Abstract
Aims
Single dose pharmacokinetics and safety of irbesartan, an angiotensin II receptor antagonist, were evaluated in healthy young and elderly male and female subjects.
Methods
Irbesartan was administered as two 25 mg capsules after a 10 h fast to 12 young men, 12 young women, 12 elderly men and 12 elderly women. Serial blood and urine sample were collected up to 96 h after the dose. Plasma and urine samples were analysed for irbesartan by h.p.l.c./fluorescence methods.
Results
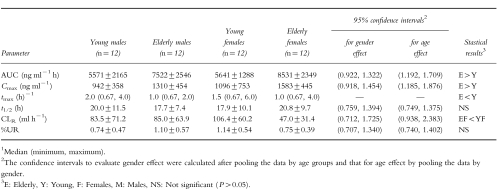
No statistically significant gender effects were observed in peak plasma concentration (Cmax), area under the curve (AUC), and terminal elimination half-life (t1/2) of irbesartan. The geometric mean AUC and Cmax increased by about 43% and 49%, respectively, in the elderly subjects. Also the time to peak was significantly shorter in the elderly subjects compared with that observed in the young subjects. Renal clearance of irbesartan was significantly reduced in the elderly females but this reduction is not likely to be of any clinical relevance since less than 3% of the administered dose of irbesartan is excreted unchanged in the urine.
Conclusions
Although there was an effect of age on the pharmacokinetics of irbesartan, based on the safety and efficacy profile, no adjustment in irbesartan dosage is necessary with respect to age or gender.
Keywords: age, gender, irbesartan, pharmacokinetics
Introduction
The recent development of several imidazole angiotensin II receptor antagonists offers several advantages over ACE inhibitors in the treatment of hypertension [1–3]. Irbesartan, is a potent, long-acting angiotensin II receptor antagonist (AT1 subtype) [4] that effectively decreases blood pressure in a dose-dependent manner in patients with mild-to-moderate hypertension [5, 6] and exhibits linear kinetics over the therapeutic dose range [7]. The principal objective of the study was to assess the effects of age and gender on the single dose pharmacokinetics of irbesartan.
Methods
Subjects
A total of 48 healthy subjects participated in the study after giving a written informed consent. The criteria for selecting subjects were no history or evidence of heart disease, renal or hepatic disease, pulmonary obstructive disease, bronchial asthma or hypertension. Subjects with a history of drug or alcohol use were excluded from the study. Pregnant, nursing women or those using oral contraceptive drugs were not included in the study. Subjects with blood pressure < 90/60 mmHg or >140/100 mmHg, or pulse rates < 50 beats min−1 or >90 beats min−1 were also excluded. The mean (s.d.) body weight (kg) and age (years) of young males were 76 (7.5) and 30 (6.7), respectively, and those of young females were 61 (9.8) and 31 (6.8), respectively. Corresponding values for elderly males were 71 (9.5) and 72 (3.5) and those for elderly females were 61 (9.8) and 69 (4.3), respectively.
Study design
This was an open-label, single dose, parallel group design. The study protocol was approved by the Institution Review Board of the Besselaar Clinical Research Units, West Palm Beach, FL. Each subject received a single 50 mg dose (2 × 25 mg capsules) of irbesartan with 250 ml water after an overnight (10 h) fast. The subjects continued to fast for 4 h after administration. Serial blood and urine samples were collected for 96 h after administration for analysis of unchanged irbesartan. Vital sign measurements were taken during the entire study; clinical laboratory tests and physical examinations were also performed to monitor subjects’ safety.
Sample collection and handling
Approximately 10 ml blood was collected into Vacutainers® (Becton and Dickinson, Rutherford, NJ) containing tripotassium ethylenediamine tetraacetic acid (K3EDTA) as an anticoagulant. Blood samples were collected before and at 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 30, 36, 48, 60, 72, 84, and 96 h after dose administration. The plasma was separated and transferred to screw-capped polypropylene tube and stored at or below −20° C until analysis.
Urine samples were collected over the following specified time intervals: predose, and 0–4, 4–8, 8–12, 12–24, 24–36, 36–48, 48–60, 60–72 and 72–96 h after administration. Each urine sample consisted of the total urine voided over the specified collection interval. At the end of each interval, a 5 ml aliquot was transferred to a prelabeled storage tube and frozen at or below −20° C until analysis.
Sample assay methods
Plasma and urine samples were analysed fro irbesartan by a validated h.p.l.c./fluorescence procedure [8]. The lower limit of quantification (LLQ) was established at 1 ng ml−1. The standard curve range was 1–1000 ng ml−1 for plasma and 1–500 ng ml−1 for urine. Plasma and urine quality control (QC) samples were prepared before the study began and stored along with the study samples. The overall within- and between-run precision for the plasma and urine QCs was less than 6% and 10% relative standard deviation, respectively. The mean predicted concentrations of the plasma and urine QC samples deviated by less than 5% and 3% from the nominal concentrations, respectively.
Pharmacokinetic analyses
The plasma concentration vs time data were analysed by using noncompartmental pharmacokinetic methods [9, 10]. The area under the plasma concentration-time curve (AUC) was calculated using a combination of the linear and log-trapezoidal rules. The AUC was extrapolated to infinity and expressed as AUC. The highest observed concentration and the corresponding sampling time were defined as Cmax and tmax, respectively. The terminal elimination half-life (t1/2) was determined from the regression line that best fit the terminal portion of the concentration-time curve. Renal clearance (CLR) was calculated as the ratio of amount excreted in urine divided by the AUC up to the same time point. Percent of dose in urine (%UR) was calculated as the cumulative amount (mg) in urine divided by the dose of irbesartan.
Statistical methods
Statistical analyses were carried out using SAS/STAT® software version 6.07 (SAS Institute, Cary, NC). The pharmacokinetic parameters of irbesartan (Cmax, AUC, tmax, t1/2, %UR and CLR) were evaluated using an analysis of variance (anova). The analysis included factors for age, gender, and age-by–gender interaction. A priori, the variables Cmax, and AUC were log transformed and tmax was rank transformed. All tests of statistical hypotheses were carried out at the two-tailed 5% significance level and all interval estimates are two-sided and constructed with 95% confidence intervals.
Results
Safety
No clinically significant abnormalities or changes in vital signs were observed after receiving irbesartan. A total of 19 episodes of adverse events were reported by 13 subjects (i.e. 27% of the total subjects). The most frequently reported adverse event was headache. A total of six subjects reported headache. The second most commonly reported adverse event was muscular/skeletal pain, reported by 6% of subjects. Other events reported were dizziness, somnolence, pharyngitis, and upper respiratory infection (2% of the total subjects per event). The investigator judged all the adverse events to be mild in terms of severity.
Pharmacokinetics of irbesartan
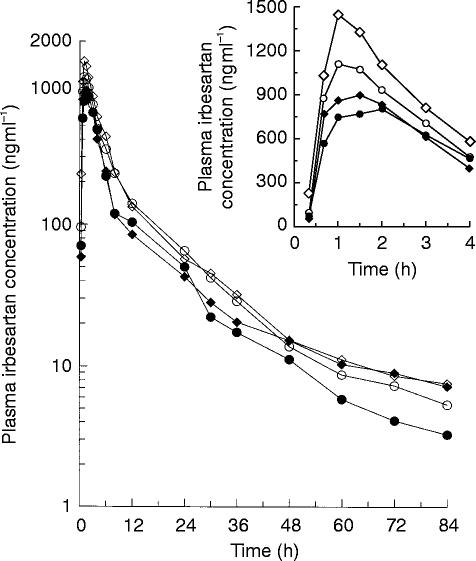
The mean plasma concentration vs time profiles are depicted in Figure 1. Mean (s.d.) pharmacokinetic parameters for irbesartan are summarized in Table 1. No statistically significant gender effects, in either young or elderly subjects, were observed for AUC, Cmax, tmax, and t1/2 of irbesartan. The mean AUC and Cmax were significantly (P ≤ 0.001) greater in elderly subjects. The geometric mean AUC and Cmax were about 43% and 49% greater, respectively, in elderly subjects. The median tmax value was significantly (P = 0.001) shorter in the elderly subjects. The t1/2 values were not influenced by age or gender. For variables percentage UR and CLR, considerable age-by–gender interactions were observed. For CLR, a statistically significant age effect was observed only in females. There was about a 56% reduction in the mean CLR in the elderly females compared with the young females.
Figure 1.
Mean plasma concentration-time profiles of irbesartan in young male (–•–) and female (–✦–) subjects, and elderly male (–○–) and female (–◊–) subjects following a single 50 mg oral dose. Inset: Mean plasma concentration-time profiles for the first 4 h after dosing on a rectilinear scale.
Table 1.
Mean±s.d. pharmacokinetic parameters of irbesartan in healthy young and elderly subjects.
Discussion
Approximately 60% of the elderly are hypertensive and women comprise about 58% of the total hypertensive population [11]. This study was designed to provide information on the safety and single dose pharmacokinetics of irbesartan, an angiotensin antagonist, in young and elderly male and female subjects.
There were no clinically important differences in the severity or frequency of the adverse events among the four demographic groups. Irbesartan was well tolerated by the elderly as well as the young male and female subjects.
Irbesartan is nearly completely absorbed following oral administration with an average absolute bioavailability of 60–80% [12]. About 25% of the administered radioactive dose is excreted in the urine and the remainder is eliminated in faeces [12]. Several determinants of drug disposition such as gastric acidity, gastric motility, glomerular filtration rate, plasma albumin, renal and hepatic blood flow are altered in the elderly subjects [13]. It is possible that one or more factors might have contributed towards the observed significant increases in the Cmax and AUC of irbesartan. A significant reduction in the CLR of irbesartan of about 56% was also observed in the elderly vs younger females but not in elderly males. However, this reduction is not likely to be of clinical relevance since < 3% of the administered dose of irbesartan is excreted unchanged in urine. Also, results obtained in renally impaired patients indicated that renal impairment had no clinically important effects on the pharmacokinetics of irbesartan [14].
Furthermore, the results obtained in two multicentre, double-blind, placebo-controlled studies in a total of 889 patients with mild-to-moderate hypertension receiving either placebo or irbesartan (1 mg to 300 mg) have clearly shown that the reductions from baseline in trough blood pressure were of similar magnitude for the subgroups of gender and age [15]. Irbesartan demonstrated an excellent tolerability profile with no evidence of dose-related toxicity in these two studies. Therefore, based on the safety and efficacy profile of irbesartan, no adjustment in irbesartan dosage is necessary with respect to age or gender.
Acknowledgments
The authors would like to acknowledge S. Chang for skilful bioanalytical assistance.
References
- 1.Johnston CJ. Angiotensin receptor antagonists: focus on losartan. Lancet. 1995;346:1403–1407. doi: 10.1016/s0140-6736(95)92411-6. [DOI] [PubMed] [Google Scholar]
- 2.Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 3.Eberhardt RT, Kevak RM, Kang PM, Frishman WH. Angiotensin II receptor blockade: an innovative approach to cardiovascular pharmacotherapy. J Clin Pharmacol. 1993;33:1023–1038. doi: 10.1002/j.1552-4604.1993.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 4.Cazaubon C, Gougat J, Bousquet F, et al. Pharmacological characterization of SR 47436, a new nonpeptide AT1 subtype angiotensin II receptor antagonist. J Pharmacol Exp Ther. 1993;265:826–834. [PubMed] [Google Scholar]
- 5.Van den Meiracker AH, Admiraal PJ, et al. Hemodynamic and biochemical effects of the AT1 receptor antagonist irbesartan in hypertension. Hypertension. 1995;25:22–29. doi: 10.1161/01.hyp.25.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Marino MR, Langenbacher KM, Raymond RH, Whigan D, Ford NF. Pharmacokinetics (PK) and antihypertensive effects of irbesartan (an AII receptor antagonist) in subjects with hypertension. J Hypertens. 1996;14:348. [Google Scholar]
- 7.Marino MR, Langenbacher KM, Ford NF, Uderman HD. Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol. 1998;38:246–255. doi: 10.1002/j.1552-4604.1998.tb04422.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang S-Y, Whigan DB, Vachharajani NN, Patel R. A high-performance liquid chromatography assay for quantitation of irbesartan (SR 47436/BMS-186295) in human plasma and urine. J Chromagr B. 1997;702:149–155. doi: 10.1016/s0378-4347(97)00387-3. [DOI] [PubMed] [Google Scholar]
- 9.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York, NY: Marcel Dekker, Inc.; 1982. pp. 409–417. [Google Scholar]
- 10.Riegelman S, Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokin Biopharm. 1980;8:509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- 11.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: Results from the third national health and nutrition examination survey, 1988–91. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 12.Vachharajani NN, Shyu WC, Chando TJ, Everett DW, Greene DS, Barbhaiya RH. Oral bioavailability and disposition characteristics of irbesartan, an angiotensin antagonist, in man. J Clin Pharmacol. 1998;38:702–707. doi: 10.1002/j.1552-4604.1998.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 13.Ritschel WA. Gerentokinetics. Caldwell, NJ: Telford Press; 1988. [Google Scholar]
- 14.Sica DA, Marino MR, Hammett JL, Ferreira I, Ford NF. Pharmacokinetics of irbesartan are not altered by renal impairment or hemodialysis. Clin Pharmacol Ther. 1997;62:902–909. doi: 10.1016/S0009-9236(97)90080-1. [DOI] [PubMed] [Google Scholar]
- 15.Pool JL, Guthrie RM, Littlejohn TN, et al. Dose-related antihypertensive effects of irbesartan in patients with mild-to-moderate hypertension. Am J Hypertens. 1998;11:462–470. doi: 10.1016/s0895-7061(97)00501-3. [DOI] [PubMed] [Google Scholar]