Abstract
Aims
Frusemide is widely used in the treatment of acute pulmonary oedema, chronic congestive heart failure and, to a lesser degree, in the treatment of hypertension. Evidence suggests that frusemide exerts an anti-vasoconstrictor effect independent of its diuretic properties. Since angiotensin II is a highly potent vasoconstrictor involved in the pathophysiology of these diseases, we have investigated the effect of frusemide on the contraction elicited by angiotensin II on human internal mammary artery (IMA) and saphenous vein (SV).
Methods
Rings of IMA and SV were suspended for isometric tension recording in organ baths. Concentration-response curves to angiotensin II were performed in the absence (control) or in the presence of frusemide (10−6 to 10−3 m). In addition, the effect of frusemide was evaluated after cyclooxygenase inhibition by indomethacin (10−6 m) and was compared with those of the other loop diuretic bumetanide (10−4 m).
Results
Frusemide induced a concentration-dependent decrease of the contraction elicited by angiotensin II on IMA and SV. On both vessels, the inhibitory effect on the maximal contraction to angiotensin II was significant with concentrations of frusemide from 10−5 to 10−3 m. Angiotensin II potency (pD2) was only reduced by 10−3 m frusemide. The effect of frusemide was not altered in the presence of indomethacin. Bumetanide was less potent than frusemide in inhibiting angiotensin II-induced contractions in both IMA and SV.
Conclusions
Frusemide, at concentrations in the therapeutic range (10−5 m), inhibits angiotensin II-induced contraction on human isolated IMA and SV. This inhibitory effect is cyclooxygenase independent and appears mediated, at least in part, by inhibition of Na+/K+/2Cl− symport. Reduction in the vasoconstrictor effect of angiotensin II may be involved in the therapeutic efficacy of frusemide.
Keywords: frusemide, angiotensin II, internal mammary artery, saphenous vein
Introduction
The potent loop diuretic frusemide is widely used in the treatment of acute pulmonary oedema, chronic congestive heart failure and, to a lesser degree, in the treatment of hypertension. Loop diuretics block the Na+/K+/2Cl− symporter in the thick ascending limb of the loop of Henle and therefore, induce volume depletion by increasing the renal excretion of solutes and water [1]. However, evidence suggests that the effect of frusemide is not solely a result of volume depletion and is also mediated by an action on the vasculature. Indeed, frusemide produces a decrease in vascular resistance that has been attributed to a constrictor-inhibitory effect independent of its diuretic properties [2]. Angiotensin II is a highly potent vasoconstrictor involved in the pathophysiology of hypertension, acute pulmonary oedema and congestive cardiac failure [3]. The decrease in arterial resistance and the increase in venous capacitance after long-term treatment with frusemide may be therefore related to reduction in the angiotensin II-dependent constrictor effect. Intravenous administration of frusemide induces a rapid but short-lived arterial constriction which contrasts with the simultaneous dilatation of veins. These opposite vascular effects have been reported to be prevented by inhibition of angiotensin II formation with captopril and the arterial constriction was attributed to a frusemide-induced renin release from the kidney and increased angiotensin II levels [4]. Similarly, frusemide has been recently shown to increase baseline pulmonary vascular resistance through a possible activation of the renin angiotensin system and angiotensin II-mediated pulmonary vasoconstriction [5]. In order to better understand the mechanisms of frusemide action, we have investigated its effect on the contraction elicited by angiotensin II on human internal mammary artery (IMA) and saphenous vein (SV).
Methods
Tissue preparation
Human internal mammary artery (IMA)
Internal mammary artery segments were harvested from 20 patients undergoing IMA coronary artery bypass. The preoperative drug therapy in these patients was as follows: four patients were on calcium antagonists, 14 patients were on β-adrenoceptor blockers, three patients were on potassium channels openers and five patients were on angiotensin converting enzyme inhibitors. Any discarded distal IMA segments were immediately placed in a container with oxygenated Belzer solution maintained at +4° C, and then transferred to the laboratory. The Belzer solution had the following composition: pentafraction (50 g l−1), lactobionic acid (35.83 g l−1), KHPO3 (3.4 g l−1), MgSO4,7 H2O (1.2 g l−1), raffinose pentahydrate (17.83 g l−1), adenosine (1.34 g l−1), allopurinol (0.136 g l−1), total gluthathione (0.922 g l−1), KOH q.s., NaOH adjusted to pH 7.4. The experiments were carried out within 24 h, meanwhile the vessels were stored in Belzer solution at 4° C. The IMA were then carefully dissected free from the surrounding connective tissue and were precisely cut into 2 mm length segments, the number of rings obtained from each IMA varied from 2 to 4. The segments were suspended on wires in a 5 ml organ bath containing Krebs solution at 37° C. The Krebs solution had the following composition: NaCl (118 mm), KCl (4.7 mm), CaCl2 (2.5 mm), MgSO4 (1 mm), KH2PO4 (1 mm), NaHCO3 (25 mm) and glucose (11 mm), and was continuously aerated with a gas mixture of 5% CO2 and 95% O2. The lower wire was fixed to a micrometer (Mitutoyo, Japan) and the upper wire was attached to a force transducer (UF-1, Pioden, UK) through which changes in isometric forces were displayed on recorders (Linseis 200, Bioblock, France). After mounting, the unstretched preparations were allowed to equilibrate for 30 min. A normalisation procedure was then performed to standardise the baseline resting internal circumference (calculated from the distance between the wires) of each ring as previously reported [6, 7]. Briefly, the rings were stretched with the micrometer in progressive steps of 2.5 g to determine the wall tension-internal circumference exponential curve for each ring. On the basis of this relationship, the distance between the wires was set at a normalised internal circumference corresponding to 90% of the internal circumference the vessel would have at a transmural pressure of 100 mm Hg. Fifty-eight rings of IMA were used in the study. The average vessel internal diameter at an equivalent transmural pressure of 100 mm Hg was 1.6±0.2 mm and the resting force tension at 0.9 D100 was 6.4±0.5 g. This degree of passive tension was maintained throughout the experiment. Resting segments were allowed to equilibrate for 60 min, and then the rings were challenged twice by the addition of KCl 90 mm. These initial maximal contractions were performed to stabilise the preparations and ensure reproducible contractile responsiveness. During the following hour, the Krebs solution was changed every 15 min.
Human saphenous vein (SV)
Human saphenous veins were obtained from seven patients undergoing bypass surgery and from 11 patients undergoing surgical removal of varicose veins. The preoperative drug therapy in these patients was as follows: seven patients were on anticoagulants, two patients were on angiotensin converting enzyme inhibitors, three were on cholesterol lowering agents. Immediately after harvesting, the discarded saphenous vein segments were placed in oxygenated Belzer solution maintained at +4° C and immediately transferred to the laboratory. The veins were cut into segments of about 5 to 6 mm length and suspended on wires in the 5 ml organ baths containing Krebs solution at 37° C and continuously bubbled with 5% CO2 and 95% O2 gas mixture. Seventy-eight rings of SV were used in the study. At the beginning of the experiments, the rings were stretched to an initial tension of 2 g and allowed to equilibrate for 1 h in the Krebs solution, which was changed every 15 min. The vein rings were then challenged twice with 90 mm KCl to stabilise the preparations. As for the IMA rings, the preparations were allowed to equilibrate again for 1 h, the Krebs solution being changed every 15 min.
Protocol
Concentration-response curves to angiotensin II were made by cumulative addition of increasing concentrations (0.5-log increments) at time intervals sufficient to reach a plateau of response before the next addition was made. Only one concentration-response curve was performed with each ring. All determinations were carried out on 2 to 4 rings from the same patient. The rings were incubated for 1 h with frusemide (10−6 m, 10−5 m, 10−4 m or 10−3 m), with bumetanide (10−4 m) or the vehicles, before cumulative addition of angiotensin II. In some experiments with frusemide (10−3 m), indomethacin (10−6 m) or its vehicle was added to the preparation 10 min before the diuretic.
Data analysis
Contractile responses were expressed as a percentage of the second response to KCl 90 mm. For each ring, the maximum effect (Emax) was the greatest response obtained with angiotensin II at 3 × 10−7 m. The concentration of angiotensin II producing 50% of the maximal effect (EC50) was determined from each curve by a logistic, curve-fitting equation. The pD2 value represents the negative logarithm of EC50 and was used to compare the shift of the concentration-response curves. Results are expressed as the mean±s.e.mean for the specified number of preparations tested. Statistical analysis was performed using one way analysis of variance (ANOVA) for multiple comparisons followed by Bonferroni corrected t-test and individual comparisons were made by Student’s t-test for unpaired data. 95% confidence intervals for the differences between the means (CI) was also calculated. P < 0.05 was considered to be significant.
Drugs
Drugs used in this study and their sources were: angiotensin II, acetylcholine chloride, indomethacin and bumetanide (Sigma, L’Isle D’Abeau, France), frusemide (Hoechst, Puteaux, France), KCl Normapur (Prolabo, Paris, France), Belzer solution (ViaSpanTM) from Dupont (Herts, Netherlands). Indomethacin and bumetanide were dissolved in ethanol, the maximum ethanol concentrations in the organ bath were 0.01% and 1%, respectively.
Results
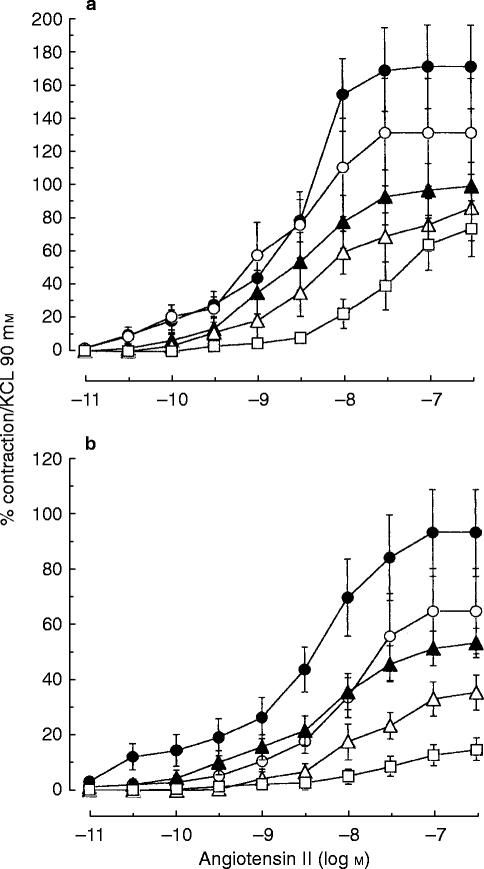
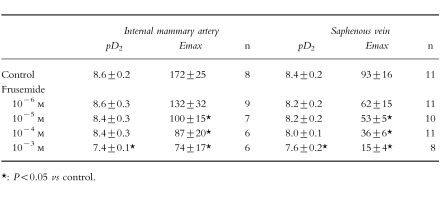
Frusemide caused a concentration-dependent decrease of the amplitude of the contraction elicited by angiotensin II on internal mammary artery (Figure 1a) and on saphenous vein (Figure 1b). In comparison with the respective controls, the maximal contraction in IMA was reduced by 24% (CI −55, 7), 30% (CI −50, −9), 50% (CI −58, −42) and 79% (CI −100, −59) and in SV by 10% (CI −40, 21), 16% (IC −40, 8), 42% (CI −67, −17), and 67% (CI −82, −52)after incubation with frusemide at the concentrations of 10−6 m, 10−5 m, 10−4 m and 10−3 m, respectively. In terms of potency, pretreatment with frusemide did not alter the apparent pD2 except after frusemide 10−3 m (Table 1).
Figure 1.
Contraction (expressed as a percentage of contraction elicited by 90 mm KCl) of human internal mammary artery (a) and of human saphenous vein (b) induced by cumulative addition of increasing concentration of angiotensin II in the absence (vehicle: (•) or the presence of frusemide 10−6 m (○), 10−5 m (▴) or 10−4 m (▵) and 10−3 m (□). Values represent means of 8 to 11 experiments. The vertical bars are s.e.mean.
Table 1.
Effect of frusemide 10−6, 10−5, 10−4 m and 10−3 m on the contraction elicited by angiotensin II in human internal mammary artery and saphenous vein. Results (mean±s.e.mean) are expressed as pD2 (−log EC50) and maximal contraction (% of response to 90 mm KCl); n represents the number of experiments.
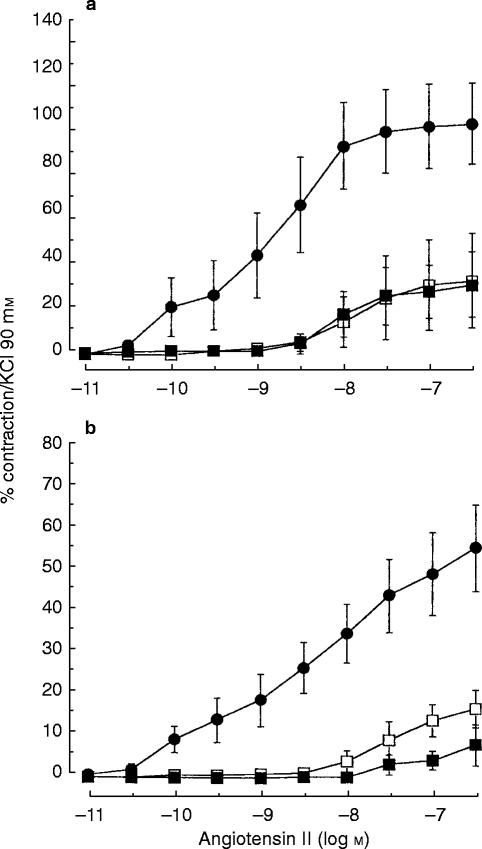
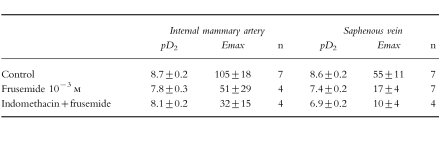
Pretreatment with indomethacin (10−6 m) did not significantly modify the inhibitory effect of frusemide 10−3 m on the contraction induced by angiotensin-II (Figures 2a and 2b, Table 2) and reduced the angiotensin II-induced contraction by 70% (CI −97, −44) in IMA and by 60% (CI −81, −39) in SV.
Figure 2.
Contraction (expressed as a percentage of contraction elicited by 90 mm KCl) of human internal mammary artery (a) and of human saphenous vein (b) induced by cumulative addition of increasing concentration of angiotensin II in the absence (vehicle: (•) or the presence of frusemide 10−3 m (□) or of frusemide 10−3 m and indomethacin 10−6 m (▪). Values represent means of 4 to 7 experiments. The vertical bars are s.e.mean.
Table 2.
Influence of indomethacin on the inhibitory effect of frusemide on angiotensin II-induced contraction in human IMA and SV. Results (mean±s.e.mean) are expressed as pD2 (−log EC50) and maximal contraction (% of response to 90 mm KCl); n represents the number of experiments.
Pretreatment with bumetanide (10−4 m) also reduced the maximal contractile responses to angiotensin II in IMA (by 22% (CI −40, −11), n = 7; P < 0.05) and in SV by 34% (CI −57, −12), n = 9; P < 0.03) but did not alter angiotensin II potencies in IMA (pD2: 8.2±0.2 (control) vs 8.4±0.2 (bumetanide) and in SV (pD2: 7.8±0.2 (control) vs 7.0±0.9 (bumetanide)).
Discussion
The present study provides evidence that frusemide exerts in vitro a potent inhibitory effect on angiotensin-II-induced contractions in human IMA and SV. This result extends to human vessels, the original observation in rat portal vein made by Blair-West et al. [8] in which the maximum contractions to angiotensin II were suppressed on the average to 48% of controls in the presence of 3 × 10−4 m frusemide. In the present study, the inhibitory effect of frusemide was significant at 10−5 m, a concentration close to plasma concentrations observed after 20 mg administered intravenously and 40 mg administered orally in patients with congestive heart failure [9]. In this regard, in patients with acute congestive cardiac failure, frusemide very rapidly decreases left atrial pressure before any diuretic effect occurs [10] and this is related to an increase in blood vessel compliance [11–14]. Since angiotensin II is involved in the pathophysiology of congestive cardiac failure, inhibition by frusemide of the vascular tone induced by angiotensin II may contribute to its beneficial effect on blood vessel compliance in this pathology. In the same way, since angiotensin II is involved in the pathophysiology of hypertension, the clinical efficacy of frusemide in this indication may be also related, at least in part, to an inhibitory effect on angiotensin II-induced vasoconstriction.
The increase in blood vessel compliance has been shown to be prevented by pretreatment with indomethacin and was not seen in anephric patients. Gerkens suggested that frusemide may release an anti-vasoconstrictor hormone from the kidney through a prostaglandin-dependent indomethacin-sensitive step [2]. Such a mechanism cannot explain our in vitro results. The reduction in vascular tone in canine isolated lung lobe [15] was prevented by indomethacin pretreatment suggesting that the direct effect of frusemide on vascular tone may be mediated through induction of prostanoid synthesis. The inhibitory effect of frusemide on angiotensin II-induced contraction was, however, not caused by the induction of relaxant prostaglandin synthesis since pretreatment with indomethacin did not alter the inhibitory effect of frusemide. In line with this finding, it has been reported that the direct dilatory effect of frusemide on rat tracheal vessel [16] as well as its relaxant effect on the vascular tone in rat isolated kidney [17] and in dog isolated pulmonary vein [18] is not attenuated by inhibition of prostanoid release by indomethacin or ibuprofen. In their study, Greenberg et al. [18] also provided evidence that frusemide may relax the dog pulmonary vein by an effect on the Na+/K+/2Cl− co-transport. This co-transport has been described for vascular smooth muscle cells, where it is inhibited by frusemide [19–22]. In this regard, it has been shown that angiotensin II strongly increases the specific activity of the Na+/K+/2Cl− co-transporter in cultured vascular smooth muscle cells [23], particularly in angiotensin-II hypertrophied vascular smooth muscle cells where this transporter is a component of the hypertrophic growth response [24]. In order to assess whether the inhibitory effect of frusemide on angiotensin II-induced contraction was related to an inhibition of the co-transport, we have tested the effects of bumetanide at a concentration (10−4 m) which is considered to be at least as potent as 10−3 m frusemide on the co-transport [1]. We found that bumetanide exerts also an inhibitory effect on angiotensin II-induced contraction indicating that inhibition of the Na+/K+/Cl− co-transporter may account for the effects of the loop diuretics. Frusemide appears more potent than bumetanide with respect to the inhibition of angiotensin II-induced contraction suggesting that frusemide may have additional properties.
In conclusion, frusemide, at concentrations in the therapeutic range (10−5 m) and at higher concentrations, exerts a dose-dependent inhibition of angiotensin II-induced contractions on human isolated IMA and SV. This inhibitory effect appears cyclooxygenase independent and mediated, at least in part, by inhibition of Na+/K+/2Cl− symport. Reduction in the vasoconstrictor effect of angiotensin II may be involved in the therapeutic efficacy of frusemide.
References
- 1.Jackson EK. The Pharmacological Basis of Therapeutics. 9. McGraw-Hill; 1995. Diuretics. In Goodman & Gilman’s; pp. 685–715. [Google Scholar]
- 2.Gerkens JF. Does furosemide have vasodilator activity? Trends Pharmacol Sci. 1987;8:254–257. [Google Scholar]
- 3.Ferrario CM. The renin-angiotensin system: importance in physiology and pathology. J Cardiovasc Pharmacol. 1990;15(Supp. 3):S1–S. [PubMed] [Google Scholar]
- 4.Johnston GD, Nicholls DP, Leahey WJ, Finch MB. The effects of captopril on the vascular responses to frusemide in man. Clin Sci. 1983;65:359–363. doi: 10.1042/cs0650359. [DOI] [PubMed] [Google Scholar]
- 5.Kiely DG, Cargill RI, Lipworth BJ. Effects of frusemide and hypoxia on the pulmonary vascular bed in man. Br J Clin Pharmacol. 1997;43:309–313. doi: 10.1046/j.1365-2125.1997.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He GW, Rosenfeldt FL, Buxton B, Wilson AC, Angus A. Reactivity of human isolated internal mammary artery to constrictor and dilator agents. Circulation. 1989;80(Suppl I):I-141–I-150. [PubMed] [Google Scholar]
- 7.Stanke F, Cracowski JL, Chavanon O, et al. Glibenclamide inhibits thromboxane A2-induced contraction in human internal mammary artery and saphenous veins. Eur J Pharmacol. 1998;341:65–71. doi: 10.1016/s0014-2999(97)01458-1. [DOI] [PubMed] [Google Scholar]
- 8.Blair -West JR, McKinley MJ, McKenzie JS. Effect of furosemide on the reactivity of rat portal vein. J Pharm Pharmacol. 1972;24:442–446. doi: 10.1111/j.2042-7158.1972.tb09029.x. [DOI] [PubMed] [Google Scholar]
- 9.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater C. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–609. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 10.Biagi RW, Bapat BN. Frusemide in acute pulmonary oedema. Lancet. 1967;i:849. doi: 10.1016/s0140-6736(67)92818-8. [DOI] [PubMed] [Google Scholar]
- 11.Dikshit K, Vyden JK, Forrestier JS, Chatterjee K, Prakash R, Swan HJC. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infaction. N Engl J Med. 1973;288:1087–1090. doi: 10.1056/NEJM197305242882102. [DOI] [PubMed] [Google Scholar]
- 12.Bourland WA, Day DK, Williamson HE. The role of the kidney in the early nondiuretic action of furosemide to reduce left atrial pressure in the hypervolemic dog. J Pharmacol Exp Ther. 1977;202:221–229. [PubMed] [Google Scholar]
- 13.Johnston GD, Hiatt WR, Nies AS, Payne NA, Murphy RC, Gerber JG. Factors modifying the early nondiuretic vascular effects of furosemide in man. The possible role of renal prostaglandins. Circ Res. 1983;53:630–635. doi: 10.1161/01.res.53.5.630. [DOI] [PubMed] [Google Scholar]
- 14.Fulgham TG, DiMarco JP, Supple EW, et al. Effect of prostacyclin on vascular capacity in the dog. J Clin Invest. 1985;76:999–1006. doi: 10.1172/JCI112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundergan CF, Fitzpatrick TM, Rose JC, Ramwell PW, Kot PA. Effect of cyclooxygenase inhibition on the pulmonary vasodilator response to furosemide. J Pharmacol Exp Ther. 1988;246:102–106. [PubMed] [Google Scholar]
- 16.Corboz MR, Ballard ST, Inglis SK, Taylor AE. Dilatory effect of furosemide on rat tracheal arterioles and venules. Am J Respir Crit Care Med. 1997;156:478–483. doi: 10.1164/ajrccm.156.2.9609123. [DOI] [PubMed] [Google Scholar]
- 17.Stephan D, Fontaine C, Grima M, Imbs JL Barthelmebs M. Vascular effects of loop diuretics: an in vivo and in vitro study in the rat. Naunyn-Schmiedeberg’s Arch Pharmacol. 1994;349:209–216. doi: 10.1007/BF00169839. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg S, McGowan C, Xie J, Summer WR. Selective pulmonary and venous smooth muscle relaxation by furosemide: a comparison with morphine. J Pharmacol Exp Ther. 1994;270:1077–1085. [PubMed] [Google Scholar]
- 19.Villamil MF, Ponce J, Amorena C, Muller A. Effect of furosemide on the ionic composition of the arterial wall. Life Sci. 1979;9:9–14. [PubMed] [Google Scholar]
- 20.Kreye VAW, Bauer PK, Villhauer I. Evidence for furosemide-sensitive active chloride transport in vascular smooth muscle. Eur J Pharmacol. 1981;73:91–95. doi: 10.1016/0014-2999(81)90150-3. [DOI] [PubMed] [Google Scholar]
- 21.Deth RC, Payne RA, Peecher DM. Influence of furosemide on rubidium-86 uptake and alpha-adrenergic responsiveness of arterial smooth muscle. Blood Vessels. 1987;24:321–333. doi: 10.1159/000158709. [DOI] [PubMed] [Google Scholar]
- 22.Tian R, Aalkjaer C, Andreasen F. Mechanisms behind the relaxing effect of furosemide on the isolated rabbit ear artery. Pharmacol Toxicol. 1990;67:406–410. doi: 10.1111/j.1600-0773.1990.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith JB. Angiotensin-receptor signaling in cultured vascular smooth muscle cells. Am J Physiol. 1986;250:F759–F769. doi: 10.1152/ajprenal.1986.250.5.F759. [DOI] [PubMed] [Google Scholar]
- 24.Tseng H, Berk BC. The Na/K/2Cl Cotransporter is increased in hypertrophied vascular smooth muscle cells. J Biol Chem. 1992;267:8161–8167. [PubMed] [Google Scholar]