Abstract
Aim
The aim of the study was to examine the pharmacokinetic and pharmacodynamic profiles of single doses of warfarin (25 mg) following administration alone, and in combination with multiple doses of donepezil HCl (10 mg day−1) in healthy volunteers.
Methods
This was an open-label, two-period crossover study in 12 healthy male volunteers, aged 18–55 years, who were randomized to one of the following treatment groups: (A) donepezil administered for 19 consecutive days with a single dose of warfarin administered on day 14. On day 20, there was a 21-day washout period after which a single dose of warfarin was again administered, and (B) an initial 13-day period with no medication, then a single dose of warfarin administered alone on day 14, followed by a 14-day washout period. Donepezil was then administered for 19 days (to day 47), with an additional single dose of warfarin administered on day 41. Serial blood samples were collected over 144 h following both warfarin administrations in each treatment group. Pharmacokinetic parameters were assessed for both (R)- and (S)-warfarin concentrations in plasma, and pharmacodynamic analyses utilizing prothrombin time were undertaken. Warfarin concentrations in plasma were determined by HPLC with fluorescence detection. The pharmacokinetic parameters Cmax, AUC(0–∞), CLT/F, Vλz/F and t½ of both (R)- and (S)-warfarin, maximum prothrombin time (Rmax) and the area under the prothrombin–time curve (AUCPT), were compared in the presence and absence of donepezil by analysis of variance (ANOVA).
Results
No statistically significant differences in (R)- or (S)-warfarin pharmacokinetics were observed when warfarin administered alone was compared to warfarin administered concurrently with donepezil. Warfarin pharmacodynamic parameters, Rmax and AUCPT, were also unchanged by concomitant administration of donepezil. No clinically significant changes in vital signs, ECG parameters or clinical laboratory tests were observed.
Conclusions
Concurrent administration of donepezil HCl does not alter the pharmacokinetic or pharmacodynamic profile of single doses of warfarin in healthy volunteers. These findings suggest that donepezil may be safely co-administered with warfarin without the need for dose modification.
Keywords: donepezil, warfarin, drug–drug interaction, acetylcholinesterase inhibitor
Introduction
Donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan) is a chemically distinct and well-tolerated inhibitor of the enzyme acetylcholinesterase (AChE). It is used clinically for the symptomatic treatment of mild–moderate Alzheimer’s disease. Current theories attribute the pathogenesis of the cognitive symptoms of Alzheimer’s disease to a deficiency in levels of the neurotransmitter acetylcholine (ACh) and a decrease in cholinergic neurotransmission. Donepezil is postulated to produce its therapeutic effect by increasing synaptic concentrations of ACh, via the hydrolysis of AChE, thereby enhancing neurotransmission [1–3]. Clinical studies have shown that once-daily administration of donepezil can significantly improve both cognitive and global function in patients with Alzheimer’s disease [4–7].
Warfarin is a coumarin anticoagulant that is widely used in the treatment and prophylaxis of atrial fibrillation, stroke, arterial and venous thrombosis [8–10]. The clinical effects of warfarin may be modified by several drugs. For example, the hypo-prothrombinaemic effects of warfarin may be increased by concomitant administration with chloral hydrate [11, 12], and marked increases in prothrombin times have been observed in patients taking phenylbutazone in addition to warfarin [13, 14]. Such interactions have historically been attributed to plasma protein binding displacement, an effect that is potentially relevant for drugs such as warfarin and donepezil that are highly protein bound (99% [15] and 95.6% [16], respectively). However, displacement from plasma proteins is rarely responsible for sustained changes in the plasma concentration of unbound drug, particularly for drugs that are restrictively cleared, such as warfarin, as the concentration increase caused by displacement is buffered by redistribution [17, 18]. Thus, although drug displacement does occur under certain circumstances for a number of highly protein-bound drugs, the effects of drug interactions are generally the result of other processes. For instance, although phenylbutazone displaces warfarin from plasma proteins, observed changes in the pharmacodynamic response of warfarin are due to inhibition of the metabolism of both its R- and, less potent, S-isomers [19, 20]. Similarly, the co-administration of non-steroidal anti-inflammatory agents causes the displacement of warfarin from plasma proteins and stereoselective inhibition of the metabolism of its (S)-enantiomer [21, 22].
Concomitant administration of warfarin and donepezil probably results in displacement from plasma protein binding, but this alone is unlikely to produce any clinically relevant effects. Nevertheless, the possibility of other interactions between these two drugs that are clinically relevant cannot be ruled out. With this in mind, the present study was undertaken to examine the effects of multiple-dose administration of donepezil HCl (to approximate steady state) on the pharmacokinetic and pharmacodynamic profiles of warfarin.
Methods
The study was conducted in accordance with Good Clinical Practice Guidelines issued by the European Commission (1990). The protocol was approved by an independent ethics committee and written informed consent was obtained from each volunteer prior to entry.
Subjects
The study population comprised healthy male volunteers between 18 and 55 years of age who were within 15% of normal weight for their height and body build based on the Metropolitan Insurance Company Height and Weight Tables (1983), and who were non-smokers. None of the subjects had evidence of clinically significant disease of any major organ system. Those with a history of severe adverse drug reactions or leucopenia, severe or multiple allergies, or habitual drug and/or alcohol abuse were specifically excluded from entering the trial, as were those who had participated in other clinical trials in the previous 3 months.
Protocol
This was an open-label, randomized, two-period, balanced, crossover study. Subjects who had fulfilled the entry criteria at a screening visit, undertaken ≤2 weeks prior to entry, were randomized to one of two treatment groups, A or B. Group A received donepezil for 19 days, at a dose of 5 mg once daily for the first week and 10 mg once daily thereafter. A single 25 mg dose of warfarin (5×5 mg Marevan® tablets, Evans Medical) was administered on day 14 followed by 7 days of blood sampling. The subjects then completed a 3-week washout period, at the end of which they received another single 25 mg dose of warfarin. They then returned to the clinic for an additional 7 days for blood sampling.
Treatment group B received no medication for the first 13 days of the trial. A single dose of 25 mg warfarin was administered on day 14 followed by serial blood sampling for 7 days. After a further 7 days of washout, on day 28, subjects began 19 days of treatment with donepezil (5 mg once daily for the first week; 10 mg once daily thereafter), until day 47. A second dose of 25 mg of warfarin was given on day 41 (14 days after the start of donepezil treatment, as for group A) and blood sampling continued until day 47.
All study medication was taken at 08:00 h. Subjects were not allowed to take any other medication, including prescription, non-prescription or investigational preparations during the study. Alcohol and caffeine-containing drinks and food were avoided from 12 h prior to the start until completion of each study period.
Sample collection and analysis
Warfarin was administered on days 14 and 41 of each treatment schedule. Blood samples for the determination of plasma levels of the (R)- and (S)-enantiomers were obtained just prior to drug administration and at 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 96, 120 and 144 h after drug administration.
Warfarin was analysed in plasma after solvent extraction followed by the formation of the diastereoisomers (R)- and (S)-warfarin. The enantiomers were separated on a silica high-performance liquid chromatography (HPLC) column, with post-column aminolysis and fluorescence detection. The lower limit of quantification for each of the diastereoisomers was 0.15 mg l−1. The coefficients of variation were < 15% for (R)-warfarin and < 10% for (S)-warfarin. The extent of bias at each concentration of the two enantiomers was ≤11%.
Pharmacokinetic assessments
Pharmacokinetic parameters were estimated separately for (R)-warfarin and (S)-warfarin. Peak plasma concentration (Cmax) and the time at which it occurred (tmax) were obtained from visual inspection of the plasma concentration–time curves. The terminal elimination rate constant (λz) was estimated using Siphar Version 4.0 software (Simed, Créteil, France). Exact sampling times were used in the calculations.
Total area under the plasma concentration–time curve (AUC(0–∞)) from time zero extrapolated to infinity was calculated as the sum of AUC(0–144), the area up to the last quantifiable time-point (144 h), and the area from the 144-h time-point extrapolated to infinity. AUC(0–144) was estimated by the trapezoidal approximation, and the extrapolated area was calculated from the final measurable concentration divided by λz.
The terminal half-life (t½) was calculated as 0.693/λz, and the total apparent oral clearance (CLT/F) was calculated as dose divided by AUC(0–∞). The apparent volume of distribution (Vλz/F) was calculated as the ratio of CLT/F to λz, where the factor F accounts for bioavailability.
Pharmacodynamic assessments
Blood samples for the determination of prothrombin times (Quick method) were taken at 0, 4, 12, 24, 36, 48, 60, 72, 96, 120 and 144 h after administration of warfarin. The maximum prothrombin time (Rmax) and the time of Rmax (tmax) were direct experimental observations. The area under the prothrombin time–time curve over the 144-h post-dose period (AUCPT) was estimated using the trapezoidal approximation. Prothrombin times are often reported as International Normalized Ratios (INR), i.e. the ratio of the patient prothrombin time to the control prothrombin time, raised to the power of the International Sensitivity Index (ISI). The ISI is the index of the reference thromboplastin used for the test compared with the World Health Organization reference reagent [23]. In this way, direct comparisons between laboratories are possible. However, in this case, all blood samples for prothrombin times were analysed at one time using a single batch of thromboplastin. Therefore, since the control prothrombin times were the same for all samples, the INR and prothrombin values are proportional. Thus, it was not necessary to report the results as INR in this study, although the INR values are indicated on the prothrombin time curve.
Safety assessments
Safety was assessed by routine haematology and biochemistry at screening, prior to warfarin administration and at the post-study visit. Vital signs were monitored at entry, prior to drug administration on each dosing day, at 4, 8, 12, 24, 36, 48, 60, 72, 96, 120 and 144 h after dosing with warfarin in each study period, and at the post-study visit. Blood pressure measurements were made in accordance with the British Hypertension Society guidelines. Readings were taken after the subjects had been sitting for 5 min and standing for 2 min. Korotkoff’s phase V sound disappearance was used to determine diastolic blood pressure. Twelve-lead ECG monitoring was performed at screening and at the study termination visit. All adverse events experienced during the course of this study were recorded, together with their times of onset and cessation, and an assessment of their severity and causality.
Statistical analysis
Analyses of variance (ANOVA) were used to compare the pharmacokinetic (Cmax, AUC(0–∞), CLT/F and Vλz/F) and pharmacodynamic (Rmax and AUCPT) parameters following administration of warfarin alone, and in the presence of donepezil. Each set of variables was log-transformed prior to analysis. The analysis of variance was undertaken using the GLM procedure of the SAS for Windows software version 6.10 (SAS Institute Inc., Cary, North Carolina, USA). It included factors for subject, treatment, period and treatment-by-period interaction. The Wilcoxon rank sums test was used to compare tmax following administration of warfarin alone and in the presence of donepezil.
Results
Subjects
Thirteen healthy male volunteers with a median age of 28 years (range 18–55 years) entered the study. Their body weights ranged from 64 to 88 kg (median 72 kg) and their heights from 166 to 191 cm (median 175 cm). Twelve subjects completed the study; one was withdrawn due to adverse events that included insomnia, headache and nausea.
Pharmacokinetics of warfarin
The mean pharmacokinetic parameters for (R)- and (S)-warfarin alone, and in the presence of donepezil, averaged over all 12 subjects in both treatment groups are summarized in Table 1. No statistically significant differences in warfarin pharmacokinetics were observed whether warfarin was administered alone or concurrently with donepezil. When warfarin was administered in the absence of donepezil, (R)-warfarin was seen to have a longer t½ than (S)-warfarin (53.0±14.0 versus 37.0±35.0 h, respectively), and a greater AUC(0–∞) (68.0±15.0 versus 51.0±59.0 mg h l−1, respectively). However, values for both enantiomers were not significantly different in the presence of donepezil.
Table 1.
Pharmacokinetic parameters for warfarin following a single dose (25 mg) administered alone and after 14 days of treatment with donepezil (mean±SD).
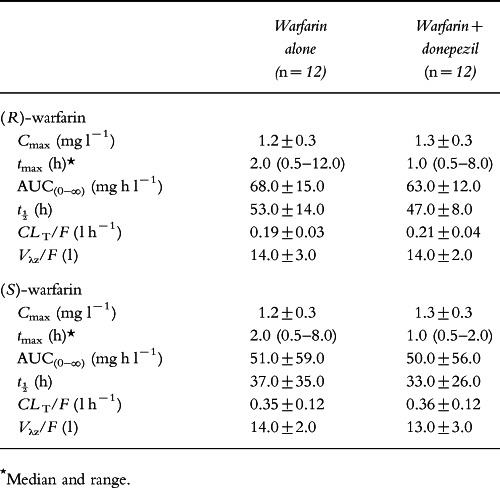
As can be seen in Figure 1, the plasma concentration–time profiles for the (R)- and (S)-warfarin enantiomers in the presence and absence of donepezil are almost superimposable. The 90% confidence intervals for the ratios of Cmax and AUC(0–∞) for (R)- and (S)-warfarin in the presence of donepezil to their respective values for each enantiomer alone were close to one (Table 2). The seemingly high inter-subject variability for the t½ and AUC(0–∞) for (S)-warfarin (Table 1) resulted from an unusually long t½ of 115 h in one of the subjects. This occurred in both treatment phases and may be due to atypical warfarin metabolism by this subject. Analysis of variance detected a statistically significant period effect for the AUC(0–∞) of (S)-warfarin (P = 0.007), but there was no accompanying treatment-by-period interaction (P = 0.576).
Figure 1.
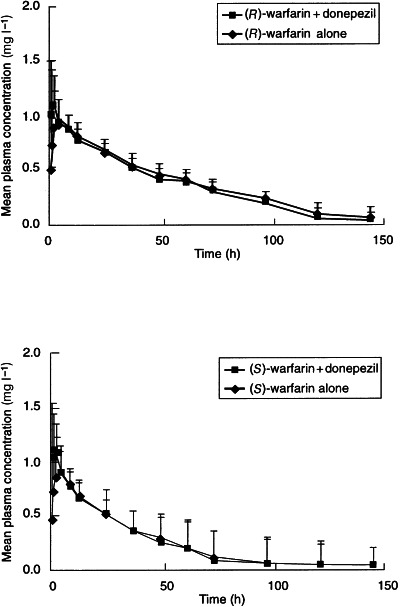
Mean (+SD) plasma concentration–time curves for (R)- and (S)-warfarin following a single dose of warfarin (25 mg) administered alone and after 14 days of treatment with donepezil.
Table 2.
The mean ratios (90% confidence intervals) of Cmax and AUC(0–∞) for (R)- and (S)-warfarin in the presence of donepezil to their respective values for (R)- and (S)-warfarin alone (n = 12).

Pharmacodynamics of warfarin
Measured values of prothrombin times are summarized in Table 3, and the effect–time curves are shown in Figure 2. The pharmacodynamic parameters for warfarin (Rmax and AUCPT) were virtually identical in the presence and absence of donepezil. Rmax values were 30.0±9.0 and 29.0±8.0 s, respectively, and AUCPT values were 3063±581 and 3032±602 s h, respectively. Furthermore, the prothrombin time curves for warfarin alone and for warfarin administered with donepezil were virtually super-imposable (Figure 2).
Table 3.
Pharmacodynamic parameters for racemic warfarin following a single dose of warfarin (25 mg) administered alone, and after 14 days of treatment with donepezil (mean±SD).
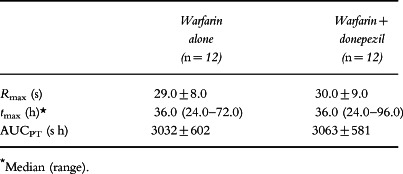
Figure 2.
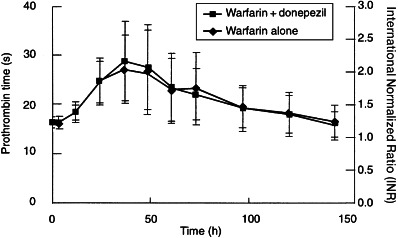
Effect of racemic warfarin on prothrombin time or international normalized ratio (mean±SD) in the presence and absence of donepezil.
No statistically significant treatment effects were detected by analysis of variance. The mean ratio of Rmax for warfarin in the presence of donepezil relative to that for warfarin alone was 1.02 (90% confidence interval 0.96–1.08). The corresponding mean ratio for AUCPT was 1.01 (90% confidence interval 0.99–1.03). Interestingly, the subject who had raised exposure to (S)-warfarin, as reflected by an AUC(0–∞) of 226.8 mg h l−1, had a prothrombin time (Rmax = 29.5 s) close to the mean value for the group. Statistically significant period effects were observed for Rmax (P = 0.005) and AUCPT (P < 0.001), but in neither case was there an accompanying treatment-by-period interaction.
Safety
Both treatments were well tolerated and there were no clinically significant changes in vital signs, clinical laboratory or ECG parameters during the course of this study. Those adverse events that were reported were transient and dissipated with continued drug administration. All were mild or moderate in intensity. These included reports of nausea (n = 18), vomiting (n = 8) and headache (n = 7).
Discussion
In this study, the pharmacokinetics and pharmacodynamics of single doses of warfarin (25 mg), administered alone, and following multiple-dose administration (to approximate steady state) of donepezil HCl (10 mg day−1) in healthy volunteers were examined.
No statistically significant differences in the pharmacokinetic parameters (Cmax, tmax, AUC(0–∞), CLT/F, Vλz/F or t½) of either (R)- or (S)-warfarin were observed when warfarin administered alone was compared with warfarin administered concomitantly with donepezil. Furthermore, the plasma concentration–time profiles for both enantiomers of warfarin alone, and in the presence of donepezil, were almost superimposable. The pharmacokinetic parameters observed for warfarin in this study are in agreement with those reported elsewhere [15], with (R)-warfarin having a somewhat longer half-life than (S)-warfarin (53 h versus 37 h, respectively). No subject had a Cmax for either warfarin enantiomer that exceeded 1.8 mg l−1. Thus, in every subject, the total warfarin Cmax (Cmax of (R)-warfarin plus Cmax of (S)-warfarin) following single-dose administration was well within the therapeutic window of 1.8–2.6 mg l−1. The stable pharmacokinetic profile of warfarin in the presence of donepezil also resulted in almost identical prothrombin times.
Although interactions between warfarin and several other drugs have been identified [11–14, 19–22], this study shows that the pharmacokinetics and pharmacodynamics of warfarin in healthy male volunteers are unaffected by the co-administration of donepezil. This does not indicate that no displacement of warfarin from plasma proteins has occurred. Indeed, as both donepezil and warfarin are highly protein-bound, concomitant administration of these drugs probably does result in protein displacement interactions. However, as outlined above, displacement from plasma protein binding results in only transient increases in the plasma concentration of unbound drug, and so is rarely associated with clinically relevant drug interactions. Rather, the unaltered pharmacodynamic and pharmacokinetic profile of warfarin on co-administration with donepezil suggests that the latter does not interfere with warfarin’s cytochrome P-450-mediated metabolism [24]. This is consistent with the results from in vitro isoform-selective substrate studies conducted in human liver microsomes (Aricept® US package insert, 1998). Determination of the donepezil plasma concentrations required for 50% inhibition (IC50) of CYP-450 enzymes 1A2, 2C9, 2C19, 2D6, and 3A4 showed that all IC50 values were greater than 100 μm. In addition, the mean ki values for CYP-3A4 and CYP-2D6 were calculated and were found to be 131 μm and 47 μm, respectively. Clinical studies have shown that steady-state Cmax concentrations for the 10 mg dose of donepezil are approximately 164 nm (data on file, Eisai Inc.). Since it is anticipated that therapeutic concentrations of donepezil are more than 280-fold lower than the lowest ki value obtained with CYP-2D6, and almost 800-fold lower than the ki observed with CYP-3A4, it is likely that donepezil will not inhibit the metabolism of other drugs metabolized by these or any other CYP-450 isoenzymes.
The results presented in this report demonstrate that no pharmacokinetic or pharmacodynamic interactions occur between donepezil and either (R)- or (S)-warfarin following single-dose (oral) administration of warfarin in the presence of steady-state donepezil plasma concentrations. These results suggest that, in clinical practice, dose modifications will not be required when donepezil is co-administered with warfarin in patients with Alzheimer’s disease.
Acknowledgments
We acknowledge the efforts of Drs P. E. Rolan and S. Toon of Medeval Ltd, University of Manchester, Skelton House, Manchester Science Park, Lloyd St North, Manchester M15 6SH, UK, who conducted this clinical trial, and the Internal Ethics Committee of Medeval Ltd (same address), who reviewed and approved the study and protocol.
References
- 1.Iimura Y, Mishima M, Sugimoto H. Synthesis of 1-benzyl-4-lsqb;(5,6-dimethoxylsqb;2−14C]-1-indanon)-2-yl]-methylpiperidine hydrochloride (E2020–14C) J Label Compounds Radiopharm. 1989;XXVII:835–839. [Google Scholar]
- 2.Rogers SL, Yamanishi Y, Yamatsu K. E2020—the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–320. [Google Scholar]
- 3.Sherman K. Pharmacodynamics of oral E2020 and tacrine in humans: novel approaches. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 321–328. [Google Scholar]
- 4.Rogers SL, Friedhoff LT the Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicenter, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 5.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer’s disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SL, Friedhoff LT. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: an interim analysis of the results of a US multicentre open label extension study. Eur Neuropsychopharmacol. 1998;8:67–75. doi: 10.1016/s0924-977x(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Atwood JE, Hirsh J, Sherman DG, Hughes RA, Connolly SJ. Stroke prevention in non-valvular atrial fibrillation. Ann Intern Med. 1991;115:727–736. doi: 10.7326/0003-4819-115-9-727. [DOI] [PubMed] [Google Scholar]
- 9.Mirsen TR, Hachinski VC. Transient ischemic attacks and stroke. Can Med Assoc J. 1988;138:1099–1105. [PMC free article] [PubMed] [Google Scholar]
- 10.Anon Management of venous thromboembolism. Lancet. 1988;i:275–277. [PubMed] [Google Scholar]
- 11.Seller EM, Koch-Weser J. Potentiation of warfarin-induced hypoprothrombinemia by chloral hydrate. N Engl J Med. 1970;283:827–831. doi: 10.1056/NEJM197010152831601. [DOI] [PubMed] [Google Scholar]
- 12.Udal JA. Warfarin–chloral hydrate interaction. Pharmacological activity and clinical significance. Ann Intern Med. 1974;81:341–344. doi: 10.7326/0003-4819-81-3-. [DOI] [PubMed] [Google Scholar]
- 13.Fox S. Potentiation of anticoagulants by pyrazole compounds. JAMA. 1964;188:320–321. doi: 10.1001/jama.1964.03060290124046. [DOI] [PubMed] [Google Scholar]
- 14.Aggeler PM, O’Reilly RA, Leong L, Kowitz PE. Potentiation of anticoagulant effect on warfarin by phenylbutazone. N Engl J Med. 1967;276:496–501. doi: 10.1056/NEJM196703022760904. [DOI] [PubMed] [Google Scholar]
- 15.Majerus P, Broze GJ, Miletich JP, Tollefsen DM. Anticoagulant, thrombolytic and antiplatelet drugs. In: Gilman AG, Molinoff PB, Ruddon RW, Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Ninth. New York: McGraw-Hill; 1995. pp. 1341–1359. [Google Scholar]
- 16.Tiseo PJ, Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br J Clin Pharmacol. 1998;46(Suppl. 1):13–18. doi: 10.1046/j.1365-2125.1998.0460s1013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sansom LN, Evans AM. What is the true clinical significance of plasma protein binding displacement interactions? Drug Saf. 1995;12:227–233. doi: 10.2165/00002018-199512040-00001. [DOI] [PubMed] [Google Scholar]
- 18.Rolan PE. Plasma protein binding displacement interactions—why are they still regarded as clinically important? Br J Clin Pharmacol. 1994;37:125–128. doi: 10.1111/j.1365-2125.1994.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly RA, Trager WF, Motley CH, Howald W. Stereoselective interaction of phenylbutazone with lsqb;12C/13C] warfarin pseudoracemates in man. J Clin Invest. 1980;56:746–753. doi: 10.1172/JCI109722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banfield C, O’Reilly RA, Chan E, Rowland M. Phenylbutazone–warfarin interaction in man: further stereochemical and metabolic considerations. Br J Clin Pharmacol. 1983;16:669–675. doi: 10.1111/j.1365-2125.1983.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day RO, Graham GG, Chapman GD, Lee E. Antirheumatic drug interactions. Clin Rheum Dis. 1984;10:251–275. [PubMed] [Google Scholar]
- 22.Furst DE. Clinically important interactions of non-steroidal antiinflammatory agents with other medications. J Rheumatol. 1988;15(Suppl. 17):58–62. [PubMed] [Google Scholar]
- 23.Poller L, Hirsh J. Special report: a simple system for the derivation of International Normalized Ratios for the reporting of prothrombin time results with North American thromboplastin reagents. Am J Clin Pathol. 1989;92:124–126. doi: 10.1093/ajcp/92.1.124. [DOI] [PubMed] [Google Scholar]
- 24.Kaminsky LS, Dunbar DA, Wang PP. Human hepatic cytochrome P-450 composition as probed by in vitro microsomal metabolism of warfarin. Drug Metab Dispos. 1984;12:470–477. [PubMed] [Google Scholar]


