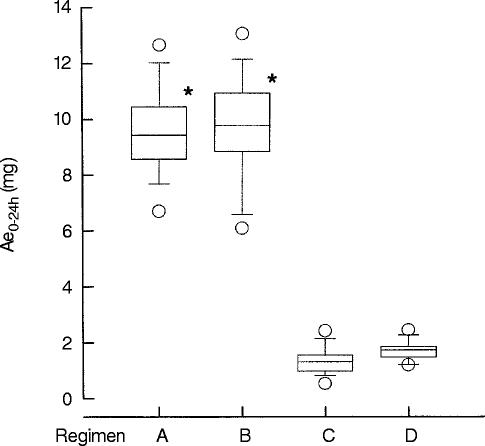
In Figure 3 the total amount of memantine excreted into the urine is shown for regimens A–D. The differences of Ae0–24 h between the regimens with an acidic urine pH (A, B) and alkaline urine pH (C, D) are statistically significant (P < 0.05). The Ae0–24 h was 5.7–7.4 fold higher in the regimens with an acidic urine pH A and B in comparison to the regimens with an alkaline urine pH C and D.
Figure 3.
Amount of memantine excreted in urine within one dosing interval (Ae0–24 h). Central line of the boxplot represents median, upper edge the 75% quartile, lower edge the 25% quartile, upper whisker the 90% quartile and lower whisker the 10% quartile. Circles represent maximum and minimum values (*P < 0.05, regimens A vs C, A vs D, B vs C, B vs D). Regimen A: acidic urine pH, low urinary flow; B: acidic urine pH, high urinary flow; C: alkaline urine pH, low urinary flow; D: alkaline urine pH, high urinary flow.
Differences between the regimens with an acidic urine pH A vs B and between the regimens with an alkaline urine pH C vs D were not found to be statistically significant.
Secondary parameters
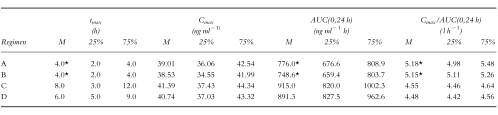
The pharmacokinetic parameters Cmax and tmax, as well as AUC(0,24h) and Cmax/AUC(0,24h) for each regimen are shown in Table 3.
Table 3.
Secondary parameters: tmax, Cmax, AUC(0,24 h) and Cmax/AUC(0,24 h) for the respective regimens A–D (median, 25% quartile, 75% quartile; (*P < 0.05, regimens A vs C, A vs D, B vs C, B vs D) regimens A: acidic urine pH, low urinary flow; B: acidic urine pH, high urinary flow; C: alkaline urine pH, low urinary flow; D: alkaline urine pH, high urinary flow.
For Cmax no statistically significant differences between the regimens were observed. The statistical analysis of tmax, AUC(0,24h) and Cmax/AUC(0,24h) resulted in significant differences (P < 0.05) between the regimens with an acidic urine pH (A, B) and the regimens with an alkaline urine pH (C, D), whereas no differences within the regimens with acidic urine conditions and alkaline urine conditions could be observed.
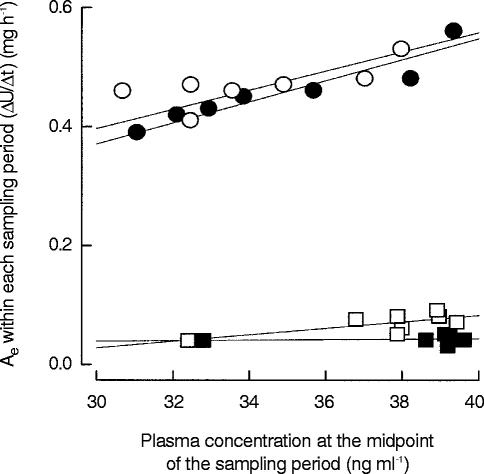
Plots of ΔU/Δt (amount excreted during one sampling period) vs plasma concentration at the midpoint of the sampling period could be fitted by linear regression, no dependence of renal clearance on memantine plasma concentration was seen (Figure 4).
Figure 4.
Memantine amount excreted in urine within each urine sampling period (ΔU/Δt) per hour vs memantine concentration in plasma at the midpoint of the same sampling period for the respective regimens A–D. (•) Regimen A: acidic urine pH, low urinary flow; (○) Regimen B: acidic urine pH, high urinary flow; (▪) Regimen C: alkaline urine pH, low urinary flow; (□) Regimen D: alkaline urine pH, high urinary flow. No dependence of CLR on memantine plasma concentration could be detected, as the correlation of ΔU/Δtvs plasma concentration at the midpoint of the sampling period can be described by linear regression in accordance with first order kinetics.
Discussion
High concentrations of memantine in plasma are correlated with a higher probability of side-effects as shown in several clinical trials [4]. As the plasma concentration is dependent on elimination it is necessary to evaluate possible influences of the elimination kinetics of memantine which is predominantly excreted via the kidneys. Major determinants for renal elimination of alkaline drugs are urine pH and urinary flow [5,6]. Therefore, we investigated the excretion of the weak base memantine (pKa = 10.27) under conditions of alkaline (pH 8) and acidic (pH 5) urine pH and high and low urinary flow rates, respectively. This range of urinary pH can be found under physiological conditions [9].
For the calculation of renal, extra-renal and total clearance of memantine under these conditions, the pharmacokinetic modelling program NONMEM [8] was used. By computation with a one-compartmental model, estimation of extra-renal and total clearance of memantine under a multiple dosing regime without detection of metabolites and memantine excreted within the faeces was possible.
The results of the study show that plasma concentrations of memantine are dependent on urine pH. Alkaline urine pH results in a reduced renal excretion and renal clearance in comparison with acidic urine pH. The reduced renal clearance at alkaline urine pH can be explained by pH-dependent tubular reabsorption under these conditions because the ratio of nonionized memantine in alkaline solutions (pH 8) is considerably higher (0.005) than in acidic urine (pH 5), where the ratio of nonionized drug is very low (0.000005). Under these conditions tubular reabsorption seems to be unlikely, in contrast tubular secretion must be taken into account, as the renal clearance of memantine at acidic pH exceeds the expected glomerular filtration rate.
Urinary flow rates are no major determinants of memantine pharmacokinetics. A high urinary flow rate results in an increase of renal clearance of about 9 ml min−1 under both acidic urine pH and alkaline urine pH. Even thought this difference between high and low urinary flow is statistically significant, it is of no clinical relevance due to its small absolute value.
No dependence of the renal clearance of memantine on plasma concentration could be detected, as the correlation of (ΔU/Δt (amount excreted during one sampling period) vs plasma concentration at the midpoint of the sampling period can be described by linear regression in accordance with first order kinetics.
Taken together, the pharmacokinetics of memantine are considerably affected by urine pH at high and low physiological values. As alkaline conditions can be found under a pure vegetarian diet and acidic conditions under a very protein rich diet [10] changes of dietary habits from very protein rich to fully vegetarian diet and vice versa during ongoing therapy might influence renal excretion of memantine. Therefore, diets should be kept stable during treatment with memantine.
References
- 1.Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. Drug Res. 1991;8:773–778. [PubMed] [Google Scholar]
- 2.Görtelmeyer R, Erbler H. Memantine in the treatment of mild to moderate dementia syndrome. Drug Res. 1992;42:904–913. [PubMed] [Google Scholar]
- 3.Pantev M, Ritter R, Görtelmeyer R. Clinical and behavioural evaluation in long-term care patients with mild to moderate dementia under memantine treatment. Zeitschr Gerontol Psychiat. 1993;6:103–117. [Google Scholar]
- 4.Freudenthaler S, Görtelmeyer R, Pantev M, Gundert-Remy U. Dose–response analysis to support dosage recommendations for memantine. Naunyn Schmiedeberg’s Arch Pharmacol. 1996;353(Suppl):R606. [Google Scholar]
- 5.Wesemann W, Sonntag K-H, Maj J. On the pharmacodynamics and pharmacokinetics of memantine. Drug Res. 1983;33:1122–1134. [PubMed] [Google Scholar]
- 6.Hertrampf R, Gundert-Remy U, Beckmann J, Hoppe U, Elsäßer W, Stein H. Elimination of flecainide as a function of urinary flow rate and pH. Eur J Clin Pharmacol. 1991;41:61–63. doi: 10.1007/BF00280108. [DOI] [PubMed] [Google Scholar]
- 7.Roy SD, Hawes EM, Midha KK. Influence of urinary pH on the disposition of methoxyphenamine and three metabolites in humans. J Pharm Sci. 1987;76:427–432. doi: 10.1002/jps.2600760603. [DOI] [PubMed] [Google Scholar]
- 8.Beal SL, Sheiner LB NONMEM Project Group. In NONMEM User’s Guide, Part I–VIII. San Francisco: University of California at San Francisco; 1992. [Google Scholar]
- 9.Wissenschaftliche Tabellen Geigy. Basle, Switzerland: Ciba-Geigy limited; 1977. Teilband Körperflüssigkeiten, Auflage. [Google Scholar]
- 10.Eckstrand J, Spak CJ, Ehrnebo M. Renal clearance of fluoride in a steady state condition in man: Influence of urinary flow and urine pH changes by diet. Acta Pharmacol Toxicol. 1982;50:321–325. doi: 10.1111/j.1600-0773.1982.tb00982.x. [DOI] [PubMed] [Google Scholar]