Abstract
Aim
The aim of this study was to characterize the pharmacokinetics and pharmacodynamics of donepezil HCl, a new, chemically distinct and specific acetylcholinesterase (AChE) inhibitor for the treatment of Alzheimer’s disease, following multiple-dose administration.
Methods
This was a double-blind, randomized, placebo-controlled, multiple-dose study in healthy male volunteers (n =27). Three dose levels were investigated in sequential order: 1, 3 and 5 mg. Each dose was administered orally, once a day, for 21 consecutive days. Donepezil concentrations in plasma were quantified by HPLC. Pharmacodynamic activity was determined by the radioenzymatic measurement of erythrocyte membrane acetylcholinesterase (rbc-AChE) inhibition.
Results
The pharmacokinetic disposition of donepezil was observed to be dose proportional. The mean terminal disposition half-life was 79.5±19.0 h which resulted in a slow approach to steady state (14–21 days). A four- to sixfold increase in donepezil plasma concentration was observed during this time; however, no further increase was evident after achievement of steady state. The mean donepezil plasma concentration at steady state (Css) was 14.2 ng ml−1. Neither the rate of accumulation nor the rate of clearance was dose dependent. Inhibition of rbc-AChE was directly correlated with donepezil concentration over a wide concentration range, with the higher concentrations showing the expected hyperbolic relationship. Donepezil was well tolerated by all subjects with no clinically significant changes in laboratory or physical parameters observed at any dose.
Conclusions
The pharmacokinetics of donepezil were found to be dose proportional following the administration of multiple doses to healthy volunteers. A predictable relationship was also observed between plasma donepezil concentrations and rbc-AChE inhibition. The half-life of donepezil makes it suitable for once-daily dosing.
Keywords: donepezil, selective acetylcholinesterase inhibitor, Alzheimer’s disease, pharmacokinetics, pharmacodynamics
Introduction
Alzheimer’s disease has been estimated to affect approximately 5–10% of the population over age 65, and as many as 50% of those older than 85 years of age [1]. Despite its prevalence, the aetiology of the condition remains poorly understood. Alzheimer’s disease is recognized pathologically by the demonstration at autopsy of amyloid plaques and neurofibrillary tangles in the areas of the cerebral cortex where cholinergic pathways have been destroyed [2]. There is a general consensus that the cholinergic deficit resulting from impairment of cholinergic neurotransmission is re- sponsible for the deficits in memory and cognition that characterize the condition [3].
Various therapeutic strategies to counteract the cognitive symptoms of Alzheimer’s disease have been investigated. These include nicotinic and muscarinic agonists, as well as neuroprotective agents. The cholinesterase inhibitors are currently the most widely investigated agents for the treatment of patients with Alzheimer’s disease. However, the use of existing agents, such as tacrine, is hampered by lack of specificity for acetylcholinesterase (AChE), resulting in unwanted peripheral cholinergic side-effects [4–7]. Tacrine and other agents are also short-acting and require administration several times a day, a factor that may compromise patient compliance and have a direct impact on therapeutic success. Further, the acridine-based cholinesterase inhibitors, such as tacrine, are associated with significant hepatotoxicity and thus require regular transaminase monitoring [4,8].
Donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan) is the product of a specific research programme undertaken to produce a treatment for Alzheimer’s disease. It was shown to have specific activity for AChE with little activity for butyrylcholinesterase (BuChE) [9–11]. The unique piperidine-based structure of donepezil makes it chemically and pharmacologically distinct from other cholinesterase inhibitors. In vitro studies have demonstrated that donepezil has a high selectivity for AChE isolated from rat brain tissue, is without effect on cholinesterases isolated from cardiac muscle or intestinal smooth muscle, and has only marginal effect on striatal muscle cholinesterase [12,13]. The ability of donepezil to increase brain acetylcholine (ACh) was demonstrated by ex vivo and in vivo measurements of brain ACh. These increases in brain ACh were associated with improved performance in behavioural, learning and memory function tests in rats with experimentally induced cholinergic hypofunction [12,14]. Furthermore, donepezil was found to be devoid of toxicity in 1-year studies in rats and dogs and, perhaps most importantly, not hepatotoxic [13].
The pharmacokinetics of donepezil following single-dose administration are reported in this supplement. The present study was designed to characterize fully the pharmacokinetics and pharmacodynamics of donepezil HCl associated with multiple-dose administration to healthy male volunteers.
Methods
Subjects
The study population comprised a total of 27 healthy, ambulatory, non-smoking men, between 19 and 40 years of age. Their body weights ranged between 65 and 85 kg, and all were within 15% of ideal weight, based on the Metropolitan Insurance Company Height and Weight Tables (1983). Upon clinical examination, subjects were found to be free from significant hepatic, gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities. None had a known or suspected history of alcohol or drug misuse and none had taken over-the-counter medications or consumed alcoholic beverages for at least 48 h prior to enrolment. No investigational or prescription medications had been taken within 1 month of entry into the study.
This study was conducted in accordance with the principles stated in the Declaration of Helsinki. The protocol was approved by the Institutional Review Board for Investigations Involving Human Subjects, Harris Laboratories, Lincoln, Nebraska, USA, and all subjects gave written informed consent prior to participation.
Protocol
The study was double-blind, randomized and placebo-controlled. Donepezil was administered in oral doses of 1, 3 and 5 mg once daily for 21 days, according to a sequential-group design. The dose range chosen for the study was based upon the results of Japanese phase I studies and on the results of a single-dose study conducted in the USA [15]. A sufficient number of subjects was enrolled to allow eight subjects to complete each dose evaluation period. Within each cohort of eight subjects, two were randomized to receive placebo and six to receive donepezil. The trial was blinded by using a standard daily dosage of three tablets per patient. Each dose comprised donepezil (1 mg or 2 mg) and/or placebo tablets depending upon the treatment that was being administered. Progression to the next higher dose level required demonstration that the previous dose had been well tolerated.
On days 1, 7, 14 and 21, the subjects observed a 12 h fast from food and fluids (except water) prior to dose administration and collection of blood samples for clinical laboratory and analytical evaluations. The subjects remained awake and in an upright position (>45° angle from supine) for 4 h after drug administration. The subjects stayed at the investigational site for 30 days until all of the follow-up assessments had been completed. During this time they abstained from caffeine-containing beverages, and physical exertion was limited to normal walking.
Sample collection and analysis
Venous blood samples (7 ml) for the measurement of donepezil concentrations in plasma and AChE activity in red blood cell membranes (rbc-AChE) were collected in evacuated heparinized tubes at 1, 2, 2.5, 3, 3.5, 4, 6, 8, 12 and 18 h following drug administration on days 1, 7, 14 and 21. Follow-up samples were collected 24, 48, 96 and 120 h after the final dose. On all other days, a single trough sample was collected just prior to drug administration.
Blood samples were placed on ice immediately after collection. Plasma was then separated from the cellular components by centrifugation. The plasma–rbc interface was marked on the tube and the 2 ml plasma phase was then harvested and stored at −20° C until analysis.
Following the removal of the plasma for pharmacokinetic measurement, the white blood cell/platelet layer and the top 3 mm of the rbc layer were removed using a Pasteur pipette and discarded. The remaining rbcs were gently vortexed before being stored in an upright position at −20° C in polypropylene Sarstedt tubes until analysis. Plasma concentrations of donepezil were quantified using a specific and sensitive high-performance liquid chromatography (HPLC) method with ultraviolet (UV) detection [16]. The lowest quantifiable concentration was 2.0 ng ml−1. The pharmacodynamic activity of donepezil was assessed by measuring AChE activity in peripheral rbc membranes.
Pharmacokinetic assessments
Model-independent pharmacokinetic parameters were determined using standard techniques. The peak plasma concentration (Cmax), minimum plasma concentration (Cmin), and the time at which the peak concentration occurred (tmax) were determined by inspection of individual subject data. The area under the donepezil concentration versus time curve (AUC(0–24)) was calculated by the linear trapezoidal rule using the data collected on days 1, 7, 14 and 21. On each of these study days, the preterminal disposition rate constant of donepezil (λ1) was estimated by least-squares regression of the natural logarithm of donepezil plasma concentration versus time. The terminal disposition rate constant (λz) was estimated following the last dose on day 21. The terminal half-life (t½) was calculated as 0.693/λz.
Time-averaged total clearance at steady state (CLss/F), adjusted for systemic bioavailability (using factor F), was expressed as the ratio of the daily dose divided by the day 21 value of AUC(0–24). The apparent volume of distribution (Vλz/F) (also adjusted for bioavailability using factor F) was estimated from the daily dose divided by the product of day 21 AUC(0–∞) and λz. The average steady-state concentration of donepezil (Css) attained in each subject was estimated as the day 21 AUC(0–24)divided by 24 h, on the assumption that steady-state conditions had been achieved by the end of the third week of drug administration.
The accumulation (RA) of donepezil at steady state was calculated as AUC(0–24)on day 21 divided by AUC(0–24) on day 1. These values were compared with the predicted accumulation which was estimated from the terminal disposition rate constant and the dosing interval (i.e. 1/1−e−λzτ).
Pharmacodynamic assessments
The pharmacodynamic activity of donepezil was based upon the inhibition of rbc-AChE activity measured using a specific and sensitive radioenzyme assay [17]. In this method, [3H-acetyl]choline was incubated at 4° C with homogenized erythrocytes containing unknown concentrations of donepezil. After 30 s, the hydrolytic product was extracted into a scintillation cocktail and total radioactivity was counted. Activity from patient samples was quantified using standard curves of percent rbc-AChE inhibition versus the natural logarithm of the donepezil concentration (ng g−1 erythrocytes). Parameters calculated were minimal, maximal and steady-state values of AChE inhibition (Emin, Emax and Ess, respectively), the concentration of donepezil required to reduce AChE activity by 50% (EC50), and the area under the effect curve (AUE). Values are expressed as percent inhibition of pre-drug rbc-AChE activity.
Safety assessments
All adverse events that were spontaneously reported by a subject or elicited/observed by the investigator were recorded, together with times of onset and cessation, and assessments of severity and causality. Sitting blood pressures and radial pulse rates were measured using the procedures described in the American Heart Association recommendations. Assessments were made every 6 h, commencing 4 h after the first dose and continuing until day 30.
A standard 12-lead ECG was recorded at screening and at 2-day intervals beginning on day 1 and ending on day 21. ECGs were recorded at times scheduled to correspond with the tmax of donepezil (approximately 4 h after dose administration).
Routine haematology, clinical chemistry and urinalysis tests were performed following a 12 h fast on days 1 (pre-drug), 7, 14 and 21. Haematological assessments included haemoglobin, haematocrit, red blood cell count, platelet count, white blood cell count and differential. Clinical chemistry assessments included liver function (alanine transaminase, aspartate transaminase, alkaline phosphatase and total bilirubin), renal function (creatinine, blood urea nitrogen), metabolic status (glucose, total protein, albumin, cholesterol, triglycerides), electrolytes (sodium, potassium, chloride, phosphorus, calcium) and cardiac enzymes (lactate dehydrogenase and creatine kinase). Routine urinalysis was performed (pH, glucose, protein, haemoglobin or blood, ketones), together with specific gravity and microscopic examination of the urine sediment.
Statistical analysis
Data obtained from placebo-treated subjects were pooled across doses for statistical comparison. The relationships between λz, AUC, and dosage and duration of treatment were assessed by analysis of variance and multiple linear regression. Associations between Css and AUC at steady state, and daily dosage were assessed by linear least-squares regression. Plasma concentrations of donepezil on days 1 and 21 were examined by orthogonal least-squares regression.
Areas under the effect–time curve (AUE(0–24)) for rbc-AChE inhibition were calculated using the linear trapezoidal rule with data obtained on days 1, 7, 14 and 21. Orthogonal linear least-squares regression analysis was used to compare these integrated measures of effect with overall systemic exposure to donepezil (AUC(0–24)).
The relationship between rbc-AChE inhibition and donepezil plasma concentration was examined individually in each subject, as well as in data pooled across subjects and doses. Both linear and classic Emax models were fit to the data. Non-linear regression analyses were used to evaluate the relationships.
The incidence of adverse events in donepezil- and placebo-treated subjects within each dose group were compared using Fisher’s exact test. Haematology, clinical chemistry and routine urinalysis results were also compared with normal ranges for deviations. Shift tables were used to identify trends in the data.
Results
Subjects
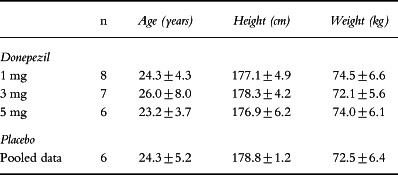
A total of 27 male subjects were enrolled in this study. The three dose groups were comparable with respect to age, height and weight (Table 1). All of the subjects assigned to treatment with placebo were Caucasian. Two subjects treated with donepezil at 1 mg day−1 and one receiving the 5 mg day−1 dose were black. Two subjects were withdrawn from the study as a result of abnormal baseline laboratory values and did not receive any medication. A third subject was withdrawn after receiving ten doses of medication because of his unstable alkaline phosphatase values. The remaining 24 subjects completed the study without incident.
Table 1.
Demographic data (mean±SD).
Pharmacokinetics
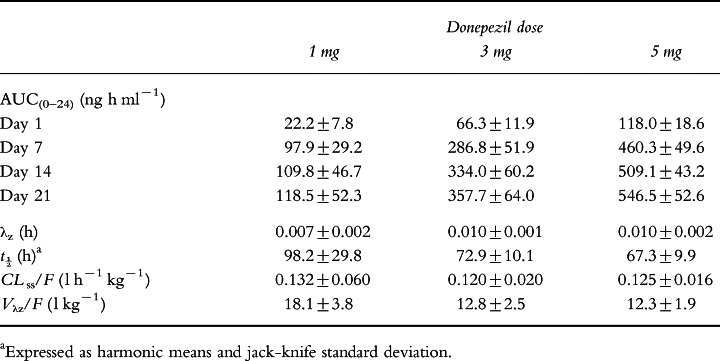
Model-independent pharmacokinetic parameters for donepezil during 21 days of once-daily oral dosing are summarized in Table 2. Regression analysis of individual subject data indicated that the daily dose and duration of administration were both significant determinants of AUC (P <0.001 for both). AUC(0–24) increased as treatment continued but remained proportional to dose at each interval. Full steady state was achieved between days 14 and 21.
Table 2.
Model-independent pharmacokinetic parameters for donepezil during once-daily oral administration for 21 days (mean±SD).
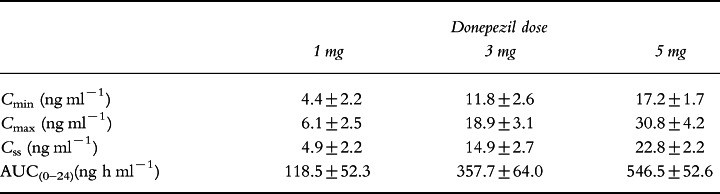
As shown in Table 3, Css and AUC(0–24) were dose proportional. There were statistically significant correlations between AUC(0–24) on days 1 and 21; the regression coefficient (r2) for this correlation was 0.89 (P <0.0001) and for the correlation between AUC(0–24) on day 1 and at steady state it was 0.82 (P <0.0001). Similarly, there were linear relationships between AUC(0–24) at steady state and Cmin (r2 =0.91; P <0.0001) as well as Cmax (r2 =0.83; P <0.0001) on day 1.
Table 3.
Plasma concentrations of donepezil at steady state (mean±SD).
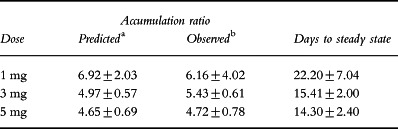
As shown in Table 4, there was good agreement between the observed accumulation of donepezil in plasma and that predicted on the basis of λz. The observed accumulation factor was independent of dosage (ANOVA, P>0.5).
Table 4.
Accumulation of donepezil in plasma during once-daily dosage for 21 days in healthy male volunteers (mean±SD).
The clearance of donepezil during once-daily administration was linear, as evidenced by significant regression coefficients for the correlations between dose and AUC(0–24) at steady state (r2 =0.92; P <0.0001) and between dose and Css (r2 =0.92; P <0.0001). The estimated terminal disposition rate constants for donepezil were dose dependent. However, since clearance was unchanged, this difference was probably related to a reduced Vλz/F at the higher doses, as this value was significantly larger in the 1 mg group (18.1 l kg−1) than in the 3 and 5 mg groups (12.8 and 12.3 l kg−1, respectively; P <0.005; ANOVA). The change in λz resulted in a calculated mean half-life (±SD) of 98.2±29.8, 72.9±10.1 and 67.3±9.9 h in the 1, 3 and 5 mg dose groups, respectively (Table 2).
Pharmacodynamics
In parallel with the increasing AUC(0–24), the extent of AChE inhibition increased throughout the treatment period (Figure 1). AUE was dependent on both dose and duration of treatment (P <0.0001, two-way ANOVA). Pairwise comparisons showed that the AUE on day 7 was significantly greater than on day 1. However, there were no significant differences in the values of AUE on days 7, 14 and 21, suggesting that pharmacodynamic steady state was achieved after 7 days on donepezil treatment.
Figure 1.
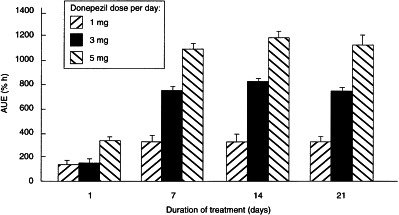
The effect of duration of treatment with donepezil on AUE for rbc-AChE inhibition.
In the majority of subjects there was a predictable relationship between rbc-AChE inhibition and plasma concentration of donepezil, and fluctuations in rbc-AChE inhibition within the dosing interval mirrored fluctuations in donepezil concentration. AUE was strongly correlated with AUC irrespective of the dose or duration of treatment (P <0.0001; Figure 2). The regression coefficient for this association (r2 =0.919) suggests that approximately 90% of the inter-individual differences in AChE inhibition can be ascribed to inter-subject variability in donepezil disposition.
Figure 2.
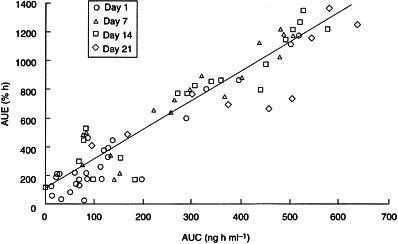
Relationship between AUE and AUC during administration of donepezil to healthy male volunteers for 21 days.
In nine of 12 subjects receiving the lower donepezil doses of 1 or 3 mg day−1,the effect–plasma concentration profiles were best approximated by linear model kinetics. The mean slope of the lines obtained for these nine subjects was 2.8±1.2% ng−1 ml, corresponding to the predicted mean (±SD) increase in AChE inhibition per unit (ng ml−1) increase in donepezil plasma concentration. Further, their mean y-axis intercept (1.1±2.2%) was not statistically different from zero.
On the other hand, the effect–plasma concentration profiles for all six subjects receiving the higher 5 mg day−1 dose of donepezil, as well as three of those in the 3 mg day−1 group were best described by the Emax model, as expected for enzyme activity. Estimates from the Emax model in these subjects suggested that a mean plasma concentration of 28.7±3.9 ng ml−1 donepezil was required to produce EC50, and that the theoretical value of Emax was 105.7±16.2%. This mean value for Emax was in good agreement with the theoretically predicted value of 100% inhibition at high donepezil concentrations.
When the individual concentration–effect data from all subjects were pooled, the Emaxmodel was shown to be slightly superior to the linear effect model in fitting the pooled data (Figure 3). The linear effect model then predicted an average increase in AChE inhibition per unit (ng ml−1) increase in donepezil plasma concentration of 2.0±0.03%, with an increased y-axis intercept of 4.6±0.4%. Similarly, the Emax model for all the individual data from the 18 subjects yielded values of 26.2±1.2 ng ml−1 and 104.4±1.1% for EC50 and Emax, respectively (Figure 3).
Figure 3.
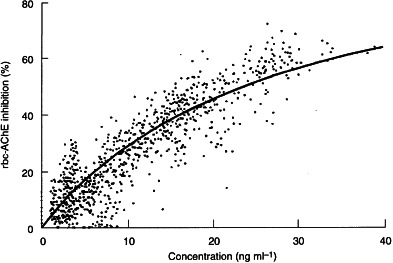
The relationship between rbc-AChE inhibition and donepezil plasma concentration described by the Emax model.
Safety
Donepezil was well tolerated by all subjects, with no clinically significant changes in laboratory, ECG or vital sign parameters observed at any dose. Adverse events that were spontaneously reported or observed by the investigator included nausea, insomnia, diarrhoea, headache and dizziness. These were all mild in intensity and transient. The difference in the incidence of adverse events between donepezil- and placebo-treated subjects within each dosage group was not statistically significant (P>0.05, Fisher’s exact test).
Discussion
The disposition of donepezil, administered at 1, 3 or 5 mg oral doses once daily for 21 consecutive days, appeared to be dose proportional. Small but statistically significant dose-dependent effects were observed in the terminal disposition rate constant and in the apparent volume of distribution. These differences may reflect concentration-dependent binding to tissue or blood components. However, these effects offset one another and, as such, no dose-dependent behaviour was observed for total clearance. The area under the concentration–time curve was linearly related to the administered dose. The long half-life of donepezil (80 h) resulted in a slow approach to steady state. Steady state was reached between 2 and 3 weeks after initiation of dose administration. Once-daily dose administration resulted in a four- to sixfold accumulation in plasma donepezil concentration (until steady state), with the ratio of accumulation being consistent across doses. Strong linear correlations (r2>0.8) were observed for concentration determinations on day 21 (AUC or Css) compared with day 1 (peak concentration, trough concentration or concentration 4 h after drug administration). The strongest relationship was between steady-state AUC or Css and day 1 trough concentration. These observations suggest that (a) the pharmacokinetics of donepezil are unchanged during a 3-week course of administration, and (b) the eventual steady-state concentration may be predicted with a high degree of accuracy from a single concentration determined on the first day of drug administration.
It is generally recognized that cholinesterase inhibitors that have a clinical effect in patients with Alzheimer’s disease counteract the cholinergic deficit in the central nervous system by inhibiting the hydrolytic action of AChE rather than BuChE [12]. Donepezil has been shown to be 1200 times more selective for AChE than for BuChE [11]. In this study, inhibition of rbc-AChE in healthy volunteers was used as a marker for assessing the pharmacodynamic action of donepezil. It was found that the extent of rbc- AChE inhibition tended to increase in parallel with the duration of drug administration. This finding was directly related to the time required to achieve steady-state plasma concentrations of donepezil. Mean maximum enzyme inhibition was achieved after the first week of dose administration and was maintained until the end of the study.
Increasing the daily dose of donepezil from 1 to 3 mg resulted in an approximately proportional increase in the pharmacodynamic effect, although a further increase to 5 mg produced an increase in pharmacodynamic effect that was less than expected on the basis of a proportional relationship. This reflects the hyperbolic nature of enzyme inhibition. The extent of AChE inhibition expressed as the AUE was related to the AUC(0–24) for donepezil in plasma and to the absolute plasma concentration of donepezil, suggesting that in clinical practice the response can be adjusted by altering the dose. The data revealed no time dependencies or evidence of hysteresis in pharmacological response.
Modelling was conducted to characterize the relationship between AChE inhibition and donepezil concentration. The linear-effect model was, as expected, useful at mid-range concentrations (from 10 to 30 ng ml−1). However, it tended to underpredict the effect of donepezil at concentrations ranging from 10 to 20 ng ml−1, while overpredicting its effect at concentrations >30 ng ml−1. This pattern of bias was expected since enzyme activity classically approaches minimal and maximal values asymptotically. Thus, the concentration–effect data were better approximated by an Emaxmodel, particularly at the higher donepezil concentrations.
Donepezil was well tolerated. Reported adverse events were consistent with an increase in cholinergic activity. No clinically significant changes in vital signs, ECG or clinical laboratory parameters were observed during the course of this study.
The results of this study demonstrate that once-daily administration of donepezil allows achievement of significant AChE inhibition throughout the dosing interval, even after the first dose administration. Moreover, repeated administration of oral doses of 1–5 mg of donepezil once daily is characterized by predictable pharmacokinetic and pharmacodynamic profiles that are uncomplicated by dose-limiting toxicity. Steady-state concentrations of the drug are reliably achieved after 14–21 days of once-daily administration. The strong correlations between pharmacodynamic effect and plasma donepezil concentration, and the predictable relationship between first-dose and steady-state plasma concentrations suggest that the steady-state pharmacodynamic effect can be expected on the basis of plasma concentration or rbc-AChE inhibition measured after the initial dose of donepezil. It is suggested that this stable pharmacokinetic and pharmacodynamic profile during repeated administration may simplify the use of donepezil in clinical practice.
Acknowledgments
We acknowledge the efforts of Dr James Kisicki, Harris Laboratories Inc, 624 Peach Street, Box 80827, Lincoln, NE 68501, USA, who conducted this clinical trial, and Harris Laboratories Institutional Review Board, who reviewed and approved the study and protocol.
References
- 1.Kaplan HI, Sadock BJ, editors. Comprehensive Textbook of Psychiatry. Sixth. Baltimore, Maryland: Williams & Wilkins; 1995. p. 2562. [Google Scholar]
- 2.Katzman R, Saitoh T. Advances in Alzheimer’s disease. FASEB J. 1991;5:278–286. [PubMed] [Google Scholar]
- 3.Becker RE. Therapy of the cognitive deficit in Alzheimer’s disease: the cholinergic system. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 1–30. [Google Scholar]
- 4.Taylor P. Anticholinesterase agents. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. Eighth. New York: McGraw-Hill; 1993. pp. 131–149. [Google Scholar]
- 5.Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986;315:1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Thal LJ, Gamzu ER, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N Engl J Med. 1992;327:1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- 7.Stern Y, Sano M, Mayeux R. Long-term administration of oral physostigmine in Alzheimer’s disease. Neurolog. 1988;38:1837–1841. doi: 10.1212/wnl.38.12.1837. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA. 1994;271:992–998. [PubMed] [Google Scholar]
- 9.Cardozo MG, Iimura Y, Sugimoto H, Yamanishi Y, Hopfinger AJ. QSAR analysis of the substituted indanone and benzylpiperidine rings of a series of indanone-benzylpiperidine inhibitors of actylcholinesterase. J Med Chem. 1992;35:584–589. doi: 10.1021/jm00081a022. [DOI] [PubMed] [Google Scholar]
- 10.Cardozo MG, Kawai T, Iimura Y, Sugimoto H, Yamanishi Y, Hopfinger AJ. Conformational analysis and molecular shape comparisons of a series of indanone-benzylpiperidine inhibitors of acetylcholinesterase. J Med Chem. 1992;35:590–601. doi: 10.1021/jm00081a023. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and anti-acetylcholinesterase activity of 1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine hydrochloride (E2020) and related compounds. Biorg Med Chem Lett. 1992;2:871–876. doi: 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- 12.Yamanishi Y, Ogura H, Kosasa T, Araki S, Sawa Y, Yamatsu K. Inhibitory action of donepezil, a novel acetylcholinesterase inhibitor, on cholinesterase. Comparison with other inhibitors. In: Nagatsu T, Fisher A, Yoshida M, editors. Basic, Clinical, and Therapeutic Aspects of Alzheimer’s and Parkinson’s Diseases. Vol. 2. New York: Plenum Press; 1990. pp. 409–413. [Google Scholar]
- 13.Rogers SL, Yamansihi Y, Yamatsu K. E2020—the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–320. [Google Scholar]
- 14.Ogura H, Kosasa T, Araki S, Yamanishi Y, Yamatsu K. Behavioral study of E2020, a novel centrally acting acetylcholinesterase inhibitor. Soc Neurosci Abstr. 1988;14:60–60. (27.21) [Google Scholar]
- 15.Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):1–6. doi: 10.1046/j.1365-2125.1998.0460s1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JW, Rogers SL, Friedhoff LT, Stiles MR, Cooper NM. Validation and application of an HPLC method for the determination of 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl] methyl piperidine HCl (E2020) in human plasma. Pharm Res. 1992;9 [Google Scholar]
- 17.Hulse JD, Rogers SL, Friedhoff LT, Sukovaty R, Pedersen JE, Lee JW. A radioenzyme assay of acetylcholinesterase activity in red blood cells and its correlation with 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl] methyl piperidine HCl (E2020) Pharm Res. 1992;9:338. [Google Scholar]


