Abstract
Aim
The aim of the study was to evaluate the pharmacokinetics of theophylline administered alone, and in combination with donepezil HCl, following multiple-dose administration of both drugs in healthy volunteers.
Methods
This was an open-label, randomized, two-period crossover study in healthy male volunteers (n = 12). During each treatment period, subjects received either titrated-dose theophylline alone, or in combination with donepezil (5 mg, once daily) for 10 consecutive days. On day 10 of each treatment period, serial blood samples for the determination of theophylline concentrations in plasma were measured up to 24 h. Treatment periods were separated by a 3-week, drug-free washout. Plasma concentrations of theophylline were determined by HPLC with UV detection.
Results
No statistically significant differences in theophylline pharmacokinetics (Cmax, AUC or tmax) were observed between theophylline administered alone and in combination with donepezil. No clinically significant changes in vital signs, ECG parameters or clinical laboratory tests were observed in any subject during any treatment period.
Conclusions
Concurrent administration of donepezil HCl does not alter the pharmacokinetic profile of theophylline following multiple-dose administration of both drugs in healthy volunteers. These findings suggest that donepezil may be safely co-administered with theophylline without a need for dose modification or additional monitoring procedures.
Keywords: donepezil, theophylline, drug–drug interaction, acetylcholinesterase inhibitor
Introduction
Donepezil HCl (also known as E2020 or Aricept®, the registered trademark of Eisai Co. Ltd, Tokyo, Japan) is a piperidine-based inhibitor of the enzyme acetylcholinesterase (AChE) [1, 2]. It has recently been approved for marketing in the USA, Canada and several EU member states, including the UK, for the symptomatic treatment of mild–moderate Alzheimer’s disease. In vitro studies have demonstrated that donepezil has a significantly greater degree of selectivity for AChE in the central nervous system (CNS) than for butyrylcholinesterase (BuChE) in the periphery [2, 3]. Clinical trials undertaken in the USA and Europe have demonstrated that donepezil (5 mg or 10 mg, once daily) significantly improves cognitive and global function in patients with Alzheimer’s disease [4–7]. Furthermore, these studies have shown that donepezil is well tolerated and is not associated with the hepatotoxicity that is commonly seen with acridine-based cholinesterase inhibitors, such as tacrine [8]. Phase I studies conducted in the USA [9] have demonstrated that donepezil pharmacokinetics are linear and dose proportional, and are characterized by slow plasma clearance and a long half-life (70–80 h) [10, 11]. Although donepezil is metabolized primarily by the P-450 isoenzyme CYP-3A4, and to a lesser extent by CYP-2D6, compromised hepatic function does not significantly affect its pharmacokinetic profile [12].
Since its recognition as a broncodilator almost 50 years ago, theophylline, a methylxanthine alkaloid, has played an important role in the management of both acute and chronic reversible airway obstruction, in particular asthma and chronic bronchitis. However, it has a narrow safety window, which, along with its variability in disposition, makes dosing difficult to predict and toxicity difficult to prevent. Symptoms of toxicity include tachycardia, severe restlessness, agitation, nausea and vomiting, particularly at high blood concentrations [13, 14].
The physical status of a patient can greatly alter theophylline metabolism and/or elimination, resulting in toxic plasma levels. Several disease states, as well as advanced age, smoking and obesity, have been shown to influence the pharmacokinetics of theophylline [13]. Previous reports have also demonstrated that the half-life of theophylline is prolonged, and plasma clearance decreased, in patients with liver disease, acute pulmonary oedema and congestive heart failure [13, 14].
Theophylline is prone to clinically significant interactions with other drugs. For instance, the clearance of theophylline is increased substantially during the concomitant administration of phenytoin [15]. Rifampin and oral contraceptives produce smaller but appreciable increases in theophylline clearance, whereas cimetidine and erythromycin have been reported to reduce the clearance of theophylline [15]. With this in mind, the present study was designed to determine whether concurrent, multiple-dose administration of sustained-release theophylline with donepezil would produce clinically relevant changes in theophylline pharmacokinetics in healthy volunteers.
Methods
This study was conducted in accordance with Good Clinical Practice Guidelines issued by the European Commission (1990). The protocol was approved by an independent ethics committee and written informed consent was obtained from each volunteer prior to participation in any study activities.
Subjects
The study population comprised 12 healthy male subjects between 18 and 36 years of age who were within 15% of normal weight for their height and body build, based on the Metropolitan Insurance Company Height and Weight Tables (1983). The study subjects were all non-smokers and each had normal blood pressure and ECG at screening. Subjects with evidence of clinically significant cardiovascular, respiratory, gastrointestinal, hepatic, endocrine, renal, neurological, psychiatric and/or haematological disease were excluded from the trial. Subjects with a history of severe adverse drug reactions or leucopenia, severe or multiple allergies or habitual drug and/or alcohol abuse were also excluded from entering the trial, as were those who had participated in other drug trials in the previous 3 months.
Protocol
This was an open-label, randomized, two-period, balanced crossover study. Prior to initiation of the first treatment period, a 1-week pre-trial titration was conducted to determine the dose of theophylline needed to achieve plasma concentrations within the accepted therapeutic range of 10–20 mg l−1 for each subject. During this titration phase, subjects attended the clinic each day at 08:00 h to receive their daily dose of theophylline (TheoDur®, Astra Pharmaceuticals Ltd). On alternate days, their trough (pre-dose) plasma theophylline levels (Cmin) were measured. The starting dose of theophylline was 200 mg, twice daily. This was titrated upwards every second day according to the measured plasma levels in order to achieve the target theophylline Cmin of approximately 10 mg l−1. In cases where Cmin was below 5 mg l−1, the dose of theophylline was doubled. For values of Cmin between 5 mg l−1 and 10 mg l−1, a proportionate adjustment was made according to availability of different-sized tablets. Volunteers were excluded from the study if they presented with unacceptable side-effects during the titration phase. Following completion of the titration phase, there was a 1-week, drug-free washout period prior to initiation of the first treatment period.
The two treatments administered in this study were: (1) theophylline, and (2) the combination of theophylline+ donepezil (5 mg, once daily), with subjects receiving the treatments in random order. The 5 mg day−1 dose of donepezil chosen for this study is the approved recommended starting dose in the USA and Europe, and was selected on the basis of its clinical efficacy [4, 6, 7]. Treatment periods were 10 days in duration and separated by a 3-week, drug-free washout period. During the first 4 days of each treatment period, the dose of theophylline was re-titrated to the intended final dose in order to reduce the incidence of adverse events. This titration scheme was as follows: administration of a quarter of the final theophylline dose for the first 2 days, half the final dose for the next 2 days and the full dose for the remaining 6 days of each 10-day treatment period. The theophylline dose was taken twice daily. Each day at 08:00 h and following an 8-h overnight fast, subjects were administered theophylline alone or theophylline+donepezil, depending on the treatment being administered, with 240 ml of water. Subjects were then permitted to return home, where they took the remainder of their daily theophylline dose at 20:00 h.
Subjects were prohibited from taking any other medication, including non-prescription preparations, from 48 h prior to entering the study until completion of the safety monitoring visit 2 weeks after the last dose of trial medication. Subjects abstained from alcohol and caffeine-containing drinks and food from 24 h prior to the start until completion of each study period.
Sample collection and analysis
Venous blood samples for the determination of theophylline concentrations were taken at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h after the morning dose. Additional 0- and 24-h blood samples were collected for the determination of donepezil plasma concentrations.
Theophylline Cmin values were measured on days 2, 4, 6, 7, 8 and 9, approximately 12 h after dose administration. Theophylline was analysed in plasma after solid-phase extraction, using a standard high-performance liquid chromatography (HPLC) method with UV detection. The lower limit of quantification was 0.74 mg l−1. Titration-phase concentrations of theophylline were assessed using the Beckman Synchron CX5 theophylline reagent (P/N 442897). There was good correlation between this method and the HPLC assay used to measure concentrations during the treatment periods.
Pharmacokinetic assessments
Pharmacokinetic parameters for theophylline were estimated using model-independent methods. The data were analysed separately for the 0–12 h and 12–24 h dosing intervals. Peak plasma concentration (Cmax) and the time at which peak plasma concentration occurred (tmax) were obtained from inspection of the plasma concentration–time curves, with tmax recorded separately for each dose of theophylline. The interdosing area under the plasma concentration–time curve (AUC) was estimated using the linear trapezoidal approximation for the intervals 0–12 h (AUC(0–12)) and 12–24 h (AUC(12–24)).
Safety assessments
All adverse events experienced by the subjects and/or observed or elicited during physical examination were recorded together with times of onset and cessation, and an assessment of their severity and causality. Systolic and diastolic blood pressure and pulse rate were monitored at each visit according to the guidelines of the British Hypertension Society. Measurements were taken after the subject had been sitting for 5 min and standing for 2 min. The point of disappearance of Korotkoff’s phase V sounds was used to determine diastolic pressure. A standard 12-lead ECG was recorded at the screening visit and on completion of the trial.
Routine haematology, clinical chemistry and urinalysis tests were performed prior to and 24 h after drug administration on day 10 of both treatment periods. Haematological assessments included haemoglobin, haematocrit, red blood cell count, platelet count, white blood cell count and differential. Clinical chemistry included liver function (aspartate transaminase, alanine transaminase, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin), renal function (urea, creatinine, uric acid), metabolic status (glucose, total protein, albumin, globulin) and blood electrolytes (sodium, potassium, chloride, bicarbonate, calcium, phosphate). Routine urinalysis (Bayer Multistix®) was also performed (pH, glucose, protein, haemoglobin or blood, ketones), together with specific gravity and microscopic examination of the urine sediment.
Statistical analysis
Cmax and AUC for theophylline administered alone and in combination with donepezil were compared using analysis of variance (ANOVA). The analysis was undertaken on log-transformed data using the GLM procedure of the SAS for Windows 6.10 software (SAS Institute Inc., Cary, North Carolina, USA). It included factors for subject, treatment, period and treatment-by-period interaction. The Wilcoxon rank sums test was used to compare tmax between the treatment regimens.
Results
Subjects
Twenty-six volunteers were screened: 16 entered the dose titration period and 13 were recruited into the study. Subjects were between 19 and 36 years of age (median 24 years); 63 and 101 kg in weight (median 73 kg); and between 174 and 194 cm in height (median 181 cm).
One subject had a positive drug screen during the study and was withdrawn from further treatment. Data from the remaining 12 volunteers who completed all phases of the study were used in the pharmacokinetic analyses.
Pharmacokinetics of theophylline
The pharmacokinetic parameters for theophylline alone and in the presence of donepezil are summarized in Table 1. No statistically significant differences in theophylline pharmacokinetics were observed whether theophylline was administered alone or concurrently with donepezil. The values (±SD) of Cmax (11.45±1.52 and 11.01±1.45 mg l−1), and AUC(0–12) (112.0±15.0 and 113.0±18.0 mg h l−1) were virtually identical for the theophylline and the theophylline+donepezil groups, respectively. The 90% confidence intervals for the ratios of Cmax and AUC for the theophylline+donepezil group relative to the theophylline only group were close to one (Table 1). There were no statistically significant treatment-by-period interactions for any of the pharmacokinetic parameters. Plasma concentration–time profiles for theophylline alone and theophylline in the presence of donepezil are presented in Figure 1.
Table 1.
Pharmacokinetic parameters of theophylline in healthy volunteers (n = 12) following twice-daily administration of sustained-release theophylline tablets administered alone or concurrently with donepezil (5 mg day−1) for 10 days (mean±SD).
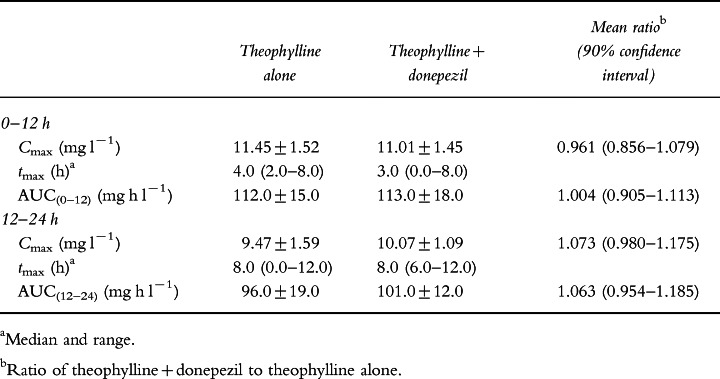
Figure 1.
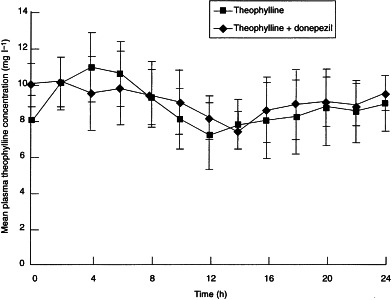
Mean (±SD) theophylline plasma concentration–time curves following 10 days of twice-daily administration of theophylline sustained-release tablets taken alone or in the presence of donepezil (5 mg day−1) by 12 healthy male volunteers.
Safety
Both treatments were well tolerated and no clinically significant adverse events were reported during the course of the study. Those events that were reported were mild in severity and transient. When theophylline was administered alone, adverse events included nausea, headache, dizziness and feelings of agitation. During the theophylline+donepezil treatment phase, they included nausea, light-headedness, insomnia, heart palpitations and pruritus.
No clinically significant changes in vital signs, ECG parameters or clinical laboratory tests were observed.
Discussion
The objective of this study was to investigate the possibility of a pharmacological interaction between donepezil and theophylline following concurrent multiple-dose administration of these drugs in healthy volunteers. This is an important study in view of the potential severity of theophylline toxicity coupled with the reduction in theophylline clearance in the elderly [16], the target population for donepezil. The rate of decrease in theophylline clearance has been estimated to be 1.25% per year between the ages of 18 and 77, and the average reduction in theophylline clearance in elderly relative to young healthy volunteers is 34% [17].
Various pharmacokinetic interactions are known to exist between theophylline and commonly used drugs. Perhaps the most serious of these is the interaction observed with the quinolone antibiotics, enoxacin and ciprofloxacin, both of which substantially reduce the clearance of theophylline. In some patients, this has resulted in plasma concentrations of theophylline above the generally accepted level of 20 mg l−1 [18]. This pharmacokinetic interaction precipitates theophylline-induced convulsions which, in some cases, are difficult to control with anticonvulsant therapy [13]. Although such interactions between theophylline and other drugs have been identified, it was anticipated that concurrent administration of donepezil would not alter the pharmacokinetics of theophylline in this study. This assumption was based on findings from in vitro studies with donepezil (Aricept® US package insert, 1998). Isoform-selective substrate studies were conducted in human liver microsomes and the concentrations of donepezil required for 50% inhibition (IC50) of CYP-450 enzymes 1A2, 2C9, 2C19, 2D6 and 3A4 were calculated. All IC50 values were greater than 100 μm. In addition, the mean ki values for CYP-3A4 and CYP-2D6, the primary isoenzymes responsible for donepezil metabolism, were calculated to be 131 and 47 μm, respectively. Clinical studies have shown that steady-state Cmax concentrations for the 10 mg dose of donepezil are approximately 164 nm at the level of the hepatocyte. Since it is anticipated that therapeutic concentrations of donepezil are more than 280-fold lower than the lowest ki value obtained with CYP-2D6 and almost 800-fold lower than the ki observed with CYP-3A4, it is unlikely that donepezil will inhibit the metabolism of other drugs metabolized by these or any other CYP-450 isoenzymes.
The results of the present study demonstrate that donepezil does not alter the pharmacokinetic or safety profile of sustained-release theophylline in healthy volunteers. The larger values of Cmax and AUC(0–12) and the smaller value of tmax seen after the morning dose of theophylline reflect previously reported diurnal variation between morning and evening doses [19].
In conclusion, the concurrent administration of theophylline with donepezil (5 mg day−1) does not alter the pharmacokinetics of sustained-release theophylline in healthy volunteers. Thus, the concurrent administration of theophylline and donepezil in clinical practice is unlikely to increase theophylline concentrations. This suggests that donepezil may be safely administered to Alzheimer’s disease patients who are also taking stable doses of theophylline, without the need for dose modification or additional monitoring.
Acknowledgments
We acknowledge the efforts of Drs P. E. Rolan and S. Toon of Medeval Ltd, University of Manchester, Skelton House, Manchester Science Park, Lloyd St North, Manchester M15 6SH, UK, who conducted this clinical trial, and the Internal Ethics Committee of Medeval Ltd, who reviewed and approved the study and protocol.
References
- 1.Iimura Y, Mishima M, Sugimoto H. Synthesis of 1-benzyl-4-[(5,6-dimethoxy[2−14C]-1-indanon)-2-yl]methylpiperidine hydrochloride (E2020–14C) J Label Compounds Radiopharm. XXVII:835–839. [Google Scholar]
- 2.Rogers SL, Yamanishi Y, Yamatsu K. E2020—the pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 314–320. [Google Scholar]
- 3.Sherman K. Pharmacodynamics of oral E2020 and tacrine in humans: novel approaches. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 321–328. [Google Scholar]
- 4.Rogers SL, Friedhoff LT the Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicenter, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 5.Rogers SL, Friedhoff LT. Long-term efficacy and safety of donepezil in the treatment of Alzheimer’s disease: an interim analysis of the results of a US multicentre open label extension study. Eur Neuropsychopharmacol. 1998;8:67–75. doi: 10.1016/s0924-977x(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SL, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer’s disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 7.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA. 1994;271:992–998. [PubMed] [Google Scholar]
- 9.Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol. 1998;46(Suppl. 1):7–12. doi: 10.1046/j.1365-2125.1998.0460s1007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers SL, Walters EJ, Friedhoff LT. The pharmacokinetics (PK) and pharmacodynamics (PD) of E2020 [(R,S)-1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine hydrochloride] a novel inhibitor of acetylcholinesterase (AChE): implications for use in the treatment of Alzheimer’s disease. Neurobiol Aging. 1992;13(Suppl. 1):S125–S126. [Google Scholar]
- 11.Rogers SL, Friedhoff LT. The pharmacokinetic (PK) and the pharmacodynamic (PD) profile of donepezil HCl (E2020) following single and multiple oral doses. Clin Pharmacol Ther. 1997;61 181 (PII–63) [Google Scholar]
- 12.Tiseo PJ. Vargas R, Perdomo CA, Friedhoff LT. An evaluation of the pharmacokinetics of donepezil HCl in patients with impaired hepatic function. Br J Clin Pharmacol. 1998;46(Suppl. 1):51–55. doi: 10.1046/j.1365-2125.1998.0460s1051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafin WE. Drugs used in the treatment of asthma. In: Gilman AG, Molinoff PB, Ruddon RW, Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Ninth. New York: McGraw-Hill; 1995. pp. 659–682. [Google Scholar]
- 14.Griffin JP, D’Arcy PF, Speirs CJ. Manual of Adverse Drug Interactions. Fourth. London: John Wright; 1988. Theophylline and derivatives; pp. 427–439. [Google Scholar]
- 15.Reynolds JEF. Martindale, The Extra Pharmacopoeia. 31. London: The Pharmaceutical Press; 1996. pp. 1657–1665. [Google Scholar]
- 16.Daigneault EA, Hamdy RC, Ferslew KE, et al. Investigation of the influence of acetylsalicylic acid on the steady state of long-term therapy with theophylline in elderly male patients with normal renal function. J Clin Pharmacol. 1994;34:86–90. doi: 10.1002/j.1552-4604.1994.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Chen G. Evaluation of theophylline population pharmacokinetics in adult patients using NONMEM analysis. Chung Kuo Yao Li Hsueh Pao. 1994;15:267–270. [PubMed] [Google Scholar]
- 18.Schwartz J, Jauregui L, Lettieri J, Bachmann K. Impact of ciprofloxacin on theophylline clearance and steady-state concentrations in serum. Antimicrob Agents Chemother. 1988;32:75–77. doi: 10.1128/aac.32.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison LI, Kehe CR, Ekholm BP, Chang SF, Lavoie KA, Kisicki JC. Comparative pharmacokinetics of morning and evening doses of once-a-day theophylline capsules. J Pharm Sci. 1994;83:1171–1174. doi: 10.1002/jps.2600830818. [DOI] [PubMed] [Google Scholar]


