Introduction
Peroxides represent a new chemical class of antimalarial drugs (Figure 1). The first generation compounds are all derivatives of artemisinin, the active principle of a Chinese herbal remedy [1]. Artemisinin, or qinghaosu (green plant extract), is extracted from Artemesia annua, a plant which grows widely in South China. The first generation compounds currently in use are derivatives of the lactol, dihydroartemisinin, which is a major metabolite for each of the derivatives, and may be the most important chemical species for antimalarial activity in vivo. The promise of these compounds lies in their effectiveness against chloroquine-resistant parasites and their rapid action against malaria. Moreover, they have been used for the treatment of malaria in three million patients with few reports of clinically significant toxicity [2].
Figure 1.
Chemical structures of ‘First’ and ‘Second’ generation peroxides. The fenozan, tetroxane and tricyclic trioxanes are totally synthetic compounds prepared from readily available starting materials. The first generation DHA derivatives and the carba derivatives (e.g. C-10-Carba) are compounds prepared directly by manipulation of the parent drug artemisinin.
The drawback with artemisinin and related compounds is that they suffer from poor bioavailability: the relative bioavailability of oral artemisinin (vs intramuscular) and intramuscular artemether (vs oral) and the bioavailability of artesunate are all within a range of 15–30% [3–5]. This may be one of the reasons for the relatively high rate of recrudescence associated with peroxide monotherapy. Their complex chemical structures are not essential for pharmacological activity and there has, therefore, been a drive to design and synthesize simpler endoperoxides which have greater in vivo potency. To date, more than 1000 new endoperoxides belonging to several chemical classes have been prepared. A number of ‘second generation’ peroxides have been developed, including arteflene (Figure 1). This is a synthetic peroxide developed from yingzhaosu A, the complex active constituent of the Chinese plant Artabotrys uncinatus, after it was shown to have antimalarial activity [6]. It has been tested in clinical trials in Africa.
The ‘first generation’ artemisinin derivatives, as described above, are chemically simple esters and ethers of the parent lactol, dihydroartemisinin (Figure 1) [7, 8]. These compounds are generally more potent than artemisinin both in vitro and in vivo, and provide a wider choice of formulations which are more easily administered to patients in the field. Artemether and arteether are more oil soluble than artemisinin and are currently undergoing clinical trials under WHO sponsorship [8]. Other first generation derivatives include sodium artesunate, the DHA β-O-succinate half ester sodium salt, and sodium artelinate, the corresponding DHA β-O-p-carboxybenzyl ether sodium salt. Both of these compounds, which are water soluble, are currently under investigation as intravenous treatments for severe malarial infections [8].
Given the obvious pharmacokinetic limitations of the ‘first generation’ analogues and the realization that the peroxide bridge is the essential pharmacophore for biological activity, medicinal chemists have developed a range of second generation derivatives. Representative compounds include the bicyclic fenozan type derivatives, synthesized by Jefford et al. [9], the tetroxanes synthesized by Vennerstrom [10] and tricyclic simplified derivatives prepared by Posner and coworkers [11]. The discovery that several of the first generation artemisinin derivatives are metabolised extensively to DHA has prompted other workers to prepare C-10-carba deoxy derivatives (for a review see Meshnick et al. [1]). These compounds cannot be metabolised to DHA, which undergoes fairly rapid clearance [8], and should therefore have longer half-lives in vivo.
The initial focus of the medicinal chemist is clearly directed towards the discovery of simpler (hence often less expensive) and more potent compounds, which provides not only lead compounds for therapeutic use, but also pharmacological tools to define the mechanism of action of this class of agent and the essential pharmacophore. A QSAR study of antimalarial activity indicates that docking between an active trioxane and the receptor, haem, could be the crucial step preliminary to drug action [12].
Drug safety is an important consideration which has to be evaluated in concert with efficacy at the earliest possible stage in drug development. It is therefore essential to define the potential hazards associated with second or third generation peroxides that present greater and more prolonged systemic exposure and design appropriate test systems for screening for drug toxicity. The specific questions which need to be addressed during further chemical development of peroxide antimalarials are as follows.
Will such chemical modifications lead to loss of the high degree of selective therapeutic potency noted for first generation peroxide antimalarials?
Will chemical modification result in greater exposure of mammalian systems to hazards posed by the peroxide group such as the neurotoxicity observed in rodents given first generation peroxides?
The purpose of this review is to address each of these questions from chemical and clinical perspectives.
Clinical efficacy and safety
Numerous studies have shown that artemisinin compounds have several advantages over other antimalarial drugs: these include the more rapid clearance of parasites in the periphery [2], more rapid resolution of fever [13, 14], and a reduction in the transmission potential of falciparum malaria [15]. Despite these apparent advantages, no study has yet shown a beneficial effect of artemisinin compounds on survival, although this may largely be due to the fact that most studies performed so far have been under-powered to detect differences in mortality [16]. Importantly, the peroxides are effective against Plasmodium falciparum strains resistant to other antimalarials [17], and there have been no reports of parasite resistance [2, 7].
The artemisinin compounds have become first-line drugs in several regions in South-east Asia. The main clinical disadvantage of artemisinin and its derivatives is the high rate of recrudescence when compared with other antimalarials [2]; the reasons for this are unclear, but partly reflect the low bioavailability and short half-lives of these compounds. In addition, it has been found that artemisinin areas under the plasma concentration curves decrease during repeated drug administration, indicating that auto-induction of hepatic drug-metabolising enzymes may contribute to incomplete parasite clearance [18]. In vivo potency with respect to parasite elimination is also a problem with second generation peroxides. A comparison of single dose therapies in children with uncomplicated P. falciparum malaria in Gabon showed that arteflene was ineffective in contrast to mefloquine, which proved to be highly effective [19].
Despite their extensive use, there have been very few reports of clinically significant toxicity with the peroxide antimalarials. Minor gastro-intestinal adverse effects such as nausea, diarrhoea and abdominal pain have been reported in some patients; tenesmus occurs in 6% of patients administered artemisinin suppositories [2]. Transient increases in serum transaminases, and decreases in the reticulocyte and neutrophil counts have also been encountered, but these have been of little clinical significance [2]. There have also been isolated reports of rash but a causal association was unlikely [20]. Interestingly, 25% of volunteers receiving artemisinin had an episode of drug-induced fever [2, 16] in some studies, though the significance of this is unclear.
Given that cardiotoxicity is a potential worry with many of the antimalarial drugs [21], and QT prolongation has been reported in animals treated with artemether [2], many of the clinical studies have actively monitored for potential cardiotoxicity. To date, most of the abnormalities detected have been minor and of little clinical significance. For example, transient first-degree heart block was noted in 1 of 82 patients receiving artesunate, and 3 of 39 patients receiving artemether [2]. In a recent study, it was demonstrated that 25% of 284 adults treated with intramuscular artemether for severe falciparum malaria had a QTc interval of more than 0.5 s [22]. However, it is possible that this was caused by the disease rather than the treatment [23].
Given the findings in animals (see below), the major concern with this class of compounds has been their potential to cause neurotoxicity. Clinical trials to date have not identified any cases of neurotoxicity [2] which can definitely be attributed to the artemisinin compounds. However, the recent reports of a prolonged length of time to recovery from coma (when compared with patients treated with quinine) in Vietnamese [22] and Gambian [24] patients, and an increased frequency of seizures in artemether-treated patients in Gambia [24], have raised concerns of the neurotoxic potential of these compounds [25]. In addition, there has recently been one report of the occurrence of an acute cerebellar syndrome in a 36-year-old previously healthy man after five doses of artesunate, the features of which were still present (although improved) 4 months later [26]. Clearly, one such report does not constitute proof of a causal association [27], but the possibility that repeated administration of artemisinin compounds in malaria endemic areas will result in cumulative damage cannot be ruled out [2, 28], especially since these drugs are often freely available for use in tropical countries [29]. Another possibility that cannot be discounted at this stage is that some patients may have suffered subclinical neurological toxicity which would have required sophisticated neurological and neuropsychological tests for detection. The potential of the drugs to cause neurotoxicity is discussed in greater detail below.
Fear of potential toxicity has limited the use of the artemisinin derivatives in pregnancy since animal studies have documented teratogenicity when the drugs are administered at high doses [30]. At present documentation of the safety of artemisinin derivatives in the treatment of malaria in pregnant women is limited and it has therefore been recommended that these drugs should be used in pregnancy only as a last resort [31].
Thus, there is a need to develop better peroxide compounds, the ideal properties of which would be (a) a pharmacological profile which allows for shorter durations of treatment with fewer doses, (b) greater bioavailability to ensure sufficient parasite exposure, (c) a predictable pharmacokinetic profile such that the drug is retained for sufficient lengths of time to ensure that it kills the parasites, but does not accumulate to any extent to cause damage to the host, (d) low interindividual pharmacokinetic variability and (e) lack of toxicity. Rational molecular design must be based on both structure-activity and structure-toxicity/metabolism relationships.
Mechanism of action of peroxide antimalarials: role of parasite bioactivation
The essential chemical features required for pharmacological activity are simply a peroxide moiety, which is sterically hindered, and in the case of many second generation peroxides a lipophilic aromatic ring system (Figures 1 and 2). Artemisinin contains a peroxide bridge within a more complex 1,2,4-trioxane structure. A comparison with the second generation peroxides indicates that the 1,2,4-trioxane structure per se is not essential for pharmacological activity in the parasite, although it has not yet been established whether the overall structure provides (a) ‘protection’ for the host by restricting the intracellular (e.g. in the nervous system) bioactivation of the peroxide, and (b) alternative routes of metabolism in man (i.e. direct detoxication). Artemether undergoes several hydroxylations in vivo, most of which lead to a decrease in pharmacological activity [32]. Dihydroartemisinin undergoes extensive glucuronidation [33], which again results in loss of activity [34].
Figure 2.
Structural features required for activity. In both peroxide drugs, C-centred radicals can be formed following iron (II)-mediated one-electron reduction by haem and subsequent rearrangement of the initially formed oxyl radical (see Figure 5 for more detail). In arteflene, the crucial endoperoxide pharmacophore is linked to a lipophilic bistrifluorinated ring system as shown.
The parasite digests haemoglobin to obtain amino acids but releases haem, a potentially toxic species (Figure 3). Haem is detoxified in the parasite by the formation of a nontoxic but not entirely unreactive polymer, the malaria pigment, haemozoin. Free haem catalyses the conversion of peroxides into free radicals and other electrophilic intermediates which alkylate haem and specific parasite proteins, and thereby it is supposed, kill the parasite [35, 36]. As well as the formation of carbon-centred radicals there is also the formation of a high valent iron-oxo species (FeIV = O) (Figure 4).
Figure 3.
General scheme for the mechanism of action of antimalarial peroxides. The critical step for activity of the drug is believed to be the formation of a C-centred radical that is proposed to kill the parasite by alkylation.
Figure 4.
Chemical details of the iron-mediated bioactivation reactions of artemisinin that are believed to be critical for antimalarial activity. At the top of the scheme, the initially formed oxyl radical rearranges by a 1,5-H shift to produce a C-centred radical that is then ultimately transformed into the hydroxy deoxy derivative (HODEOXART) as shown. Note that this pathway may also produce a high valent Fe(IV)O species which is proposed to oxidatively damage cellular macromolecules [52]. At the bottom of the scheme, association of Fe(II) with O1 and scission of the alkyl bond produces a primary radical, which in the absence of a protein or porphyrin acceptor is further transformed into the THF-acetate derivative as shown.
In vitro studies have shown that all endoperoxide antimalarial compounds share common targets with respect to protein alkylation [35]. It has been demonstrated that dihydroartemisinin associates with parasite membranes, haemozoin and mitochondria; damage occurs in the mitochondria, rough endoplasmic reticulum and the nuclear envelope, and there are also changes in the nucleus, ribosomes and food vacuole [36].
Antioxidants such as catalase, dithiothreitol and α– tocopherol have been shown to antagonise the antimalarial action of artemisinin [37]. The role of iron was defined in experiments which showed that iron chelators antagonise the antimalarial activity of an artemisinin derivative, arteether [38].
Toxicology in animal models
Undoubtedly the major concern raised by preclinical studies in animals is that of the neurotoxicity which has been observed in rats, dogs [39, 40] and more recently in monkeys [41]. It seems to be dose-dependent and has occurred at relatively high doses (20–50 mg kg−1 day−1); the currently recommended dose for both artemether and artesunate in man is 10 mg kg−1 day−1. However, relative systemic exposure to drug in man and in the animal models has not been determined. A progressive syndrome of clinical neurological deficits with cardio-respiratory collapse (with prolongation of the QTc interval) and death was noted in dogs dosed for 8 days with arteether (20 mg kg−1). Prominent neuropathologic lesions were limited to the pons and the medulla [39]. In the rat, brain stem histological lesions have been seen in the absence of neurologic signs or deficits in behavioural performance [1]. Toxicity has been confirmed in vitro in Neu2a neuroblastoma cells; these cells took up much less dihydroartemisinin than P. falciparum-infected red blood cells, suggesting that the compounds may show selective toxicity for the parasite in vivo. As pointed out earlier, despite many years of use there is little evidence of neurotoxicity in man. An opportunistic postmortem examination of the brain stem of a patient who took single-dose artesunate showed no abnormalities [42].
Artemisinin derivatives have been shown to be toxic to neuroblastoma cells in vitro, as measured by either radiolabelled precursor incorporation or neurite outgrowth. Toxicity is specific to neuronal cells and does not affect glial cells [43]. Structure-toxicity relationships indicate that the chemistry of the d-ring is an important determinant of in vitro toxicity, with dihydroartemisinin being considerably more toxic than other derivatives [43, 44]. However, whether this toxicity derives from an inherently more reactive peroxide group or the formation of neurotoxic ketones through the rapid isomerization of dihydroartemisinin that occurs in the presence of iron (II), has not been defined (Maggs et al. unpublished). Addition of haemin to in vitro systems can markedly potentiate the neurotoxicity of peroxides indicating a role for iron-catalysed radical formation [45].
Arteflene (Figure 1) is a synthetic peroxide which, as mentioned earlier, was brought forward for preclinical and clinical evaluation from a series of yingzhaosu A derivatives [6]. The compound was selected on the basis of its greater chemical stability than artemisinin and thus its anticipated improved pharmacokinetic properties. Arteflene was found to be negative in a battery of mutagenicity tests and did not produce any neurotoxicity when given in high doses to dogs (up to 700 mg kg−1). However, reproductive studies revealed clear embryolethal effects (37–57% intrauterine deaths) in rats at 20 mg kg−1 and a slight effect in rabbits at 40 mg kg−1. Autopsy examination of the foetus showed the presence of blood vessel anomalies and fused sternebrae in the 20 mg kg−1 dose group [30]. The embryotoxicity was comparable with the effects seen with artemisinin. Pregnant rats given extremely high doses (up to 3000 mg kg−1) of artemisinin within 6 days of gestation had normal foetuses; however, the drug caused foetal death and resorptions in rodents, even at relatively low doses when given after the sixth day of gestation [46, 47]. There was no evidence of teratogenicity in the offspring that survived organogenesis. No such abnormalities have been reported in man, but the safety data are extremely limited [30]. While such effects may not be considered a handicap for possible use in the treatment of malaria [6], such findings must necessarily preclude the use of these compounds for prophylaxis in women unless specific contraceptive precautions are undertaken.
The acute toxicity of artemisinin in mice which involves the gastrointestinal tract is antagonised by an iron chelator [38], indicating a role for iron-catalysed bioactivation of the peroxide group. This may reflect the availability of free iron in the gut. However, the role for peroxide bioactivation in particular, and metabolism in general, in chronic toxicity (neurotoxicity and reproductive toxicity) is not known. Since neurotoxicity is not observed with arteflene [6], this would suggest that the toxicity when observed after administration of artemisinin and its derivatives is not simply a function of the presence of a peroxide group. In vitro studies with neuroblastoma cells have shown that iron-catalysed activation of artemether significantly enhances the neurotoxicity of artemether with concomitant formation of a rearrangement product, artemether tetrahydrofuran acetate [48]. The antioxidants ascorbic acid and glutathione afforded protection against the toxicity.
Although it is thought that products of iron-catalysed bioactivation are responsible for both the parasite and mammalian toxicity of peroxides, it is also possible that the radicals might damage iron-containing proteins and thereby release iron which could be a cause of oxidative damage [49]. Iron is thought to be a crucial factor in reactive oxygen metabolite-induced cytotoxicity. In order to induce lipid peroxidation, superoxide and/or hydrogen peroxide must react with iron to generate hydroxyl radicals via the iron-catalysed modified Haber-Weiss reaction [50, 51]:-
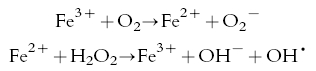 |
(Fenton reaction)
Thus, it is possible that the disposition of iron at both the cellular and molecular levels might be intimately involved in the bioactivation of peroxides and their subsequent biological effects.
Biomimetic Fe(II) catalysed reactions and biomarkers of bioactivation
As mentioned earlier, the malaria parasite Plasmodium falciparum, in the intraerythrocytic stage of its life cycle, digests haemoglobin, which results in the generation of ferriprotoporphyrin IX (haem). Since ‘free haem’ is toxic to the parasite, it is normally removed by polymerization to haemozoin, an insoluble pigment. A number of model studies utilizing iron (II) salts and haem iron (II) have been reported for artemisinin [36] with several intermediates being proposed as the ultimate cytotoxic species (Figure 4). Association of either of the peroxide oxygens in the trioxane skeleton produces oxyl radicals that can either participate in a 1,5-H-shift or carbon C-C bond scission to produce a carbon-centred radical species. Also implicated in the mechanism of action of artemisinin is the formation of a high valent iron-oxo species produced by -scission of the C-4 radical intermediate (X) as shown [52] (Figure 4).
More recently, we have proposed a mechanism of action for arteflene based on similar mechanistic studies using Fe(II) salts [53]. Arteflene, on reaction with FeCl2.4H2O, yielded two products, the two-electron reduction diol product and the enone, a surrogate marker for the formation of the cyclohexyl radical (Figure 5).
Figure 5.
Proposed mechanism of action of arteflene [53]. Arteflene by Fe(II)-mediated one-electron reduction can produce a C-centred radical and a potentially toxic α,β-unsaturated enone. This enone, if generated in the parasite food vacuole, might act as a Michael acceptor of thiols. The enone product, in effect, is a surrogate marker of bioactivation. Ar = 2, 4-bistrifluoromethyl benzene.
The formation of the THF acetate from artemisinin and the enone products from arteflene may be effective surrogate markers of bioactivation. However, further work is required to determine whether the generation of these entities in a mammalian system leads to radical-induced toxicity. In theory, such radicals might cause toxicity which would be partly determined by the availability of iron (II) and its tissue distribution.
Two distinct (but not exclusive) mechanisms for the iron-mediated activation of peroxides in mammalian tissues are envisaged. Firstly, simple nonenzymic reactions, involving free iron and/or iron bound to protein, although the chemical reactivity of peroxides with iron associated with, for example, transferrin has not been defined. Secondly, reactions catalysed by haemthiolate enzymes, and specifically by the microsomal cytochrome P450 enzymes [54] and the prostacyclin and thromboxane synthases [55]. The synthases, in particular, catalyse isomerizations of endogenous prostaglandin H2 endoperoxide which are analogous in detail to the iron-mediated isomerizations of artemisinin.
Disposition of peroxides in relation to human safety
The main safety concern for present and future peroxide antimalarials is whether the peroxide pharmacophore can act as a toxicophore and if so, under what circumstances could this occur. At present, little is known about the metabolic fate of peroxides. Important questions which need to be addressed are:-
does the peroxide group per se undergo a direct detoxication biotransformation in mammalian systems?
does the peroxide bridge undergo bioactivation in vivo in mammalian cells?
what are the biological consequences of bioactivation?
is the development of peroxides with longer half-lives, for more sustained antimalarial activity, likely to be associated with a greater risk of toxicity?
can prolonged exposure to free radicals exhaust the scavenging or repair systems resulting in host cell damage and if so, can drugs with longer half-lives be (clinically) more toxic?
In order to address these issues, it is necessary to define all the major and minor routes of metabolism. Of particular relevance are metabolites (including rearrangement products and fragments) which indicate bioactivation of the peroxide group; and also metabolites which signal the formation of potentially toxic species such as aldehydes and enones. However, peroxide antimalarials were introduced into clinical use without any formal knowledge of either disposition or metabolism [56] because of the lack of suitable analytical techniques. Thus, assessment of the risk to man of findings in animals by normal pharmacokinetic analysis was precluded. Furthermore, the importance of development age and disease with respect to toxicological risk had not been defined.
Recently, specific and sensitive h.p.l.c. and LC-MS methods [3, 33, 57] have been developed for the analysis of artemisinin and related compounds in plasma, and for the characterization and quantification of metabolites. Following intravenous administration, artesunate is rapidly (t1/2 3 min) hydrolysed to dihydroartemisinin which in turn has a relatively short half-life of only 40 min [4]. In both the rat [33] and man (Maggs et al. unpublished), the principal pathway of metabolism for dihydroartemisinin (Figure 6) is glucuronidation. It is possible that the rate of glucuronidation may serve to limit the therapeutic effectiveness of the drug with respect to disease recrudescence, as glucuronides are inactive [34], but it may also preclude the neurotoxicity observed with high doses of first generation peroxides in the rat and dog by preventing isomerization of DHA to neurotoxic products. Further studies are required to test this hypothesis. In addition, glucuronidation serves to trap the unstable lactol moiety, in a chemical sense, and thus prevents the rearrangement of the unstable dihydroartemisinin to ketone intermediates which are potentially neurotoxic (although so far only observed in in vitro systems). There is no evidence for detoxication of the peroxide group per se, by reduction, for example, as has been described for prostaglandin peroxides [58].
Figure 6.
Chemical mechanisms for the formation of metabolites derived from dihydroartemisinin (DHA). DHA can undergo either direct glucuronidation or Fe(II)/Fe(III)-catalysed reactions, as shown, to give deoxydihydroartemisinin (DEOXDHA) and a ring contracted derivative (DHATHF).
A key question is whether bioactivation of peroxides catalysed by iron occurs in vivo during therapeutic administration. The body takes great care to safely sequester iron and copper in non or poorly reactive forms [59]; the body store is divided between essential iron-containing compounds and excess iron which is held in storage [60]. Iron disposition is extremely tightly controlled, with only 10% of total stores being excreted per year. The major form of essential iron is haemoglobin, although there are a variety of haem-dependent enzymes including the cytochrome P450 family. Ferritin is the intracellular storage protein, and may contain 4000 atoms of iron (III) per ferritin molecule. Aggregated ferritin (haemosiderin) constitutes about one third of normal stores. The two predominant sites of iron storage are hepatocytes and the reticuloendothelial system. Internal distribution and disposition of iron is facilitated by the plasma protein transferrin, which has two binding sites for ferric iron. Iron is delivered to intracellular sites via specific transferrin receptors and subsequent endocytosis; iron is then released in the endosome.
Iron can catalyse the rapid bioactivation of all peroxide antimalarials to chemically reactive radical intermediates [36]. However, this ability depends upon the regeneration of iron (II), which may be prevented by binding of Fe(III) to a carrier protein [49]. Iron complexed to haem also catalyses the bioactivation of peroxide antimalarials [53], and this activity appears to be preserved in haemothiolate enzymes [55]. In contrast, cytochrome P450 enzymes can effect the hydroxylation of either artemisinin or arteflene at a site close (<2 A°) to the peroxide bridge without necessarily activating the peroxide bridge [54]. Incubation of haemoglobin with organic peroxides releases iron [59].
Ferritin contains the greatest part of iron found in the brain, and the release of iron stores from ferritin has an essential role in iron-dependent lipid peroxidation. Stimulation of cultured microglia by phorbol myristate acetate causes both iron mobilization and superoxide production [61]. It has been postulated that such iron release may be partly responsible for neurodegenerative disorders such as Parkinson’s disease. Histochemical studies have shown that there is considerable variation in the iron content throughout the brain, and there is also interspecies variation in transferrin levels [62]. Interestingly, it has been shown that both iron uptake and the iron concentration are relatively high during neonatal development in the rat [63]. There is thus circumstantial evidence to relate the disposition of iron to the two major toxicities observed in vivo, neurotoxicity and embryotoxicity.
The key questions that must be answered with respect to safety evaluation are:
does peroxide bioactivation cause toxicity in man, including neurotoxicity and reproductive toxicity?
is there species variation in the bioactivation of peroxides?
are there species differences in the detoxication of peroxides?
Evidence for peroxide bioactivation in vivo can be obtained by using specific metabolites as biomarkers. Parallel in vitro studies can be performed to define the role of disposition in toxicity and the chemical species which is responsible. Such information would allow extrapolation of the toxicities observed in animals to man.
Conclusions
Malaria remains a major health problem world-wide, with more than 200 million people infected at any time and at least one million deaths annually. Chloroquine has been a cheap and effective treatment for malaria for several decades but there is now widespread resistance to this and newer antimalarials such as halofantrine and mefloquine.
Peroxides are an important new chemical class of antimalarials which are at present undergoing extensive clinical evaluation and further chemical development. No major forms of human toxicity have been detected for the peroxides, although assessment of long-term human safety is limited by a situation in which the clinical use of the drug is not accompanied by the standard practice of postmarketing surveillance. The concern over long-term toxicity in man is exacerbated by the practice of self-medication and uncontrolled drug use in many parts of the world. Laboratory studies have identified a number of potential hazards associated with long-term use, the most notable being reproductive toxicity and neurotoxicity. To define the human risk posed by peroxides, it is still necessary to define the chemical entities and chemical reactions which initiate the pathological processes, and then determine the propensity of individual peroxide drugs to undergo such bioactivation in vulnerable patient groups, and the foetus, by the use of chemical and molecular analysis. In order to develop new, longer-acting peroxide antimalarials, it will be necessary to define molecular characteristics which determine parasite-selective bioactivation and those which preclude the risk of serious human toxicity (Figure 7).
Figure 7.
Careful risk-benefit analysis of the peroxide antimalarials will be essential in order to maximize their ability to eliminate parasites, while at the same time minimize their potential for toxicity, particularly with regard to neurotoxicity and embryotoxicity.
BKP is a Wellcome Principal Research Fellow. The authors also wish to acknowledge the support of Roche Pharmaceuticals (PMO).
References
- 1.Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer HAC, Zuidema J, Kager PA, Wetsteyn JCFM, Lugt CB, Merkus FWHM. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J Pharm Pharmacol. 1990;42:810–813. doi: 10.1111/j.2042-7158.1990.tb07030.x. [DOI] [PubMed] [Google Scholar]
- 4.Batty KT, Thu LTA, Davis TME, et al. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br J Clin Pharmacol. 1998;45:123–129. doi: 10.1046/j.1365-2125.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teja-Isavadharm P, Nosten F, Kyle DE, et al. Comparative bioavailability of oral, rectal, and intramuscular artemether in healthy-subjects—use of simultaneous measurement by high-performance liquid-chromatography and bioassay. Br J Clin Pharmacol. 1996;42:599–604. doi: 10.1111/j.1365-2125.1996.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofheinz W, Burgin H, Gocke E, et al. Ro 42–1611 (arteflene), a new effective antimalarial: chemical structure and biological activity. Trop Med Parasitol. 1994;45:261–265. [PubMed] [Google Scholar]
- 7.Meshnick SR, Jefford CW, Posner GH, Avery MA, Peters W. Second-generation antimalarial endoperoxides. Parasitol Today. 1996;12:79–82. doi: 10.1016/0169-4758(96)80660-0. [DOI] [PubMed] [Google Scholar]
- 8.de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jefford CW, Velarde JA, Bernardelli G, Bray DH, Warhurst DC, Milhous WK. Synthesis, structure and antimalarial activity of tricyclic 1,2,4-trioxanes related to artemisinin. Helv Chim Acta. 1993;76:2775–2778. [Google Scholar]
- 10.Vennerstrom JL, Fu HN, Ellis WY, et al. Dispiro-1,2,4,5-tetraoxanes—a new class of antimalarial peroxides. J Med Chem. 1992;35:3023–3027. doi: 10.1021/jm00094a015. [DOI] [PubMed] [Google Scholar]
- 11.Posner GH, Oh CH, Webster HK, Ager AL, Rossan RN. New, antimalarial, tricyclic 1,2,4-trioxanes—evaluations in mice and monkeys. Am J Trop Med Hyg. 1994;50:522–526. doi: 10.4269/ajtmh.1994.50.522. [DOI] [PubMed] [Google Scholar]
- 12.Grigorov M, Weber J, Tronchet MJ, Jefford CW, Milhous WK, Maric D. A QSAR study of the antimalarial activity of some synthetic 1,2,4-trioxanes. J Chem Inf Comput Sci. 1997;37:124–130. doi: 10.1021/ci9601168. [DOI] [PubMed] [Google Scholar]
- 13.Sy ND, Hoan DB, Dung NP, et al. Treatment of malaria in Vietnam with oral artemisinin. Am J Trop Med Hyg. 1993;48:398–402. [PubMed] [Google Scholar]
- 14.Qinghaosu Antimalarial Coordinating Research Group. Antimalarial studies on qinghaosu. Chin Med J. 1979. pp. 811–816. [PubMed]
- 15.Price RN, Nosten F, Luxemburger C, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 16.White NJ. Artemisinin: current status. Trans R Soc Trop Med Hyg. 1994;88:S3–S4. doi: 10.1016/0035-9203(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 17.Hassan-Ali M, Bjorkman A, Ashton M. In vitro activity of artemisinin, its derivatives, and pyronaridine against different strains of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1990;84:635–637. doi: 10.1016/0035-9203(90)90129-3. [DOI] [PubMed] [Google Scholar]
- 18.Ashton M, Hai TN, Sy ND, et al. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:2601–2605. [PubMed] [Google Scholar]
- 19.Radloff PD, Philipps J, Nkeyi M, Sturchler D, Mittelholzer ML, Kremsner PG. Arteflene compared with mefloquine for treating Plasmodium facliparum malaria in children. Am J Trop Med Hyg. 1996;55:259–262. doi: 10.4269/ajtmh.1996.55.259. [DOI] [PubMed] [Google Scholar]
- 20.Barradell LB, Fitton A. Artesunate: A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. doi: 10.2165/00003495-199550040-00009. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SHL. Drugs, QT interval abnormalities and ventricular arrhythmias. Adverse Drug React Toxicol Rev. 1994;13:77–102. [PubMed] [Google Scholar]
- 22.Hien TT, Day NPJ, Phu NH, et al. A controlled trial of artemether or quinine in vietnamese adults with severe falciparum-malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 23.Von Seidlein L, Jaffar S, Greenwood B. Prolongation of the QTc interval in African children treated for falciparum malaria. Am J Trop Med Hyg. 1997;56:494–497. doi: 10.4269/ajtmh.1997.56.494. [DOI] [PubMed] [Google Scholar]
- 24.Vanhensbroek MB, Onyiorah E, Jaffar S, et al. A trial of artemether or quinine in children with cerebral malaria. N Engl J Med. 1996;335:69–75. doi: 10.1056/NEJM199607113350201. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman SL. Artemether in severe malaria—still too many deaths. N Engl J Med. 1996;335:124–126. doi: 10.1056/NEJM199607113350209. [DOI] [PubMed] [Google Scholar]
- 26.Miller LG, Panosian CB. Ataxia and slurred speech after artesunate treatment for falciparum malaria. N Engl J Med. 1997;336:1328. doi: 10.1056/NEJM199705013361818. [DOI] [PubMed] [Google Scholar]
- 27.Davis TME, Edwards GO, McCarthy JS. Artesunate and cerebellar dysfunction in falciparum malaria. N Engl J Med. 1997;337:792. doi: 10.1056/NEJM199709113371116. [DOI] [PubMed] [Google Scholar]
- 28.Gachot B, Eliaszewicz M, Dupont B. Artesunate and cerebellar dysfunction in falciparum malaria. N Engl J Med. 1997;337:792–793. [PubMed] [Google Scholar]
- 29.Miller LG, Panosian CB. Artesunate and cerebellar dysfunction in falciparum malaria—reply. N Engl J Med. 1997;337:793. doi: 10.1056/NEJM199709113371116. [DOI] [PubMed] [Google Scholar]
- 30.Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf. 1996;14:131–145. doi: 10.2165/00002018-199614030-00001. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. The role of artemisinin and its derivatives in the current treatment of malaria (1994–95) 1994.
- 32.Chi HT, Ramu K, Baker JK, et al. Identification of the in vivo metabolites of the antimalarial arteether by thermospray high-performance liquid chromatography/mass spectrometry. Biol Mass Spectrom. 1991;20:609–628. doi: 10.1002/bms.1200201006. [DOI] [PubMed] [Google Scholar]
- 33.Maggs JL, Madden S, Bishop LP, O’Neill PM, Park BK. The rat biliary metabolites of dihydroartemisinin, an antimalarial endoperoxide. Drug Metab Dispos. 1997;25:1200–1204. [PubMed] [Google Scholar]
- 34.Ramu K, Baker JK. Synthesis, characterization, and antimalarial activity of the glucuronides of the hydroxylated metabolites of arteether. J Med Chem. 1995;38:1911–1921. doi: 10.1021/jm00011a011. [DOI] [PubMed] [Google Scholar]
- 35.Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumming JN, Ploypradith P, Posner GH. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism (s) of action. Adv Pharmacol. 1996;37:253–297. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 37.Krungkrai SR, Yuthavong Y. The antimalarial action on Plasmodium falciparum of quinghaosu and artesunate in combination with agents that modulate oxidative stress. Trans R Soc Trop Med Hyg. 1987;81:710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 38.Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Iron-dependent free radical generation and the antimalarial artemisinin (qinghaosu) Antimicrob Agents Chemother. 1993;37:1108–1114. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brewer TG, Grate SJ, Peggins JO, et al. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 40.Kamchonwongpaisan S, McKeever P, Hossler P, Ziffer H, Meshnick SR. Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg. 1997;56:7–12. doi: 10.4269/ajtmh.1997.56.7. [DOI] [PubMed] [Google Scholar]
- 41.Petras JM, Kyle DE, Gettayacamin M, et al. Arteether: Risks of two-week administration in Macaca mulatta. Am J Trop Med Hyg. 1997;56:390–396. doi: 10.4269/ajtmh.1997.56.390. [DOI] [PubMed] [Google Scholar]
- 42.Davis TME, Breheny FX, Kendall PA, et al. Severe falciparum malaria with hyperparasitaemia treated with intravenous artesunate. Med J Austral. 1997;166:416–418. doi: 10.5694/j.1326-5377.1997.tb123192.x. [DOI] [PubMed] [Google Scholar]
- 43.Wesche DL, Da Coster MA, Tortella FC, Brewer TG. Neurotoxicity of artemisinin analogs in vitro. Antimicrob Agents Chemother. 1994;38:1813–1819. doi: 10.1128/aac.38.8.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fishwick J, McLean WG, Edwards G, Ward SA. The toxicity of artemisinin and related compounds on neuronal and glial cells in culture. Chem Biol Interact. 1995;96:263–271. doi: 10.1016/0009-2797(94)03597-2. [DOI] [PubMed] [Google Scholar]
- 45.Smith SL, Fishwick J, McLean WG, Edwards G, Ward SA. Enhanced in vitro neurotoxicity of artemisinin derivatives in the presence of haemin. Biochem Pharmacol. 1997;53:5–10. doi: 10.1016/s0006-2952(96)00591-6. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q-C. The antimalarial and toxic effects of artesunate on animal models. J Trad Chin Med. 1982;22:99–103. [PubMed] [Google Scholar]
- 47.China Co-operative Group on Qinghaosu and its derivatives as antimalarials. Chemical studies on qinghaosu (artemisinin) J Trad Chin Med. 1982. pp. 3–8. [PubMed]
- 48.Smith SL, Maggs JL, Edwards G, Ward SA, Park BK, McLean WG. The role of iron in neurotoxicity—a study of novel antimalarial drugs. Neurotoxicol. 1998 in press. [PubMed] [Google Scholar]
- 49.Buettner GR. The reaction of superoxide, formate radical, and hydrated electron with transferrin and its model-compound, Fe(III)-ethylenediamine- N,N′-bis[2-(2-hydroxyphenyl) acetic acid] as studied by pulse-radiolysis. J Biol Chem. 1987;262:11995–11998. [PubMed] [Google Scholar]
- 50.Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salt. Proc R Soc A Math Phys Sci. 1934;147:332–351. [Google Scholar]
- 51.Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salt is a feasible source of hydroxyl radicals in vivo. Biochem J. 1982;205:461–462. doi: 10.1042/bj2050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posner GH, Park SB, Gonzalez L, et al. Evidence for the importance of high valent Fe = O and of a diketone in the molecular mechanism of action of antimalarial trioxane analogs of artemisinin. J Am Chem Soc. 1996;118:3537–3538. [Google Scholar]
- 53.O’Neill PM, Bishop LP, Searle NL, et al. The biomimetic iron-mediated degradation of arteflene (Ro-42–1611), an endoperoxide antimalarial: implications for the mechanism of antimalarial activity. Tetrahedron Lett. 1997;38:4263–4266. [Google Scholar]
- 54.Baker JK, Yarber RH, Hufford CD, Lee IS, Elsohly HN, McChesney JD. Thermospray mass-spectroscopy high-performance liquid-chromatographic identification of the metabolites formed from arteether using a rat-liver microsome preparation. Biomed Environ Mass Spectrom. 1989;18:337–351. doi: 10.1002/bms.1200180509. [DOI] [PubMed] [Google Scholar]
- 55.Ullrich V, Brugger R. Prostacyclin and thromboxane synthase—new aspects of hemethiolate catalysis. Ange Chem Int Ed Engl. 1994;33:1911–1919. [Google Scholar]
- 56.White NJ. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans R Soc Trop Med Hyg. 1994;88:S41–S43. doi: 10.1016/0035-9203(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 57.Thomas CG, Ward SA, Edwards G. Selective determination, in plasma, of artemether and its major metabolite, dihydroartemisinin, by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1992;583:131–136. doi: 10.1016/0378-4347(92)80355-t. [DOI] [PubMed] [Google Scholar]
- 58.Burgess JR, Yang H, Chang M, Rao MK, Tu CP, Reddy CC. Enzymatic transformation of PGH2 to PGF2-alpha catalysed by glutathione transferases. Biochem Biophys Res Commun. 1987;142:441–447. doi: 10.1016/0006-291x(87)90294-4. [DOI] [PubMed] [Google Scholar]
- 59.Gutteridge JMC. Metalloproteins as donors of metal ions for oxygen chemistry. In: Davies KJA, editor. Oxidative Damage and Repair. Oxford: Pergamon Press; 1990. pp. 355–363. [Google Scholar]
- 60.Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 61.Yoshida T, Tanaka M, Sotomatsu A, Hira S. Activated microlgia cause superoxide-mediated release of iron from ferritin. Neuroscience Lett. 1995;190:21–24. doi: 10.1016/0304-3940(95)11490-n. [DOI] [PubMed] [Google Scholar]
- 62.Koeppen AH, Dickson AC, McEvoy JA. The heterogeneous distribution of brain transferrin. J Neuropathol Exp Neurol. 1995;54:395–403. doi: 10.1097/00005072-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Connor JR, Pavlick G, Karli D, Menzies SL, Palmer C. A histochemical study of iron-positive cells in the developing rat brain. J Comp Neurol. 1995;355:111–123. doi: 10.1002/cne.903550112. [DOI] [PubMed] [Google Scholar]









