Abstract
Aim
The aim of this study was to investigate the metabolism and elimination of donepezil HCl in humans, following the administration of a single 5 mg (liquid) oral dose containing a mixture of unlabelled and 14C-labelled donepezil.
Methods
This was an open-label, non-randomized study in healthy male volunteers (n = 8). Characterization of donepezil metabolism and elimination was performed by analysing blood, urine and faecal samples collected over a 10-day period following drug administration. Each collected sample was assayed for total radioactivity, and aliquots from specified time-points and/or pooled samples were assayed for the presence of donepezil metabolites by thin-layer chromatography (TLC). Donepezil concentrations in plasma were determined by HPLC.
Results
Recovery of radioactivity in subject samples averaged 72% of the administered dose. Recovery of the administered dose in urine (57%) was significantly greater than that recovered in faeces (15%). Unchanged donepezil accounted for the largest component of the recovered dose in each matrix. Three metabolic pathways were identified: (i) O-dealkylation and hydroxylation to metabolites M1 and M2, with subsequent glucuronidation to metabolites M11 and M12; (ii) hydrolysis to metabolite M4; and (iii) N-oxidation to metabolite M6.
In plasma, the parent compound accounted for about 25% of the dose recovered during each sampling period, as well as of the cumulative dose recovered. The recovered residue showed higher levels of the hydroxylated metabolites M1 and M2 than of their glucuronide conjugates M11 and M12, respectively. In urine, the parent compound accounted for 17%, on average, of the dose recovered from each pooled sample, as well as of the total recovered dose. The major metabolite was the hydrolysis product M4, followed by the glucuronidated conjugates M11 and M12. In faeces, the parent compound also predominated, although it accounted for only 1% of the recovered dose. A large percentage of the radioactivity in faeces consisted of unidentified very polar metabolites, which were retained at the TLC origin. Of the extracted metabolites, the hydroxylation products M1 and M2 were the most abundant, followed by the hydrolysis product M4 and the N-oxidation product M6.
Conclusions
Donepezil is hepatically metabolized and the predominant route for the elimination of both parent drug and its metabolites is renal, as 79% of the recovered dose was found in the urine with the remaining 21% found in the faeces. Moreover, the parent compound, donepezil, is the predominant elimination product in urine. The major metabolites of donepezil include M1 and M2 (via O-dealkylation and hydroxylation), M11 and M12 (via glucuronidation of M1 and M2, respectively), M4 (via hydrolysis) and M6 (via N-oxidation).
Keywords: donepezil, metabolism, elimination, Alzheimer’s disease
Introduction
Pre-clinical metabolism and elimination studies in rats and dogs have shown that donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan) undergoes significant first-pass metabolism. Following administration of a single oral dose of 14C-donepezil in dogs, 55% of the radioactivity is present in the blood as metabolites, predominantly the O-glucuronide form, rather than intact drug (data on file, Eisai Inc.). These same studies found that faecal elimination of donepezil, accounting for 59.7% of the dose, predominated over renal excretion (39.2% of dose). Human pharmacokinetic studies confirm that donepezil undergoes first-pass hepatic metabolism following oral administration, but in contrast to findings in animal studies, the primary route of elimination in humans was renal [1, 2].
In vitro studies using human hepatic microsomes have identified the cytochrome P-450 isoenzyme CYP-3A4 as primarily responsible for the metabolism of donepezil, with CYP-2D6 playing a lesser role (Aricept® US package insert, 1998). Pharmacokinetic and clinical studies with Alzheimer’s patients indicate that the clearance of donepezil from plasma is unaffected by age, and that dose modification is not necessary in the elderly [1]. Other studies, reported in this supplement, indicate that donepezil does not interact adversely with other hepatically metabolized drugs such as cimetidine, ketoconazole, digoxin, warfarin and theophylline, and that the kinetics of single doses of donepezil are not significantly changed in patients with impaired hepatic and renal function.
Although much pharmacokinetic and pharmacodynamic data on donepezil has been collected from patients suffering from Alzheimer’s disease, as well as from normal volunteers, a thorough investigation of the metabolism and elimination of this compound has not yet been conducted. The purpose of this study was to identify and characterize the primary routes of metabolism and elimination of donepezil HCl in humans, following the administration of a single 5 mg oral dose of 14C-labelled donepezil.
Methods
Subjects
Eight healthy male subjects between 19 and 45 years of age participated in this study. Their body weights ranged from 60 to 85 kg and were within 20% of ideal weight for their height and body build based on the Metropolitan Insurance Company Height and Weight Tables (1983). None of the subjects had clinical evidence of significant gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities. Subjects who had a known or suspected history of alcohol or drug misuse or a positive urine drug screen were excluded from entry, as were those who had donated blood within 1 month of entry, or who had participated in other radiolabelled drug studies during the previous 12 months. Patients who were smokers or who were taking products containing nicotine were also excluded.
The study was conducted in accordance with the principles stated in the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board for Investigations Involving Human Subjects, Harris Laboratories, Lincoln, Nebraska, USA. All subjects gave written informed consent prior to participation.
Protocol
This was an open-label, non-randomized study. Subjects were admitted to the study site on the evening before drug administration. Baseline measurements (routine physical examination, weight and vital signs) were obtained upon admission. A urine drug screen was also performed, and baseline urine and faecal samples were obtained. The following morning, after an 8-h fast, subjects received a single 5 mg (liquid) oral dose of donepezil. This dose was a 20 ml aqueous solution containing 4.6 mg of unlabelled donepezil and 0.4 mg (50 μCi) of 14C-labelled donepezil (55 μCi mmol−1, Amersham International, UK), followed by 250 ml of tap water (three 30 ml washes of the glass vial plus 160 ml). This radiochemical form of donepezil was produced by substituting 14C at position 2 in the indanone ring. Following drug administration, the subjects remained in an upright position (>45° angle from supine) and continued to fast from food and fluids for a further 4 h. Subjects remained as in-patients for 10 days following drug administration. Urine, faecal and blood samples were collected at specified intervals throughout the 10-day period for the measurement of 14C-labelled donepezil and for metabolic profiling. The subjects were discharged from the study site on the morning of day 11, following a final discharge evaluation.
Throughout the study, subjects abstained from physical activity other than normal walking. None of the subjects had received any prescription or investigational drugs for at least 1 month prior to entering the trial. There was no consumption of over-the-counter products, recreational drugs or alcohol from 72 h prior to admission until the end of the study. In addition, all subjects abstained from caffeine-containing foods or beverages for the duration of the study.
Sample collection and analysis
Venous blood samples were collected prior to drug administration on day 1 (−1 h), and at 1, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216 and 240 h following drug administration. The samples were collected in three separate, heparinized, evacuated tubes for radioactivity counting, analysis of donepezil plasma concentrations and metabolic profiling.
Urine samples were collected continuously throughout the 10-day study period. On day 1, samples were collected in 4 h pooled aliquots, on day 2 samples were collected in 6 h pooled aliquots, and for the remaining 8 days, samples were collected in 24 h pooled aliquots.
Subjects were required to provide a baseline faecal sample at admission to the study site. During the study, faecal samples were pooled for each 24-h period. Samples were collected in tarred, wide-mouth polypropylene–polyethylene jugs and frozen at −20° C following collection. Plasma concentrations of donepezil HCl were measured using high-performance liquid chromatography (HPLC) with UV detection (315 nm) [3]. Concentrations were interpolated from calibration curves shown to be linear from 1 ng ml−1 to 100 ng ml−1. The lowest quantifiable concentration for the validated assay was 2 ng ml−1.
Pharmacokinetic analysis
Characterization of donepezil and 14C-donepezil pharmacokinetics was conducted using data from samples collected over a 144-h period following drug administration. Pharmacokinetic parameters were estimated using a non-compartmental model. The donepezil plasma concentration and total radioactivity data from each subject were plotted as log concentration–time curves. Plasma radioactivity was expressed as ng donepezil equivalents per ml (ng equiv ml−1). The peak plasma concentration (Cmax) and time to peak plasma concentration (tmax) were recorded from the observed values. The terminal disposition phase was identified by visual inspection of each subject’s semi-logarithmic plot at points between 24 h and 144 h post-dose. The terminal disposition rate constant (λz) was estimated as −2.303 times the slope of the best-fit linear regression line of the terminal phase. The plasma half-life (t½) was calculated as 0.693/λz. The area under the curve (AUC) from 0 to 144 h (AUC(0–144)) was calculated by using the trapezoidal rule and extrapolated to infinity (AUC(0–∞)), by adding the ratio of the last non-zero concentration occurring up to 144 h post-dose to λz. Clearance (CL T/F) was calculated as dose divided by the product of AUC(0–∞) and F, a factor to account for bioavailability. The apparent volume of distribution (Vλz/F) was calculated as CL T/F divided by λz.
Analysis of metabolites
Characterization of 14C-donepezil metabolism and elimination was based upon analysis of blood, urine and faecal samples collected over the 10-day period following drug administration. Each collected sample was assayed for total radioactivity. Samples from specified time intervals for each matrix were used for metabolic profiling. Faecal samples were homogenized in water (4:1 water:faeces). Urine, faecal and plasma samples from specific time intervals were pooled where indicated and stored frozen (−20° C) until metabolite profile determination.
Total radioactive residue (TRR) in each pooled sample was determined for mass balance purposes. TRR in faecal samples was determined by combustion to 14CO2. TRR in pooled urine samples was determined by liquid scintillation counting (LSC). TRR in pooled plasma samples was calculated from volumes and radioactivity concentrations determined on individual samples to conserve radioactivity for profiling. Pooled samples were extracted, hydrolysed with β-glucuronidase (containing partial sulphatase activity) and re-extracted, resulting in an aqueous phase, two organic phases and a post-extraction solid phase (PES; faeces and some plasma) for each sample. Both organic phases were chromatographically analysed by one-dimensional thin layer chromatography (1D-TLC). Extracts were co-chromatographed and Rf values (ratio to front value for TLC) compared with authentic standards. Aqueous phases, considered to contain polar materials, were assayed by LSC, but not analysed further.
Safety assessments
All adverse events reported spontaneously by the subjects or observed during physical examination were documented, together with times of onset and cessation, and assessment of severity and causality. Vital signs were monitored at regular intervals during the study. Laboratory evaluations were conducted just prior to drug administration on day 1.
Results
Subjects
Eight healthy Caucasian male subjects were enrolled into the trial and all completed the 11-day monitoring period. Their ages ranged from 19 to 43 years (mean 27 years), their body weights from 73.5 to 85.0 kg (mean 78.6 kg), and their heights from 175 to 183 cm (mean 180.1 cm).
Metabolism and elimination
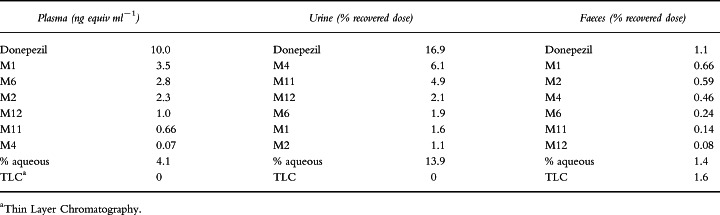
Plasma, urine and faecal samples for analysis were collected at specified intervals during the 10-day study period. Total recovery of radioactivity in subject samples averaged 72% of the administered dose (50 μCi). The average recovery in urine was 57%, which was significantly greater than the average recovery in faeces (15%). Donepezil and donepezil metabolite concentrations for the eight subjects over all time intervals are summarized in Table 1.
Table 1.
Concentrations of donepezil and its metabolites found in plasma, and as a percentage of the total recovered dose in urine and faeces. The numbers shown are average values for all eight subjects over the 10-day collection period.
Metabolite profile in plasma
Due to the low levels of radioactivity recovered in plasma samples (approximately 1500 dpm ml−1), it was necessary to pool samples collected from several time-points in order to provide the necessary counts for metabolic profiling. Time-points were pooled to provide metabolite profiles during specific phases of the plasma concentration–time curve (absorption, Cmax, distribution and elimination) as follows: 1, 2, 3 and 4 h; 6 and 8 h; 12, 18 and 24 h; 36, 48 and 72 h; 96–240 h.
Plasma radioactivity concentrations peaked in the 6–8 h time interval at 10.09 ng equiv ml−1. Donepezil, which was found to be the principal component during all of the plasma time intervals, accounted for 4.07 ng ml−1 during this interval. In general, donepezil accounted for about 25% of the recovered radioactivity in each time interval, with the exception of the 6–8 h interval, where it accounted for about 40% of the total radioactive residue.
The recovered residue from plasma contained higher levels of the hydroxylated metabolites M1 and M2 than of their respective glucuronide (or sulphate) conjugates, M11 and M12. For example, the 4 h sample pool contained 0.65 and 0.69 ng equiv ml−1 of M1 and M2, respectively, while M11 and M12 were present at concentrations of 0.33 and 0.38 ng equiv ml−1, respectively. Of the metabolites found in the initial 4 h sample pool, M6 was present in significant quantities (1.53 ng equiv ml−1), although its concentration steadily decreased in subsequent samples and was below detection limits (< 0.05 ng equiv ml−1) in the 96–240 h pool. In contrast, the hydrolysis product M4 was not present in plasma in significant concentrations (≤0.1 ng equiv ml−1) for any time interval.
As many as four unknown compounds were observed in plasma, each of which was present at concentrations < 0.9 ng equiv ml−1, on average, for all time intervals. The aqueous phase, containing very polar residues, accounted for an average of 1.29 ng equiv ml−1 of radioactivity across all time intervals. Mass balances accounted for an average of >70% of the total radioactive residue in each pooled sample.
Metabolite profile in urine
Donepezil was the predominant compound excreted in urine by all subjects, over all time intervals. On average, it accounted for one-third of the total radioactive residue at each time interval. Therefore, of the total recovered dose in urine (which was 57% of the administered dose), a cumulative total of 16.9% was donepezil. The aqueous phase accounted for approximately 14% of the recovered dose. The major metabolite present in urine was the hydrolysis product, M4, which accounted for approximately 6.1% of the dose.
The hydroxylation metabolites M1 and M2 were present principally as their glucuronide (or sulphate) conjugates, M11 and M12, respectively. M11 and M12 accounted for 4.9% and 2.1% of the dose, respectively, over the 10-day collection period, while M1 and M2 accounted for only 1.6% and 1.1% of the dose. The N-oxidation product M6 accounted for approximately 2% of the dose in urine (Table 1).
As many as six unknown compounds were observed in urine, each accounting for ≤2% of the dose, and accounting for no more than 4.5% of the dose collectively. Mass balances accounted for an average of 93.5% of the total radioactive residue in each pooled sample.
Metabolite profile in faeces
Faecal samples were collected throughout the 10-day study period, but were pooled because of variability in sample production between subjects and because of the low levels of radioactivity in each sample. Two-day intervals were chosen to examine the metabolic profile of donepezil during the early (days 2 and 3), middle (days 6 and 7) and late (days 9 and 10) phases of the sample collection period.
Approximately 15% of the administered dose was recovered in faeces during the 10-day period. The faecal extracts contained proportionately less radioactive residue than the other matrices. The faecal matrix was the most difficult to extract of the three matrices, making chromatographic resolution and co-chromatographic resolution difficult. Analysis of faecal samples was also hampered by large amounts of polar materials that did not migrate from the TLC origin and could not be identified.
Relatively little donepezil was measured in the faeces, although it represented the largest percentage of the known compounds identified during each of the three assessment intervals (1.1% of the recovered dose). On average, donepezil represented 0.75% of the dose during the early phase (days 2 and 3), declining to 0.06% by the late phase (days 9 and 10). The most significant of the known metabolites in this matrix were the hydroxylation metabolites M1 and M2 (0.66% and 0.59% of the recovered dose, respectively). On average, these two metabolites represented < 0.4% of the dose per time interval. The glucuronide (or sulphate) conjugates of these metabolites, M11 and M12, respectively, were relatively insignificant and accounted for ≤0.1% of the dose for each time interval. The hydrolysis metabolite M4 accounted for an average of 0.24% of the dose during the early phase, decreasing to 0.05% during the late phase. The N-oxidation product, M6, accounted for slightly lower levels (0.13% for the early phase, 0.02% for the late phase).
As many as five unknown compounds were observed in the faeces, each averaging < 0.2% of the dose for each time interval. A significant amount of radioactive residue was retained at or near the TLC origin, indicating unknown polar compounds unique to faeces (1.6% of recovered dose). These metabolites could not be identified. Additional radioactivity was retained in the aqueous phase that also represented very polar residues (1.4% of the dose). These residues accounted for 0.76% of the dose during the early phase and 0.27% of the dose during the late phase. Mass balances, including combustion of post-extraction solids, accounted for >70% of the total radioactive residue for each time interval.
Pharmacokinetic data
Mean plasma concentration–time curves for donepezil and 14C-donepezil in plasma are shown in Figure 1. The tmax values for donepezil and for 14C-donepezil (total radioactivity) were very similar. The Cmax and AUC values, however, were markedly higher for 14C-donepezil than for donepezil, demonstrating the presence of both the parent compound as well as donepezil metabolites (Table 2). AUC(0–144) for 14C-donepezil was 663.5±23.0 ng h ml−1, 87% greater than for unlabelled donepezil (355.0±20.7 ng h ml−1). The AUC(0–∞) for 14C-donepezil was 897.3± 36.1 ng h ml−1, 77% greater than for unlabelled donepezil (507.4±31.8 ng h ml−1). The ratio of donepezil AUC(0–144) to total 14C-donepezil AUC(0–144) was 0.54.
Figure 1.
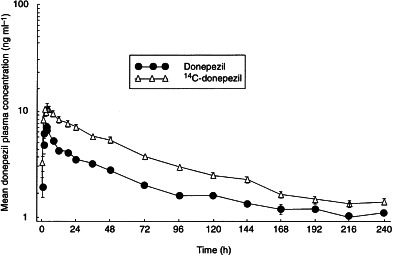
Mean plasma concentration–time curves for donepezil and 14C-donepezil following administration of single oral doses of donepezil 5 mg to healthy male volunteers.
Table 2.
Comparison of pharmacokinetic parameters (mean±SE) for donepezil and 14C-donepezil (including metabolites).
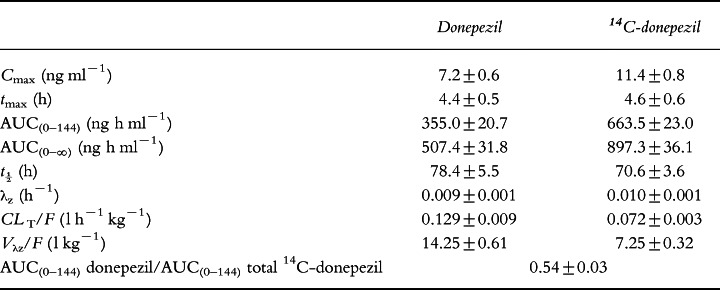
Donepezil was cleared from plasma faster than 14C-donepezil (clearance rates 0.129±0.009 and 0.072±0.003 l h−1 kg−1, respectively). Donepezil also had an apparent volume of distribution, which was twice that calculated for 14C-donepezil (Table 2).
Safety
Donepezil was well tolerated by all subjects, and there were no clinically significant changes in vital sign parameters during the course of the study. Four subjects reported mild headache during the study of which three reports were considered to be possibly related to donepezil.
Discussion
The results presented herein indicate that the primary route for the elimination of donepezil and its metabolites in human subjects is renal rather than biliary. This contrasts with findings from animal studies showing biliary excretion to be the primary route of elimination (unpublished observations).
In total, 72% of the administered dose was recovered, with approximately 57% recovered in the urine and 15% in the faeces. These findings are in agreement with previous studies, which showed that approximately four times more radioactivity was found in the urine than in the faeces [1, 2].
Although unchanged 14C-donepezil was present in both urine and plasma throughout the 240-h collection period, the results presented here demonstrate that donepezil undergoes significant hepatic metabolism. Three metabolic pathways are proposed on the basis of the results presented: (i) O-dealkylation followed by hydroxylation to metabolites M1 and M2, with partial subsequent glucuronidation to metabolites M11 and M12; (ii) hydrolysis to metabolite M4; and (iii) N-oxidation to metabolite M6 (Figure 2). Additional metabolic pathways may be operative, as represented by the number of unknown compounds observed. However, as each unknown metabolite represented an average of < 2% of the dose, these pathways are considered minor contributors to the metabolic process.
Figure 2.
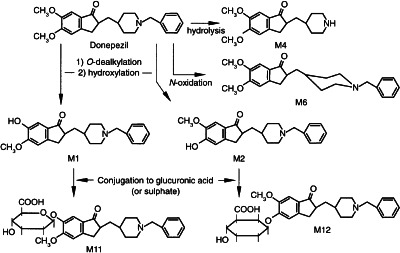
Proposed metabolic pathways for donepezil.
Plasma levels of total radioactivity were significantly greater at the first blood sampling time-point following drug administration (1 h) than those of total donepezil (3.3 ng equiv ml−1 versus 1.6 ng ml−1), suggesting that first-pass hepatic metabolism plays an important role. However, in contrast to the pre-clinical studies, the primary metabolites observed in plasma were not the O-glucuronide conjugates, but instead the hydroxylation products M1 and M2 and the N-oxidation product M6. Total radioactivity levels in plasma remained consistently higher than total donepezil levels throughout the 10-day sampling period, suggesting that the metabolic products of donepezil have a t½ similar to that of the parent compound. The consistent presence of metabolites throughout the plasma life span of donepezil is demonstrated by markedly larger AUC values for total radioactivity, as well as the value of 0.54 for the donepezil AUC(0–144) to total 14C-donepezil AUC(0–144) ratio.
In urine, the glucuronidated metabolites M11 and M12 (glucuronidated products of M1 and M2, respectively) predominated, as did the hydrolysis product M4. All three of these metabolites were found in very low quantities in plasma, suggesting rapid elimination of these products following their formation. As in plasma, unchanged donepezil accounted for a large component of the recovered dose in urine. The metabolites M1, M2 and M6, which were predominant in plasma, were present only at very low levels in this matrix.
Faecal extracts contained proportionally less radioactive residue than the other matrices examined. Analysis of faecal samples was also hampered by large amounts of polar materials, which did not migrate from the TLC origin and which could not be identified, and by a significant amount of matrix interference which made chromatographic resolution and co-chromatographic resolution difficult. These phenomena were unique to this matrix. Of the products that could be identified in faeces, unchanged donepezil predominated, although to a much smaller degree (1.1% of the recovered dose) than for plasma and urine. The hydroxylation products M1 and M2, which were found at very low levels in the urine, were the predominant metabolites found in the faeces, suggesting that their lack of hydrophilicity prevented them from being cleared renally. This also appeared to be the case for the N-oxidation product M6. The glucuronide metabolites M11 and M12 appear to be eliminated primarily in the urine, as only trace amounts were found in the faeces.
In summary, the data reported here show that donepezil undergoes significant first-pass hepatic metabolism following oral administration, and that the drug and its metabolites are eliminated primarily in the urine. Renal excretion of unchanged drug, glucuronidated metabolites M11 and M12 and the hydrolysis product M4 are the primary pathways for elimination of donepezil. Faecal elimination of the hydroxylation products M1 and M2 and the N-oxidation product M6 appears to play a minor role.
Acknowledgments
We acknowledge the efforts of Dr James Kisicki, Harris Laboratories Inc, 624 Peach Street, Box 80827, Lincoln, NE 68501, USA, who conducted this clinical trial, and Harris Laboratories Institutional Review Board, who reviewed and approved the study and protocol.
References
- 1.Ohnishi A, Mihara M, Kamakura H, et al. Comparison of the pharmacokinetics of E2020, a new compound for Alzheimer’s disease, in healthy young and elderly subjects. J Clin Pharmacol. 1993;33:1086–1091. doi: 10.1002/j.1552-4604.1993.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 2.Mihara M, Ohnishi A, Tomono Y, et al. Pharmacokinetics of E2020 a new compound for Alzheimer’s disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol. 1993;31:223–229. [PubMed] [Google Scholar]
- 3.Lee JW, Rogers SL, Friedhoff LT, Stiles MR, Cooper NM. Validation and application of an HPLC method for the determination of 1-benzyl-4-[(5,6-dimethoxy-1-indanon)- 2-yl] methyl piperidine HCl (E2020) in human plasma. Pharm Res. 1992;9:350. [Google Scholar]


