Abstract
Aim
The aim of this study was to examine the pharmacokinetics of donepezil HCl and digoxin separately, and in combination, following administration of single oral doses. Changes in cardiac conduction parameters following drug administration were also assessed.
Methods
This was an open-label, randomized, three-period crossover study in healthy male volunteers (n = 12). During each treatment period, subjects received a single dose of either donepezil HCl (5 mg), digoxin (0.25 mg), or a combination of both drugs. Each treatment period was followed by a 2-week, drug-free washout period.
Results
All 12 volunteers completed the study without incident. No statistically significant differences in donepezil pharmacokinetics (Cmax, tmax, AUC(0–120), AUC(0–∞) or t½) were observed when donepezil administered alone was compared with donepezil administered in combination with digoxin. Similarly, no statistically significant differences in digoxin pharmacokinetics were observed when digoxin was administered alone or in combination with donepezil. No clinically relevant changes in cardiac conduction (lead II ECG) were observed in any subject during any treatment period.
Conclusions
Co-administration of single doses of donepezil HCl (5 mg) and digoxin (0.25 mg) produced no changes in the pharmacokinetic profile of either drug. In addition, co-administration produced no changes in cardiac conduction parameters during the 24 h of telemetry monitoring following drug administration.
Keywords: donepezil, digoxin, drug–drug interaction, acetylcholinesterase inhibitor
Introduction
Although Alzheimer’s disease is one of the most common forms of dementia in the elderly, its aetiology remains poorly understood. Nonetheless, it is generally recognized that cholinergic pathways in the cerebral cortex and basal forebrain are compromised in these patients and that the resulting cholinergic deficit contributes to the cognitive impairment that characterizes the disease [1,2]. In support of this hypothesis, the cholinesterase inhibitors have demonstrated the most promising results of any class of drugs for the symptomatic treatment of Alzheimer’s disease [3]. The rationale for using these agents is that they restore the physiological effect of acetylcholine (ACh) by increasing its levels in surviving cholinergic synapses within the cerebral cortex [4]. In addition, evidence suggests that acetylcholinesterase (AChE) may be involved in the regulation of nerve growth as well as in the modulation of functional cholinergic activity [5]. This finding emphasizes the potential importance of developing new and specific AChE inhibitors for the treatment of Alzheimer’s disease.
Donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan) is the first member of a new chemical class of AChE inhibitors, the piperidines [6,7]. It has a high selectivity for AChE in the central nervous system (CNS) and is clinically effective at a dose of 5 mg day−1 [8–12]. Clinical trial results have demonstrated a statistically significant improvement in cognitive and global function at both 5 and 10 mg day−1 doses [10–12]. Donepezil is well tolerated, occasionally producing transient gastrointestinal symptoms such as abdominal pain, nausea and vomiting, which usually resolve with continued drug administration and without the need for dose adjustment. Unlike physostigmine and tacrine, there is no evidence that donepezil produces cardiovascular side-effects or has any effect on hepatic enzymes [12].
Digoxin is prescribed extensively to elderly patients for the treatment of atrial fibrillation and congestive heart failure. It has a narrow therapeutic index and optimal drug dosing is essential. Digoxin is eliminated from the body predominantly by renal excretion [13], a process which is known to be compromised by ageing [14].
The co-administration of any drug that alters the metabolism or elimination of digoxin could thus result in digoxin toxicity or, conversely, sub-therapeutic plasma levels of digoxin, leading to the re-emergence of underlying cardiovascular symptoms. Numerous interactions between digoxin and other drugs have been identified [15]. These include reduction in the bioavailability of digoxin as a result of interactions with antacid gels, antibiotics and kaolin pectate; altered steady-state serum levels when co-administered with quinidine; and altered renal excretion when administered in combination with potassium-sparing diuretics.
As Alzheimer’s disease is a condition that is more prevalent in later life, a significant percentage of Alzheimer’s disease patients will be taking digoxin for the treatment of concomitant cardiac conditions. Hence, an awareness of the potential for a drug–drug interaction is critically important. The present study was designed to determine if either a pharmacokinetic or pharmacodynamic interaction could result following the concurrent administration of single doses of donepezil HCl and digoxin.
Methods
Subjects
This study was conducted in accordance with the principles stated in the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board for Investigations Involving Human Subjects, Harris Laboratories, Lincoln, Nebraska, USA. All subjects provided written informed consent prior to participation in any study activities.
Eligibility for entry into the study was assessed at a screening visit, which was conducted a maximum of 14 days prior to administration of the first dose of trial medication. Subjects with evidence of significant hepatic, gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities were specifically excluded from the study, as were those who had a known or suspected history of alcohol or drug misuse or a positive urine drug screen. None of the subjects had donated blood or had received investigational or prescription medications within 1 month of study enrolment.
Subjects were healthy, Caucasian, male volunteers between 18 and 45 years of age whose body weights, ranging from 60 to 85 kg, were within 20% of ideal weight, based on the Metropolitan Insurance Company Height and Weight Tables (1983).
Protocol
This was an open-label, randomized, single-dose, three-period crossover study. Subjects were randomized to a particular treatment according to a computer-generated randomization schedule. The three treatments administered in this study were: (1) donepezil HCl, 5 mg tablet, (2) digoxin, 0.25 mg tablet (Lanoxin®, Burroughs Wellcome Laboratories), or (3) a combination of donepezil HCl (5 mg) and digoxin (0.25 mg). During each treatment period, subjects received a single oral dose of either donepezil or digoxin alone, or both drugs in combination. Each treatment period was 6 days in duration (drug administration plus blood sampling), followed by a 2-week, drug-free washout period. The dose of donepezil was chosen based upon clinical efficacy studies conducted in the USA, and the dose of digoxin used is the recommended clinical dose provided by the Physicians’ Desk Reference.
All volunteers were screened by medical history, ECG plus laboratory and physical examinations ≤2 weeks prior to the start of the study. During the course of the study, subjects were not allowed to consume caffeine-containing food or drinks or to sunbathe, and physical exercise was limited to normal walking. Over-the-counter products were discontinued from 72 h prior to admission into the trial until the end of the study.
For each treatment period, subjects were admitted to the study site on the evening of day 0, at least 12 h prior to drug administration. Subjects were fasted overnight (8 h), prior to receiving their first dose of medication on the morning of day 1.
Sample collection and analysis
Venous blood samples for analytical determinations of both digoxin and donepezil were collected 1 h prior to drug administration (−1 h) and at 1, 2, 3, 4, 6, 8, 12, 18, 24, 48, 72, 96 and 120 h following drug administration. Immediately after collection, all blood samples were placed on ice prior to centrifugation for 15 min (2000 g) at 4° C. Plasma was removed, transferred into polypropylene tubes and stored upright at −20° C until analysis.
Plasma donepezil concentrations were measured using a specific high-performance liquid chromatography (HPLC) method with UV detection, with a lower detection limit of 2.0 ng ml−1 [16]. Digoxin concentrations were determined by radioimmunoassay using the Becton Dickenson Digoxin Solid Phase Component System™. The assay was based on the inverse relationship between the competitive binding of [125I]-labelled digoxin and unlabelled digoxin by digoxin-specific antibodies immobilized on the wall of polypropylene sample tubes. This method had upper and lower detection limits of 2.5 and 0.1 ng ml−1, respectively.
Pharmacokinetic assessments
Characterization of donepezil and digoxin pharmacokinetics for each treatment group was conducted using plasma drug concentration data from samples collected over a 120-h period following drug administration. All volunteers who received test medication and who had analytical samples collected were evaluated.
The donepezil and digoxin plasma concentrations from each subject were plotted as log concentration versus time. The pharmacokinetic parameters were estimated by a non-compartmental method for both drugs. The peak plasma concentration (Cmax) of each drug, time of occurrence of Cmax (tmax) and the terminal disposition phase for each subject were derived from inspection of log concentration–time curves. The terminal disposition rate constant (λz) was estimated as −2.303 times the slope of the best-fit linear regression line of the terminal phase, the terminal half-life (t½) was calculated as 0.693/λz and the area under the plasma concentration–time curve from 0 to 120 h (AUC(0–120)) was estimated using the trapezoidal rule. The area under the plasma concentration–time curve extrapolated to infinity (AUC(0–∞)) was calculated as AUC(0–120) plus the last measured concentration divided by λz.
Safety assessments
All adverse events reported spontaneously by the subjects and/or observed or elicited during physical examination were documented together with times of onset and cessation, plus assessment of severity and causality. Vital signs were monitored at regular intervals during the in-house portion of each treatment period. Laboratory evaluations were conducted at the start of each treatment period. Telemetry monitoring (lead II ECG) began 1 h prior to drug administration and continued for 24 h after each dose. Lead II rhythm strips were generated prior to drug administration and 1, 2, 3, 4, 6, 8, 10, 12, 18 and 24 h post-dose. Heart rate, PR interval, QRS duration and QT interval were recorded.
Statistical analysis
An analysis of variance model (ANOVA), accounting for the effects of treatment, period, sequence and subject, was used to compare the pharmacokinetic parameters derived during the three treatment periods. The type III sum of squares for all model effects was used to determine statistical significance at the 0.05 level.
Results
Subjects
The study population comprised 12 healthy, Caucasian, male volunteers with a mean age of 28.3 years (range 19–44 years). Their heights ranged from 171 to 191 cm (mean 179.9 cm) and their body weights from 66.5 to 85.0 kg (mean 77.6 kg). Each of the 12 subjects completed the study without incident.
Pharmacokinetics of donepezil
No statistically significant differences in donepezil pharmacokinetic parameters were observed when donepezil administered alone was compared with donepezil administered concurrently with digoxin. As shown in Table 1, the values for tmax and Cmax were identical for both treatment groups (4.5±0.4 h and 6.3±0.3 ng ml−1, respectively), and the values for AUC(0–120), AUC(0–∞) and t½ differed only marginally (Table 1). In addition, there were no significant sequence or period effects observed between the treatment groups. Mean plasma concentration–time profiles of both donepezil treatment groups (donepezil alone and in combination with digoxin) are shown in Figure 1.
Table 1.
Donepezil pharmacokinetic parameters (mean±SE).
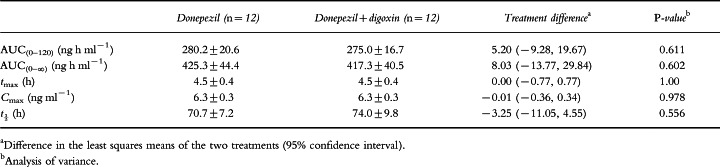
Figure 1.
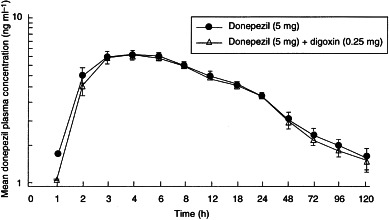
Mean (±SE) donepezil plasma concentration–time profiles for the donepezil-only and donepezil+digoxin treatment groups.
Pharmacokinetics of digoxin
No statistically significant differences in digoxin pharmacokinetic parameters were observed when digoxin administered alone was compared with digoxin administered with donepezil. As shown in Table 2, the values of Cmax were identical for both groups (0.8±0.1 ng ml−1), and the values for tmax, AUC(0–120), AUC(0–∞) and t½ differed only marginally (Table 2). In addition, there were no significant sequence or period effects observed between treatment groups. Mean plasma concentration–time profiles of both digoxin treatment groups (digoxin alone and in combination with donepezil) are shown in Figure 2.
Table 2.
Digoxin pharmacokinetic parameters (mean±SE).
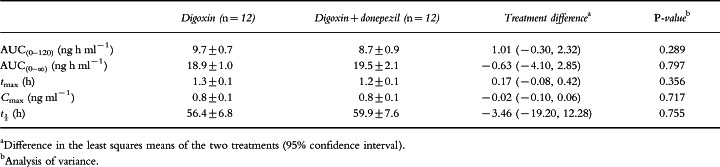
Figure 2.
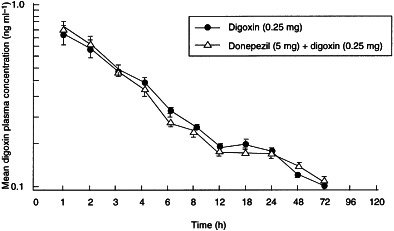
Mean (±SE) digoxin plasma concentration–time profiles for the digoxin-only and donepezil+digoxin treatment groups.
Telemetry
Increases and decreases in heart rate (bpm) and duration (s) of the PR and QRS intervals were observed for all of the subjects during the 24 h of lead II ECG monitoring following drug administration. However, these changes were typically small (±2%) and there were no discernible trends among the treatment groups which would relate these parameters to a particular time course or to a treatment effect. The observed fluctuations were judged by the investigators not to be clinically relevant.
Vital signs
The mean changes from baseline for radial pulse rate, systolic and diastolic blood pressure for each treatment group indicate that the co-administration of donepezil and digoxin does not influence blood pressure or pulse rate to a greater degree than the administration of donepezil or digoxin alone.
Adverse events
No serious adverse events were reported during this study. Only five of the 12 subjects enrolled in this study reported any adverse events during the course of this trial. These events included fatigue, headache, diarrhoea, light-headedness and vivid dreams. All reported adverse events were mild to moderate in severity.
Discussion
The primary objective of this study was to examine the pharmacokinetics of donepezil and digoxin separately, and in combination following administration of single oral doses. The basis for this approach was to determine if a pharmacokinetic interaction existed between these two drugs and also to determine if their concurrent administration produced any clinically significant changes in cardiac parameters (lead II ECG).
Although several interactions between digoxin and other drugs have been identified [15], including a reduction in bioavailability with antacid gels, antibiotics and kaolin pectate, altered steady-state serum levels with quinidine and altered renal excretion with potassium-sparing diuretics, it was not anticipated that the concurrent administration of donepezil would alter the pharmacokinetics of digoxin. This assumption was based on findings from in vitro studies with donepezil (Aricept® US package insert, 1998). Isoform-selective substrate studies were conducted in human liver microsomes and the donepezil concentrations required for 50% inhibition (IC50) of CYP-450 enzymes 1A2, 2C9, 2C19, 2D6 and 3A4 were calculated. All IC50 values were greater than 100 μm. In addition, the mean ki values for CYP-3A4 and CYP-2D6 were calculated and were found to be 131 μm and 47 μm, respectively. Clinical studies have shown that the steady-state Cmax concentration for the 10 mg dose of donepezil is approximately 164 nm. Since the anticipated therapeutic concentrations of donepezil are more than 280-fold lower than the lowest ki value obtained with CYP-2D6 and almost 800-fold lower than the ki observed with CYP-3A4, it is anticipated that donepezil will not inhibit the metabolism of other drugs metabolized by these or any other CYP-450 isoenzymes.
No statistically significant differences in donepezil or digoxin pharmacokinetic parameters (Cmax, tmax, AUC(0–120), AUC(0–∞) or t½) were observed when the pharmacokinetic profiles of these drugs administered alone were compared with the profiles of the drugs administered in combination. In addition, no clinically significant changes in cardiac conduction were observed during the 24 h of post-drug telemetry monitoring (lead II ECG), nor were there any clinically significant changes in vital signs during the course of the study. These data demonstrate that no pharmacokinetic interaction follows concurrent single-dose, oral adminstration of these drugs. In addition, the absence of an interaction following single-dose administration suggests the same lack of effect on the plasma levels of these drugs at steady state.
Acknowledgments
We acknowledge the efforts of Dr James Kisicki, Harris Laboratories Inc, 624 Peach Street, Box 80827, Lincoln, NE 68501, USA, who conducted this clinical trial, and the Institutional Review Board of Harris Laboratories, who reviewed and approved the study and protocol.
References
- 1.Katzman R, Saitoh T. Advances in Alzheimer’s disease. FASEB J. 1991;5:278–286. [PubMed] [Google Scholar]
- 2.Becker RE. Therapy of the cognitive deficit in Alzheimer’s disease: the cholinergic system. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 1–30. [Google Scholar]
- 3.Giacobini E. Pharmacotherapy of AD: new drugs and novel strategies. In: Corain B, Iqbal K, Nicolini M, Winblad B, Wisniewski HM, Zatta PF, editors. Alzheimer’s Disease—Advances in Clinical and Basic Research. New York: Wiley; 1993. pp. 529–538. [Google Scholar]
- 4.Giacobini E. Therapy for Alzheimer’s disease: symptomatic or neuroprotective? Mol Neurol. 1994;9:115–117. doi: 10.1007/BF02816110. [DOI] [PubMed] [Google Scholar]
- 5.Small DH, Michaelson S. Do cholinesterases have functions unrelated to neurotransmission? Today’s Life Sci. 1995;7:24–30. [Google Scholar]
- 6.Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and anti-acetylcholinesterase activity of 1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine hydrochloride (E2020) and related compounds. Bioorg Med Chem Lett. 1992;2:871–876. doi: 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- 7.Iimura Y, Mishima M, Sugimoto H. Synthesis of 1-benzyl-4-[(5,6–dimethoxy[2−14C]-1-indanon)-2-yl]-methylpiperidine hydrochloride (E2020-14C) J Label Compounds Radiopharm. 1989;XXVII:835–839. [Google Scholar]
- 8.Rogers SL, Yamanishi Y, Yamatsu K. E2020—the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–320. [Google Scholar]
- 9.Sherman K. Pharmacodynamics of oral E2020 and tacrine in humans: novel approaches. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 321–328. [Google Scholar]
- 10.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicenter, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 12.Rogers SL, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer’s disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 13.Roden DM. Antiarrythmic drugs. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. pp. 839–874. [Google Scholar]
- 14.Nies AS, Spielberg SP. Principles of Therapeutics. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. pp. 43–62. [Google Scholar]
- 15.Marcus FI. Pharmacokinetic interactions between digoxin and other drugs. J Am Coll Cardiol. 1985;5:82A–90A. doi: 10.1016/s0735-1097(85)80466-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Rogers SL, Friedhoff LT, Stiles MR, Cooper NM. Validation and application of an HPLC method for the determination of l-benzyl-4-[(5,6-dimethoxy-1-indanon)- 2-yl] methyl piperidine HCl (E2020) in human plasma. Pharm Res. 1992;9:350. [Google Scholar]


