Abstract
Aims
The primary objective of this study was to characterize the pharmacokinetics and pharmacodynamics of single daily doses of donepezil (5 and 10 mg) each evening for 28 consecutive days. A secondary objective was to measure the plasma protein binding of donepezil at steady state.
Methods
This was a double-blind, randomized, multiple-dose study in healthy male (n = 13) and female (n = 3) volunteers. Subjects were randomized to receive, once daily, either oral doses of 5 mg donepezil for 28 days or doses of 5 mg donepezil for 7 days followed by 10 mg donepezil for 21 days. All doses were administered in the evening. Donepezil concentrations and protein binding in plasma were determined by HPLC with UV detection and equilibrium dialysis, respectively. Inhibition of acetylcholinesterase (AChE) activity in red blood cell (rbc) membranes was assessed using a specific radioenzyme assay.
Results
The pharmacokinetics of donepezil were linear, dose proportional and stationary over the course of the study. Mean Cmax, tmax, AUC(0–24), t½ and Vλz/F at steady state were 34.1 ng ml−1, 3.0 h, 634.8 ng h ml−1, 72.7 h, and 11.8 l kg−1, respectively, for the 5 mg group and 60.5 ng ml−1, 3.9 h, 1127.8 ng h ml−1, 73.5 h and 11.6 l kg−1, respectively, for the 10 mg group. Accumulation of the drug was observed for 14–21 days, until steady state was achieved. A direct consistent relationship was observed between donepezil plasma concentration and percentage rbc-AChE inhibition during each 24 h evaluation period, indicating no hysteresis in donepezil pharmacodynamics. The pharmacodynamic parameters, Emin, Emax and Ess, were 62.2%, 71.8% and 65.3%, respectively, for the 5 mg donepezil dose, and 74.7%, 83.6% and 77.8%, respectively, for the 10 mg donepezil dose. Donepezil was 95.6% bound to plasma protein at steady state. The binding was high capacity and low affinity, and neither concentration nor time dependent. Both dosage regimens were well tolerated; no clinically significant changes in laboratory or vital sign parameters were observed in any subject.
Conclusions
The measured pharmacokinetic and pharmacodynamic parameters for both 5 and 10 mg day−1 donepezil administered in the evening are in good agreement with previous results obtained with morning administration, indicating no time of dosing effect.
Keywords: donepezil, pharmacokinetics, pharmacodynamics, evening dose
Introduction
Donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan), a chemically distinct and highly selective inhibitor of the enzyme acetylcholinesterase (AChE) [1, 2] has recently been approved for marketing in the USA, Canada and several European countries, including the UK, for treatment of the symptoms of mild–moderate Alzheimer’s disease.
Results obtained in phase II [3] and phase III [4–6] studies demonstrated that donepezil at doses of 5 and 10 mg day−1 was associated with positive therapeutic benefits, as indicated by statistically significant improvements in both cognition and global function. There was also a significant positive correlation between the percentage inhibition of AChE activity in erythrocyte membranes (rbc-AChE) and improvement on the Alzheimer’s Disease Assessment Scale cognitive subscale (ADAS-cog) [3, 5, 6]. These data support the hypothesis that the clinical effect produced by donepezil results from AChE inhibition, with a resultant enhancement of cholinergic transmission in the CNS.
Over 400 000 patient-days of exposure were accumulated in the pre-registration clinical development programme. The effects of donepezil have also been evaluated during long-term treatment [7, 8]. The findings of an interim analysis of these long-term data show that, compared with untreated historical control patients, improvements in cognitive and global function achieved during short-term donepezil treatment are sustained at the same level for over 2 years. Other than cholinergically mediated, transient gastrointestinal complaints, there have been no specific adverse events considered likely to be related to donepezil treatment.
Although donepezil was administered to patients in the evening during clinical trials, no formal study of its pharmacokinetics with evening administration had been conducted. This study was designed to characterize the pharmacokinetics and pharmacodynamics associated with repeated evening administration of single daily doses of 5 or 10 mg donepezil. A secondary objective was to measure donepezil plasma protein binding at steady state.
Methods
Subjects
Healthy male and female subjects who were 18 years of age or older, and within 20% of ideal weight for their height and body build based on the Metropolitan Insurance Company Height and Weight Tables (1983), were eligible to enrol in the study. Female subjects were required to be either surgically sterile or a minimum of 2 years post-menopausal.
None of the subjects had clinical evidence of gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities. Subjects who had a known or suspected history of alcohol or drug misuse, or a positive urine drug screen, were excluded from entry, as were those who had donated blood within 1 month of study commencement. Patients who were smokers or who were taking products containing nicotine were also excluded.
The study was conducted in accordance with the principles stated in the Declaration of Helsinki and the US Code of Federal Regulations. The study protocol was approved by the Institutional Review Board for Investigations Involving Human Subjects, Harris Laboratories, Lincoln, Nebraska, USA. All subjects gave written informed consent prior to study screening.
Protocol
This was a randomized, double-blind, multiple-dose study. Subjects were admitted to the study site at least 12 h prior to drug administration. A urine sample for drug screening was collected on admission. Subjects were provided with a standard supper 4 h before dose administration, after which an absolute fast from food and fluids (except water) was maintained.
The first dose of donepezil was administered with 250 ml of tap water at 22:00 h. Volunteers remained awake and in an upright position (>45° angle from supine) for 1 h after dose administration. Fasting continued until the following morning when breakfast was served, 8 h after drug administration. After breakfast, the standard meal schedule for the study site was followed. Drug administration continued at 22:00 h each evening for an additional 27 days.
Each blinded dose of donepezil consisted of two tablets. To minimize reactions to acute, extensive inhibition of AChE, all subjects initially received one 5 mg donepezil tablet and one matching placebo tablet. After 7 days, patients randomized to the 10 mg dose received two 5 mg donepezil tablets for the remaining 21 days of the study.
The subjects stayed at the study centre as in-patients for a total of 37 days in order to ensure compliance with the protocol. During this period they avoided caffeine-containing foods or beverages and abstained from physical activity other than normal walking. None of the subjects had received any prescription drugs for at least 1 month prior to entering the trial. Over-the-counter products and alcohol consumption were prohibited from 72 h prior to admission until the end of the treatment period.
Sample collection and analysis
Venous blood samples for analytical determinations were collected in 7 ml evacuated, heparinized tubes, at the following time-points: 1 h prior to drug administration on day 1 and 1, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 18 and 24 h post-dose. This same time course for sample collection was repeated on dose administration days 7, 14, 21 and 28 to complete the profile. After the final dose (day 28), additional samples were collected at 48, 72, 120, 168 and 216 h (day 37). Pre-dose samples were taken immediately prior to drug administration on all dose administration days.
Blood samples for protein binding studies were collected in 10 ml, heparinized, evacuated tubes, immediately prior to administration of the final dose (day 28) and at 4, 12, 24, 72, 120 and 168 h (day 35) afterwards.
After collection, the blood samples were centrifuged for 15 min at 2000 g at 4° C. Plasma was removed, transferred into polypropylene Sarstedt storage tubes and stored in an upright position at −20° C until analysis. Following the removal of plasma for donepezil measurements, the white blood cell and platelet layer and the top 3 mm of rbc were removed from the same tube, using a Pasteur pipette. The remaining rbcs were gently vortexed, transferred into a polypropylene Sarstedt storage tube, and stored in an upright position at −20° C until analysis. These cells were used to measure rbc-AChE activity.
Plasma concentrations of donepezil HCl were measured by a specific and sensitive high-performance liquid chromatography (HPLC) method with UV detection (315 nm) [9]. The lowest quantifiable concentration was 2 ng ml−1. Protein binding was determined by equilibrium dialysis and rbc-AChE activity was measured using a specific and sensitive radioenzyme assay [10], with pre-treatment baseline activity values used to calculate percentage inhibition of activity in post-treatment samples.
Pharmacokinetic assessments
Non-compartmental pharmacokinetic parameters were computed by standard methods using WinNonlin software (version 1.0; Scientific Consultants Inc., Apex, North Carolina, USA). The area under the plasma concentration–time curve from 0 to 24 h (AUC(0–24)) was evaluated on days 1, 7, 14, 21 and 28. Plasma concentration data from days 28–37 were used to calculate the terminal disposition rate constant (λz) and half-life (t½), as well as the AUC from time zero to infinity (AUC(0–∞)).
The maximum and minimum plasma concentrations achieved in each 24 h observation period (Cmax and Cmin, respectively) and the time that Cmax occurred relative to the last dose (tmax) were determined directly from the observed data. The average steady-state concentration of donepezil (Css) attained in each subject was estimated as the day 28 AUC(0–24) divided by 24. Attainment of steady state was determined by examination of Cmin, AUC(0–24), Cmax and Css values.
Total apparent oral clearance (CL T/F) was calculated after attainment of steady state by dividing dose by the product of AUC(0–∞) and F, a factor that adjusts for systemic bioavailability. The apparent volume of distribution (V λz/F) was calculated as total clearance divided by λz. Both parameters were adjusted for body weight.
The extent of drug accumulation (RA) was calculated as the ratio of AUC(0–24) on day 28 relative to that on day 1; day 1 values were multiplied by two for the group assigned to receive the 10 mg dose as only the 5 mg dose was administered on days 1–7. Normalization of day 28 data to 5 mg demonstrated the validity of this day 1 procedure for estimating RA.
Pharmacodynamic assessments
Percentage rbc-AChE inhibition at each time-point was calculated from a standard curve fitted to a third-degree polynomial equation [10]. The overall pharmacodynamic response was evaluated by integrating percentage rbc-AChE inhibition versus time during the 24 h assessment periods on days 1, 7, 14, 21 and 28 to yield an area under the effect curve (AUE(0–24)). AUE(0–24) was compared graphically to AUC(0–24). Emin and Emax were taken from the observed data. The average percentage rbc-AChE inhibition at steady state (Ess) was computed by integrating percentage rbc-AChE inhibition versus time from 0 to 24 h, during the 24 h sampling period on day 28, and dividing by 24. The subsequent pharmacodynamic data were examined using a sigmoidal Emax model.
where Emax is the maximal inhibition of AChE, EC50 is the concentration of donepezil that reduced AChE activity by 50%, and Cp is the measured concentration of donepezil in plasma at time t.
Plasma protein binding
The binding of donepezil to plasma proteins was determined by equilibrium dialysis. This method was selected over ultrafiltration because validation studies demonstrated that donepezil adhered to the ultrafiltration membranes to an extent that prevented accurate analysis. Because the individual data provided no evidence for capacity-limited or concentration-dependent binding in the clinically relevant range of plasma concentrations, the mean percentage protein binding for each subject was calculated from the values obtained for each of their seven samples.
Safety assessments
All adverse events reported spontaneously by the subjects and/or observed or elicited by questioning during the study were documented together with times of onset and cessation, plus assessments of severity and causality. Vital signs were monitored at regular intervals during the entire treatment period.
Statistical analysis
ANOVA results were tested for attainment of steady state by successive examination of pharmacokinetic parameters on days 14, 21 and 28. Statistical models for AUC(0–24) and Cmax included terms for dose, subject (dose), study day and dose×study day. Statistical evaluation of Cmin was undertaken using terms for subject and study day. The analysis compared variables on days 14 and 29. Day 29 values were compared with the lowest mean concentrations that occurred between days 14 and 28.
Dose proportionality was examined by computing the ratio and confidence intervals for least-squares mean estimates of steady-state pharmacokinetic parameters for the 5 and 10 mg doses.
All statistical calculations were performed on non-log-transformed data using BioPak software (version 2.1; Scientific Consultants Inc., Apex, North Carolina, USA).
Results
Subject demographics and disposition
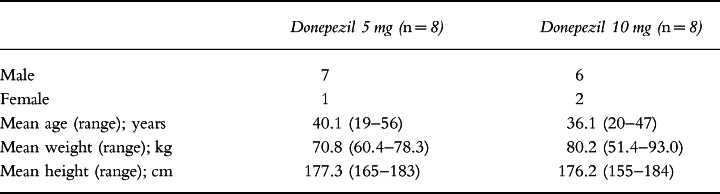
Sixteen subjects (13 male and three female), all of whom were Caucasian, entered this investigation, and 14 completed it. Their ages ranged from 19 to 56 years (mean 38.1 years) and their body weights from 51.4 to 93.0 kg (mean 75.5 kg; Table 1). The two male subjects, aged 19 and 20 years, who discontinued the study, did so after 5 days of treatment for personal reasons. As one of these subjects had been randomized to the 5 mg donepezil dose, and the other to the 10 mg donepezil dose, there were a total of seven subjects in each donepezil treatment group after their withdrawal from the study.
Table 1.
Demographic data.
Pharmacokinetics
Plasma concentration–time curves for the two dose groups are shown in Figure 1. Plasma concentrations of donepezil at steady state were observed to be dose proportional as reflected by Cmin, Cmax, Css and AUC(0–24) (Table 2). Dose proportionality was demonstrated by the ratios of the least-squares means for AUC(0–24), Cmax, tmax and clearance at steady state for the 5 mg and 10 mg doses, as shown in Table 3. The half-life of donepezil averaged 72.7±10.6 h and 73.5±11.8 h for the 5 and 10 mg donepezil doses, respectively, and time-averaged total body clearance was 0.11 l h−1 kg−1 for both donepezil dose groups. Clearance values were unchanged over the course of the study, indicating that the pharmacokinetics of donepezil are stationary, i.e. there is no evidence of saturation, product inhibition or auto-induction. The large apparent volume of distribution (11.8±1.7 and 11.6±1.9 l kg−1 for the 5 and 10 mg donepezil doses, respectively) suggests that donepezil has extensive extravascular distribution. Donepezil accumulated in plasma during the first 2–3 weeks of administration until steady state was achieved (Figure 2). The mean RA of donepezil was 7.2 at the 5 mg dose level and 6.2 at the 10 mg dose level. The difference between the RA values for the two dose groups was not statistically significant.
Figure 1.
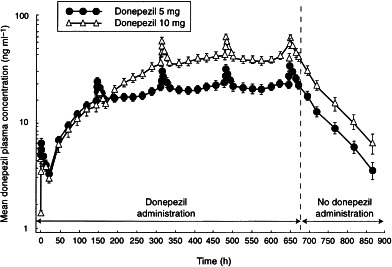
Mean (±SE) plasma concentration–time curves for 5 mg and 10 mg donepezil over the course of the 37-day study. Full pharmacokinetic profiles were undertaken on days 1, 7, 14, 21 and 28. All other time-points represent trough levels.
Table 2.
Pharmacokinetic parameters of donepezil at steady state (mean±SD).
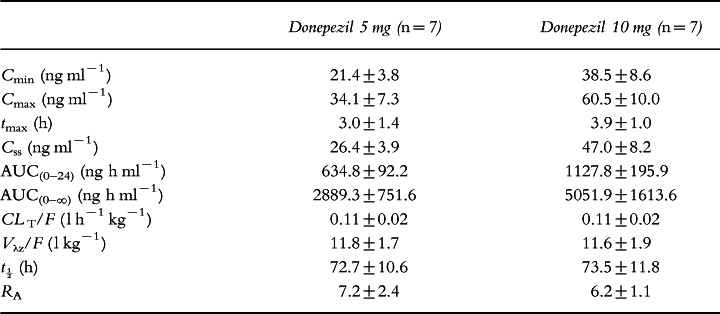
Table 3.
Dose proportionality at steady state (least-squares means).
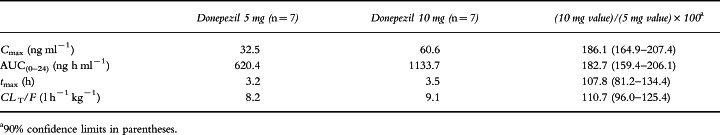
Figure 2.
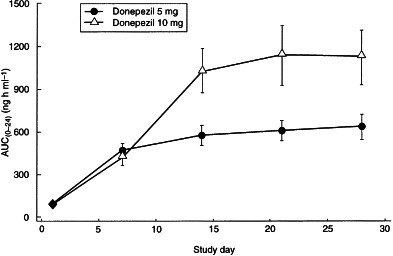
AUC(0–24) (mean±SD) for both dose levels of donepezil during the 28 days of drug administration. AUC(0–24) was calculated on days 1, 7, 14, 21 and 28.
Pharmacodynamics
The rbc-AChE inhibition–time curves are shown in Figure 3 and the rbc-AChE inhibition parameters at steady state are shown in Table 4. Emin was 62.2% and 74.7% and Emax was 71.8% and 83.6%, for the 5 mg and 10 mg dose groups, respectively. Average rbc-AChE inhibition at steady state (Ess) was 65.3% and 77.8% for the 5 mg and 10 mg dose groups, respectively. A significant positive correlation was observed between donepezil plasma concentrations and rbc-AChE inhibition (Figure 4). This relationship was consistent during each of the 24-h periods evaluated (days 1, 7, 14, 21 and 28). The relationship of AUEs demonstrates that there were no time-dependent changes in donepezil pharmacodynamics, such as hysteresis, during this 28-day period (Figure 5).
Figure 3.
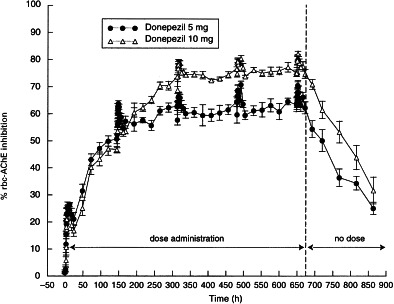
Percentage (mean±SE) rbc-AChE inhibition–time curves for the 5 and 10 mg donepezil doses during 28 days of drug administration.
Table 4.
rbc-AChE inhibition parameters at steady state (mean±SD).
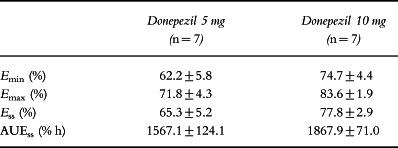
Figure 4.
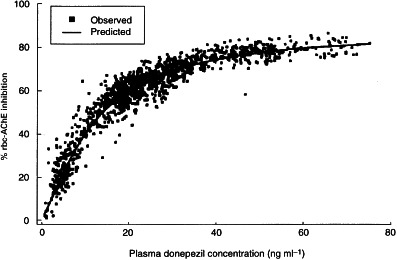
Relationship between plasma donepezil concentrations and percentage rbc-AChE inhibition measured in membranes.
Figure 5.
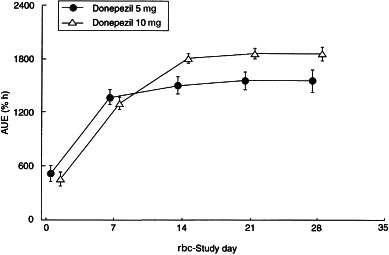
AUE (mean±SD) for rbc-AChE inhibition during the 28 days of drug administration.
Plasma protein binding
The mean (±SD) percentage plasma protein binding values for each subject (Figure 6) were used to calculate an overall mean (±SD) percentage protein binding value of 95.6±0.39%. As shown in Figure 6, protein binding was consistent among all subjects, regardless of the donepezil dose they received, time of sample collection, or the plasma concentration of donepezil in each sample. Hence, the plasma protein binding of donepezil showed no evidence of concentration dependence (saturable binding). The mean protein binding values for each subject were obtained by averaging the individual protein binding values obtained at all seven time-points.
Figure 6.
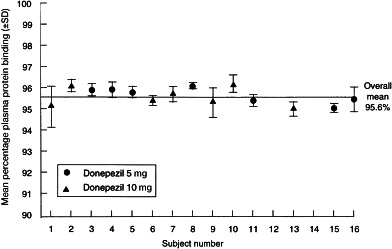
Mean percentage plasma protein binding (±SD) for donepezil in each subject.
Safety
Donepezil was well tolerated by all subjects. The most frequently reported adverse events were headache, gastrointestinal upset, sleep disturbance and lethargy. All were transient, mild and resolved during continued donepezil treatment without the need for adjunctive therapy.
Discussion
The results from this study show that the pharmacokinetics of donepezil are linear and stationary over a 28-day course of once-daily, oral evening administration. Unlike other cholinesterase inhibitors, such as tacrine [11], physostigmine [12] and rivastigmine [13], donepezil has a long mean half-life and slow clearance. However, this slow clearance is unrelated to capacity-limited metabolism. In vitro studies have indicated that donepezil is metabolized primarily by CYP-3A4 and CYP-2D6, but that its affinity for these enzymes is low. This suggests that release of donepezil from AChE and from other low-affinity binding sites (e.g. plasma proteins and rbc membranes) is largely responsible for the slow clearance. Once-daily administration results in a gradual accumulation of donepezil during the first 2–3 weeks of treatment until steady state is achieved. After that time, no additional accumulation of the drug was observed. The once-daily administration is a practical benefit in terms of patient compliance.
The pharmacokinetics of donepezil at steady state were dose proportional, as shown by a comparison of the Cmin, Cmax, Css and AUC(0–24) values for both doses, as well as by the 90% confidence intervals for the ratios of the least-squares means for AUC(0–24) and Cmax at steady state.
Donepezil has extensive extravascular distribution, as reflected by the large apparent volume of distribution (11.8±1.7 and 11.6±1.9 l kg−1 for the 5 and 10 mg donepezil groups, respectively). It was also extensively protein bound (95.6%), and its protein binding is both concentration and time independent. However, previous studies have shown this binding to be high capacity and low affinity in nature (data on file, Eisai Inc.). In vitro protein binding interaction studies with furosemide, digoxin and warfarin have provided evidence that the binding of donepezil neither disrupts, nor is disrupted by, the binding of these compounds (Aricept® US package insert, 1998). This is further supported by the clinical pharmacokinetic interaction studies presented in this supplement.
Inhibition of rbc-AChE was positively correlated with donepezil concentrations in plasma. The pharmacodynamic relationship was consistent over each of the 24-h periods evaluated, demonstrating that there are no time-dependent changes in the pharmacodynamics of donepezil.
The results presented here are consistent with previous clinical study data showing a strong positive correlation between donepezil plasma concentrations and rbc-AChE inhibition [3, 5, 6]. Steady-state rbc-AChE inhibition achieved in this study (65.3±5.2% and 77.8±2.9% for the 5 and 10 mg donepezil doses, respectively) is consistent with that reported from ex vivo measurements in patients with Alzheimer’s disease in clinical efficacy trials [3, 5, 6]. These clinical trials have also reported a significant correlation between rbc-AChE inhibition and improvements in cognitive function. Doses of 10 mg day−1 donepezil produced rates of inhibition of AChE on the upper asymptote of the enzyme inhibition curve, suggesting that further increases in dose would provide only marginal increases in activity.
In summary, the data from this study indicate that the pharmacokinetics and pharmacodynamics of donepezil after evening administration are directly comparable with those following morning administration [14]. The incidence of treatment-emergent adverse events was also similar for both dosing schedules and shows donepezil is well tolerated. Thus, donepezil can be taken either in the morning or at night, a clear benefit for patient compliance.
Acknowledgments
We acknowledge the efforts of Dr James Kisicki, Harris Laboratories Inc, 624 Peach Street, Box 80827, Lincoln, NE 68501, USA, who conducted this clinical trial, and the Institutional Review Board of Harris Laboratories, who reviewed and approved the study and protocol.
References
- 1.Yamanishi Y, Ogura H, Kosasa T, Araki S, Sawa Y, Yamatsu K. Inhibitory action of E2020, a novel acetylcholinesterase inhibitor, on cholinesterase: comparison with other inhibitors. In: Nagatsu T, Yoshida M, Fisher A, editors. Basic, Clinical, and Therapeutic Aspects of Alzheimer’s and Parkinson’s Diseases. Vol. 2. New York: Plenum Press; 1990. pp. 409–413. [Google Scholar]
- 2.Rogers SL, Yamanishi Y, Yamatsu K. E2020—the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–320. [Google Scholar]
- 3.Rogers SL, Friedhoff LT the Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicenter, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SL, Doody RS, Mohs RC, Friedhoff LT. E2020 produces both clinical global and cognitive test improvement in patients with mild to moderately severe Alzheimer’s disease (AD): results of a 30-week Phase III trial. Neurology. 1996;46:A217. (S14.001) [Google Scholar]
- 5.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer’s disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SL, Perdomo C, Friedhoff LT. Clinical benefits are maintained during long-term treatment of Alzheimer’s disease with the acetylcholinesterase inhibitor, E2020. Eur Neuropsychopharmacol. 1995;5:386. (P-8–21) [Google Scholar]
- 8.Rogers SL, Friedhoff LT. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: an interim analysis of the results of a multicentre open label extension trial. Eur Neuropsychopharmacol. 1998;8:67–75. doi: 10.1016/s0924-977x(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Rogers SL, Friedhoff LT, Stiles MR, Cooper NM. Validation and application of an HPLC method for the determination of 1-benzyl-4-[(5,6-dimethoxy-1-indanon)- 2-yl] methyl piperidine HCl (E2020) in human plasma. Pharm Res. 1992;9:350. [Google Scholar]
- 10.Hulse JG, Rogers SL, Friedhoff LT, Sukovaty R, Pederson JE, Lee JN. A radioenzyme assay of acetylcholinesterase activity in red blood cells and its correlation with 1-benzyl-4-[(5,6-dimethoxy-1-indanon)-2-yl] methyl piperidine HCl. Pharm Res. 1992;9:228. [Google Scholar]
- 11.Forgue TS, Reece PA, Sedman AJ, de Vries TM. Inhibition of tacrine oral clearance by cimetidine. Clin Pharmacol Ther. 1996;59:444–449. doi: 10.1016/S0009-9236(96)90114-9. [DOI] [PubMed] [Google Scholar]
- 12.Sharpless NS, Thal LJ. Plasma physostigmine concentrations after oral administration. Lancet. 1985;i:1397–1398. doi: 10.1016/s0140-6736(85)91827-6. [DOI] [PubMed] [Google Scholar]
- 13.Anand R, Hartman RD, Hayes PE, Gharabawi M. An overview of the development of SDZ ENA 713, a brain selective acetylcholinesterase inhibitor. In: Becker R, Giacobini E, editors. Alzheimer Disease: from Molecular Biology to Therapy. Boston: Birkhäuser; 1996. pp. 239–243. [Google Scholar]
- 14.Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):7–12. doi: 10.1046/j.1365-2125.1998.0460s1007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


