Abstract
Aims
Vigabatrin is a new antiepileptic medication consisting of a racemic mixture of 50% active S enantiomer and 50% inactive R enantiomer. Since patients suffering from epilepsy may become pregnant, it is important to understand the extent of placental transfer of such medication.
Methods
During steady-state, vigabatrin enantiomer concentrations were measured in maternal and umbilical blood and in breast milk of two patients.
Results
The concentration ratios from the umbilical vein to maternal plasma were R:0.068, S:0.16; 4h25 min after drug administration (case 1) and R:1.39, S:0.91; 9h after drug administration (case 2). The milk:plasma concentration ratio was lower than 1 at pre dose sampling in both cases, as well as 3 and 6 h post dose in one case. An estimate of the maximum amount of R and S enantiomers of vigabatrin that a suckling infant would ingest in a day is 3.6% and 1% of the weight-adjusted daily dose respectively.
Conclusions
These results would suggest a slow placental transfer of the vigabatrin enantiomers and that the quantity ingested through milk is small.
Keywords: vigabatrin, enantiomers, transfer, placenta, breast milk
Introduction
Vigabatrin is an anticonvulsant drug, supplied as a racemic mixture of two enantiomers. The S enantiomer possesses pharmacological activity, whereas the R enantiomer is inactive. At peak, (tmax R:0.8±0.2 h, S:1±0.5 h) concentrations of the R enantiomer exceeded concentrations of the S enantiomer, with an approximate ratio of 2:1 [1, 2]. The mean terminal half-life ranged from 6 to 8 h for both enantiomers in adults [1]. In children (8.7±3.8 years) the mean half-lives were similar for the S enantiomer (5.47±1.93 h) and for the R enantiomer (5.68±2.86 h). In younger children (12.1±5.9 months) the half-life was shorter for the R enantiomer (2.87±1.03 h) compared with the S enantiomer (5.65±1.52 h) [2].
A medication that has been found to be essential in the clinical management of a woman, is difficult to withdraw during a pregnancy. Vigabatrin often falls within this category and it is vital to understand the placental transfer of such a drug, in order to evaluate any possible risks involved. Although in vitro data of the placental transfer of the enantiomers of vigabatrin is available [3], in vivo data are needed to accurately assess the potential of foetal exposure.
Likewise, after confinement, any decision about breast feeding needs information on the amount of a drug which might pass into the milk, in particular if the drug could have an effect on the infant’s developing central nervous system.
This paper reports data from two patients providing some answers to these issues.
Methods
Patients
The data were not collected through a formal planned prospective study. Nevertheless both patients were fully involved in the importance of the investigation and freely gave their consent to participate.
Case 1
A 32 year old Caucasian woman, received 1000 mg twice daily vigabatrin and 200 mg three times daily carbamazepine (slow release) for previously intractable partial seizures in her final trimester.
Case 2
A 33 year old Caucasian woman, received 1000 mg twice daily vigabatrin throughout her pregnancy. The patient gave birth to a 3.2 kg newborn of 41 weeks gestation.
Sample managements
Immediately following birth a blood sample was collected from the mother and the umbilical vein (case 1) and from the umbilical vein and artery (case 2).
On the seventh day, post partum, maternal venous blood and breast milk were concomitantly collected at predose and 3 and 6 h post-dose (case 1). On the eighth day post partum maternal venous blood was collected at predose, breast milk at predose and 3 and 6 h post-dose. The milk was collected from both breasts (30 ml) using a breast-pump prior to feeding (case 2).
Analytical procedure
The concentrations of the enantiomers of vigabatrin were measured in plasma using a GC-MS method as previously described [2]; the enantiomers of vigabatrin being analysed as N-trifluoroacetyl-O-propyl ester derivatives. The procedure for the analysis in milk required an additional clean-up step which involved passing the sample extract through a conditioned Dowex microcolumn and subsequent elution of the analytes of interest. Cow’s milk was used as the blank matrix for the validation of the analytical method for use with human milk samples. Whilst the composition of cow’s milk differs, predominantly in protein content, from human milk, deproteinisation is the first step in the extraction procedure (100 μl of trichloroacetic acid 6% for 200 μl of milk). The detailed analytical method was as follows: the deproteinized milk sample was added on a preconditionned column [Dowex 50W×8 (Fluka): 200 mg, washed with HCl (3n; 1 ml) and water (1 ml)] washed with water (1 ml) and eluted with NH4 OH (3n; 1 ml). The eluate was evaporated to dryness at 40° C under a stream of nitrogen. Following this, the procedure was similar to that used for plasma.
For plasma, the standard curve was linear from 0.5 to 50 mg l−1. The coefficients of variation were less than 12.7% at all concentrations for both enantiomers. For milk the standard curve was linear from 0.2 to 10 mg l−1. The coefficients of variation were less than 6% for both enantiomers. The limits of quantification were 0.5 mg l−1 in plasma and 0.2 mg l−1 in milk.
Results
Placental transfer
The data are shown in Table 1.
Table 1.
Placental transfer.

In case 1 the maternal plasma concentrations of both enantiomers were measured 4 h 25 min after dosing (R:11.5 mg l−1, S:6.40 mg l−1). These were higher than in case 2 where concentrations were measured 9 h after dosing (R:3.50 mg l−1, S:2.19 mg l−1): this reflected the different sampling times.
The umbilical vein concentrations were either lower in case 1 (R:0.78 mg l−1, S:1 mg l−1) or similar to maternal concentrations in case 2 (R:4.87 mg l−1, S:1.99 mg l−1).
The placental transfer assessed by the umbilical vein to maternal plasma concentration ratio (F/M) showed that the pseudo equilibrium was not achieved in case 1 (F/M = R:0.068, S:0.16) but was obtained in case 2 (F/M = R:1.39, S:0.91) in the maternal foetal unit. Steady state in the foetal compartment was also evidenced (case 2) as the concentrations in the umbilical artery (R:4.80 mg l−1 and S:2.13 mg l−1) were similar to those of the umbilical vein.
Comparing case 1 (ratio F/M = R:0.068, S:0.16) to case 2 (ratio F/M = R:1.39, S:0.91) it can be inferred that placental transfer varies with time of administration. This suggests a slow transfer, the equilibrium being obtained at least 9 h after the administration of the drug.
The rate of placental transfer of the two enantiomers might be slightly different since the ratio F/M for the S active enantiomer is twice that of the R enantiomer at 4 h post dose (case 1) and 40% lower 9 h post dose (case 2).
Excretion into breast milk
The data are given in Table 2.
Table 2.
Excretion into breast milk.
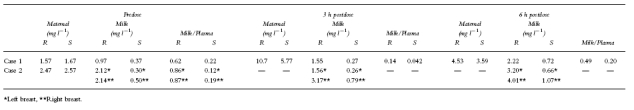
Whatever the time of collection predose or postdose (3 and 6 h), the milk plasma (M/P) concentration ratio was always lower than 1 in case 1 for both enantiomers (R enantiomer :0.62, 0.14, 0.49 and S enantiomer 0.22, 0.042, 0.20 at predose, 3 and 6 h postdose, respectively). Similar results were obtained in case 2 at predose collection (M/P R enantiomer:0.86 (left breast) and 0.87 (right breast); S enantiomer 0.12 (left breast) and 0.19 (right breast).
The data suggested that each enantiomer behaved differently. The M/P for the R enantiomer was always higher than the M/P for the S enantiomer (predose, 3 h and 6 h postdose) suggesting a lower transfer of the active S form into the milk. However no difference between the concentrations of both enantiomers in milk from the right and left breasts was seen in predose samples.
From these results, assuming the infant consumes 150 ml breast milk kg−1 day−1, the maximum dose he would receive can be estimated. In case 1, using the maximum milk concentration the amount would be for the R enantiomer 333 μg kg−1 day−1 (2.22 mg l−1×150 ml) i.e. less than 2.0% of the maternal dose and for the S enantiomer 108 μg kg−1 day−1 (0.721 mg l−1×150 ml) i.e. less than 0.6% of the maternal dose. In case 2 the same calculation would give 601 μg kg−1 day−1 (4.01 mg l−1×150 ml) and 160 μg kg−1 day−1 (1.07 mg l−1×150 ml), or 3.6% and 0.96% of the maternal dose for R and S enantiomer respectively (the maternal weight was assumed to be 60 kg).
Discussion
Since vigabatrin has a short half-life, it can be assumed that steady-state was achieved in both mothers.
At steady-state, the rise in concentration of the enantiomers of vigabatrin in the foetus is slow, which indicates that vigabatrin crosses the placenta slowly as with most lipid insoluble molecules. Vigabatrin has a low molecular weight (129), is water soluble and unbound to plasma proteins. It is transferred by simple diffusion (Fick’s law) through a hydrophilic pathway. As hydrophilic molecules do not permeate the cell membrane of the trophoblast, they are thought to cross the placenta by an extracellular route [4]. These results are in agreement with in vitro data which described a low placental transfer of both enantiomers [3]. Regarding the excretion of vigabatrin into breast milk the limited data would suggest that when vigabatrin is taken by a nursing mother, the quantity ingested through the milk is small. It would be at maximum 4% of the weight-adjusted maternal daily dose. Such a dose is unlikely to have a significant pharmacological activity in an infant following regular breast feeding from a mother on normal therapeutic doses of vigabatrin.
Acknowledgments
The authors gratefully acknowledge the secretarial assistance of Mrs Francine Gardette-Sage. This work was supported with a grant from Laboratoires Marion Merrell.
References
- 1.Haegele KD, Schechter PJ. Kinetics of the enantiomers of vigabatrin after an oral dose of the racemate or the active S-enantiomer. Clin Pharmacol Ther. 1986;40:581–584. doi: 10.1038/clpt.1986.227. [DOI] [PubMed] [Google Scholar]
- 2.Rey E, Pons G, Richard MO, et al. Pharmacokinetics of the individual enantiomers of vigabatrin (γ-vinyl-GABA) in epileptic children. Br J Clin Pharmacol. 1990;30:253–257. doi: 10.1111/j.1365-2125.1990.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challier JC, Rey E, Bintein T, Olive G. Passage of S(+) and R(−) γ-vinyl-GABA accross the human isolated perfused placenta. Br J Clin Pharmacol. 1992;34:139–143. doi: 10.1111/j.1365-2125.1992.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibley CP, Boyd RDH. In: Mechanisms of transfer accross the human placenta in fetal and neonatal physiology. Polin RA, Foed WW, editors. Philadelphia: W.D. Saunders Company; 1992. pp. 62–74. [Google Scholar]


