Abstract
Aims
Saquinavir is a potent HIV protease inhibitor whose effectiveness is limited in vivo by its low bioavailability. Since saquinavir is metabolized by CYP3A4, the effect of grapefruit juice, an inhibitor of CYP3A4, was investigated on its bioavailability.
Methods
After an overnight fast, eight healthy volunteers were treated with either 400 ml grapefruit juice or water before intravenous (12 mg) or oral saquinavir (600 mg) was administered. Serial blood samples were obtained over the following 24 h and standardized meals were served 5 and 10 h after the administration of saquinavir. The plasma concentrations of saquinavir were determined by high-performance liquid chromatography and pharmacokinetic parameters were calculated by routine methods.
Results
The AUC was not affected by grapefruit juice after intravenous administration, but it increased significantly from 76±96 (water, mean (s.d.) to 114±70 (μg l−1 h (grapefruit juice) after oral saquinavir. Similarly, the oral bioavailability of saquinavir increased by a factor of 2 with grapefruit juice (from 0.7% to 1.4%). In contrast, clearance, volume of distribution and elimination half-life of saquinavir were not affected by grapefruit juice. After oral, but not after intravenous administration, the plasma concentration-time curve showed a second peak after lunch irrespective of pretreatment, suggesting enhancement of absorption by food.
Conclusions
The studies demonstrate that grapefruit juice increases the bioavailability of saquinavir without affecting its clearance, suggesting that inhibition of intestinal CYP3A4 may contribute. Since the antiretroviral effect of saquinavir is dose-dependent, inhibition of CYP3A4 may represent a way to enhance its effectiveness without increasing the dose.
Keywords: grapefruit juice, saquinavir, HIV, protease inhibitor, pharmacokinetics, bioavailability, interaction, CYP3A4
Introduction
Human immunodeficiency virus (HIV) infection and its clinical syndrome AIDS continue to be a major health problem worldwide. Current antiretroviral therapies include dideoxynucleoside analogues which have limited activity and significant toxicity [1], and the recently developed HIV protease inhibitors. Since HIV protease is responsible for post-translational cleavage of the Gag-Pol precursor polyprotein, which is required for the formation of infectious virus [2, 3], its inhibition leads to the production of immature and non-infectious viral material.
Saquinavir is a transition-state analogue, peptide-based inhibitor of the HIV protease [4]. It is a potent inhibitor of HIV replication in vitro and suppresses viral load and increases CD4+ cell counts in HIV patients in vivo [4–6]. The major drawbacks of protease inhibitors are their high cost and for saquinavir its low and variable oral bioavailability, which is approximately 4% in the fed and 1% in the fasted state [7]. Since the effect of saquinavir on viral replication is dose-dependent [5, 6] and the therapy with saquinavir is expensive, there is an urgent need for methods to increase its low oral bioavailability.
One possibility to increase saquinavir’s bioavailability is to improve its galenical formulation. A new soft gelatin capsule is currently under investigation by the manufacturer. Another possibility is to inhibit its first-pass metabolism. Saquinavir is metabolized by the cytochrome P450 isoenzyme 3A4 (CYP3A4) [7, 8] which is expressed predominantly in liver and gut [9, 10]. Inhibition of CYP3A4 has been shown to lead to an increase in the bioavailability of many drugs metabolized by this enzyme [11–13]. Interestingly, ingestion of grapefruit juice is associated with inhibition of CYP3A4 [14, 15], which may be due to the effect of 6′,7′-dihydroxy-bergamottin [16]. Pretreatment with grapefruit juice has been shown to increase the bioavailability of a variety of drugs, including cyclosporin [17], calcium antagonists [13, 15, 18] and midazolam [19]. The aim of the current study was therefore to investigate the effect of grapefruit juice on saquinavir bioavailability in healthy volunteers.
Methods
Materials
Saquinavir mesylate (Ro 31–8959, purity >99%), its stereoisomer Ro 31–9533 (used as an internal standard for high-performance liquid chromatography) and Invirase® capsules (containing 200 mg saquinavir) were obtained from Hoffmann La Roche Ltd, Basle, Switzerland. The intravenous form was prepared by the local hospital pharmacy by dissolving saquinavir mesylate in water at a concentration of 1.2 mg ml−1. This solution was sterilized by membrane-filtration, shown to be pyrogen-free and then used for the kinetic studies.
Study design
The study was approved by the Ethics Committee of the Department of Internal Medicine of the University of Zürich. Eight healthy male volunteers (with a mean±s.d. age of 26±2 years, a body weight of 76±8 kg and a height of 182±8 cm) participated after having given written informed consent. The subjects had to avoid alcohol, caffeine, nicotine and citrus fruits throughout the study and to fast for 10 h before the administration of saquinavir.
The study had an open crossover design, and each subject was investigated during four individual sessions. The subjects were pretreated with either 200 ml of water or single strength grapefruit juice (Hitchcock, prepared freshly from concentrate by Thurella, Bischofszell, Switzerland) 45 and 15 min before the administration of intravenous (12 mg injected over 5 min through a cubital catheter in the arm not used for blood sampling) or oral saquinavir (3 capsules Invirase® containing 200 mg saquinavir each). Saquinavir was administered at 08.00 h, and standard meals were served 5 and 10 h after administration of the drug. The interval between the individual sessions was at least 1 week.
Blood sampling and determination of saquinavir plasma concentrations
For blood sampling, the subjects were in the sitting position for at least 5 min. Blood was collected through an intravenous catheter placed in a fore-arm or cubital vein into vacuum tubes containing lithium heparin. Samples were obtained before and 0.1, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 12 and 24 h after the administration of saquinavir. The samples were immediately put on ice and centrifuged (3000 g for 15 min) within 20 min. Plasma samples were stored at −20° C until analysis which was carried out within 2 months.
The saquinavir plasma concentrations were determined by high-performance liquid chromatography as described [20]. Plasma (2 ml) was washed with n-hexane and extracted with ether which was evaporated. The residue was assayed isocratically using 5 mm sulphuric acid and acetonitrile (75.5:24.5 v/v) containing 10 mm tetrabutylammonium hydrogen sulphate as a mobile phase on a Nucleosil 3C8 column kept at 45° C with u.v. detection at 240 nm. The limit of detection was 0.5 ng ml−1 and the coefficient of variation <5% in the concentration range between 5 to 110 ng ml−1 and <20% between 1 to 5 ng ml−1.
Pharmacokinetic calculations
The time to peak concentration (tmax) and the peak concentration (Cmax) were obtained from the raw data. The plasma half-life (t1/2) was calculated as:
| (1) |
where λz represents the slope of the terminal part of the plasma concentration-time curve after semilogarithmic transformation. The area under the plasma concentration-time curve (AUC) was calculated as:
| (2) |
where Cp(24h) is the plasma concentration at 24 h. AUC(0,24h) was calculated by the trapezoidal rule with linear interpolation. The bioavailability (F) of saquinavir was calculated as:
| (3) |
and the total plasma clearance (CL) after intravenous administration as:
| (4) |
The volume of distribution at steady state conditions (Vss) was calculated as:
| (5) |
with AUMC being the total area under the first moment of the plasma concentration-time curve.
Liver plasma flow and statistics
The plasma flow across the liver was estimated based on the haematocrit and assuming a liver blood flow of 21.6 ml kg−1 body weight, as published by Pirttiaho et al. [21]. Data are presented as mean±s.d. unless otherwise stated. The two pretreatments (water or grapefruit juice) were compared separately for intravenous and oral administration of saquinavir using the statistical sign test as described by Dixon & Mood [22]. An α level <0.05 was considered to be statistically significant.
Results
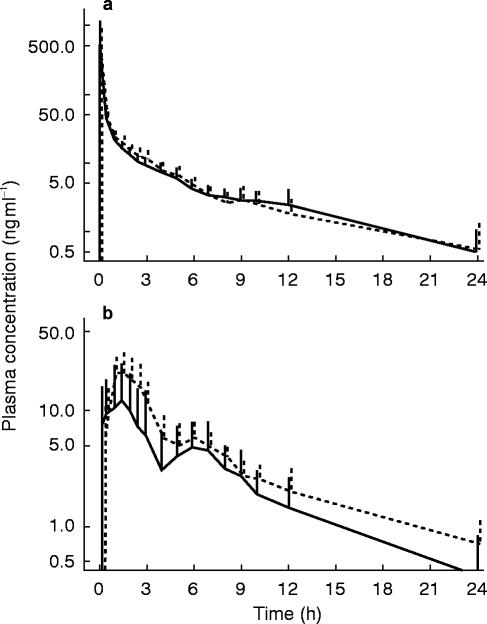
The effect of grapefruit juice on the plasma concentration-time curves of saquinavir is shown in Figure 1. After intravenous administration, saquinavir exhibits a rapid drop over the first 3 h which is followed by a linear decline in the semi-logarithmic plot, with a terminal half-life in the range of 10 to 13 h. Neither the distribution nor the elimination of intravenous saquinavir were influenced significantly by pretreatment with grapefruit juice (Figure 1 and Table 1). The ingestion of food at 5 and 10 h after intravenous administration of saquinavir had also no relevant effect on the shape of the plasma-concentration time curve.
Figure 1.
Plasma concentration-time curves of a) intravenous or b) oral saquinavir after pretreatment with water (—) or grapefruit juice (– – –): Intravenous (12 mg) or oral saquinavir (600 mg) was administered to eight healthy volunteers treated with water or grapefruit juice. Treatment with grapefruit juice does not affect the kinetics of saquinavir after intravenous administration, but leads to a significant increase in the area under the curve after oral ingestion of saquinavir. The second peak in the oral plasma concentration-time curves is associated with the lunch served at 5 h.
Table 1.
Kinetic parameters of saquinavir in normal subjects. Subjects were pretreated either with water or grapefruit juice, and saquinavir was administered either intravenously (12 mg) or orally (600 mg).
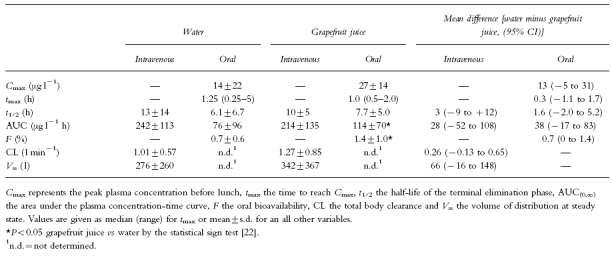
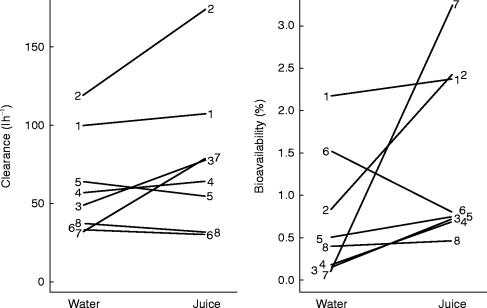
After oral administration, the saquinavir plasma concentration-time curve showed two peaks, the first one at 1.5 (water) or 1.2 h (grapefruit juice), and the second one at 6 h for both water and grapefruit juice (Figure 1). In contrast to intravenous administration, pretreatment with grapefruit juice revealed significant effects on the kinetics of oral saquinavir. The area under the plasma concentration-time curve increased by 50%, whereas Cmax, tmax and the terminal half-life were not significantly affected (Table 1). Importantly, the oral bioavailability, calculated as the dose-corrected ratio of the AUCs after oral and intravenous administration, showed an increase by 100% after pretreatment with grapefruit juice. As illustrated in Figure 2, the interindividual variability in the bioavailability of saquinavir is high and pretreatment with grapefruit juice increased the bioavailability of saquinavir in seven out of the eight subjects studied. The only exception was subject 6, who had, when compared to the other subjects, a quite high bioavailability also when pretreated with water.
Figure 2.
Total body clearance and bioavailability of saquinavir after pretreatment with water or grapefruit juice. While the clearance of saquinavir was not significantly affected by grapefruit juice, its bioavailability showed a significant increase.
Discussion
Our study confirms that saquinavir has a low oral bioavailability with a large interindividual variation and demonstrates that the bioavailability of saquinavir can be increased by a factor of 2 by the intake of grapefruit juice.
As shown in Figure 1, saquinavir is absorbed only slowly from the gastrointestinal tract, with the terminal elimination phase being reached approximately 9 h after ingestion of the capsules. The absorption is influenced by the intake of food, as illustrated by the second peak in the plasma concentration-time curve which appears after ingestion of the lunch. Since no such peak is visible after intravenous administration, the second peak probably reflects an increase by food in the absorption of previously not absorbed saquinavir and not enterohepatic cycling of the drug. This interpretation is supported by other studies, showing that the absorption of saquinavir is increased when ingested with a meal [23]. Alternatively, saquinavir may be absorbed in the intestine at different sites distant from each other.
The saquinavir clearance equalled 1.01±0.57 l min−1 and did not change significantly in the presence of grapefruit juice. This value is similar to the plasma flow across the liver calculated for the subjects studied, which equals 0.91±0.10 l min−1. Since saquinavir does not enter the erythrocytes in a relevant amount (data on file, Hoffmann-La Roche Ltd, Basle, Switzerland), plasma but not blood flow across the liver determines its hepatic clearance. Assuming that extrahepatic clearance of saquinavir is minimal, a comparison between hepatic clearance and liver plasma flow shows that saquinavir is a high-clearance drug whose metabolism is limited by liver plasma flow. However, in a previous study a clearance of 1650 ml min−1 has been reported for intravenously administered saquinavir [24], suggesting also the occurrence of some extrahepatic metabolism.
The low bioavailability is a major drawback of this effective antiretroviral drug, leading to a high number of capsules to be ingested and to high cost. Therefore, methods to increase its bioavailability would be of major importance. One possibility is to ingest saquinavir with food, as recommended by the manufacturer. Since, as discussed above, saquinavir is a high-clearance drug, its bioavailability could also be increased by inhibiting its presystemic metabolism in gut and/or liver. A simple way to inhibit CYP3A4, by which saquinavir is metabolized [7, 8], is by ingesting grapefruit juice, which increases the bioavailability of a variety of drugs [13, 15, 17–19]. Indeed, our studies show that the bioavailability of saquinavir increased in all but one of the subjects treated with grapefruit juice by an average of 100%. As saquinavir clearance did not drop after treatment with grapefruit juice, our data are compatible with the concept that this increase in saquinavir bioavailability results also from the inhibition of intestinal and not only hepatic CYP3A4 activity. Intestinal metabolism has also been shown to be important for the low oral bioavailability of cyclosporin [25] and midazolam [26], drugs which are also metabolized by CYP3A4. Interestingly, CYP3A4 is expressed in the gut of most but not all humans [10], possibly explaining our finding that in one subject grapefruit juice did not increase saquinavir bioavailability.
The currently recommended daily dose of saquinavir is three times 600 mg (a total of nine capsules per day) but three times 1200 mg may be more effective [5, 6]. Our data suggest that for most patients ingestion of saquinavir with grapefruit juice results in an increase in drug exposure similar to that expected after doubling the dose. Since preliminary studies have shown that pretreatment with grapefruit juice is associated with an increase in the AUC of orally administered saquinavir also in fed patients (data on file, Hoffmann-La Roche Ltd, Basle, Switzerland), ingestion of CYP3A4 inhibitors could represent a way to increase the effectiveness of saquinavir in patients. However, since the effect of grapefruit juice is highly variable and quite limited, other inhibitors of CYP3A4 should also be investigated. It has recently been shown that coadministration of ritonavir (another HIV-protease inhibitor which inhibits CYP3A4 [27]) to HIV-infected patients under long-term treatment with saquinavir is associated with an increase in the AUC of saquinavir by a factor of approximately sixty [28]. Since there was a large variation regarding AUC and Cmax of saquinavir, safety concerns have been raised for such combinations, necessitating further studies to define the value of combining CYP3A4 inhibitors with saquinavir in more detail.
Acknowledgments
We thank Professor P. J. Meier-Abt and H. Bühler for helpful discussions and general support, and Mrs Y. Bloemhard for help in the h.p.l.c. analysis of the plasma samples. The study was financially supported by Hoffmann-La Roche, Basel, Switzerland and by a grant from the Swiss National Science Foundation to SK (SNF 31–46792.96).
References
- 1.Hirsch MS, D’Aquila RT. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993;328:1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- 2.Kohl NE, Emini EA, Schleif WA, et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashorn P, McQuade TJ, Thaisrivongs S, Tomasselli AG, Tarpley WG, Moss B. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc Natl Acad Sci USA. 1990;87:7472–7476. doi: 10.1073/pnas.87.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts NA, Martin JA, Kinchington D, et al. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990;248:358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 5.Kitchen VS, Skinner C, Ariyoshi K, et al. Safety and activity of saquinavir in HIV infection. Lancet. 1995;345:952–955. doi: 10.1016/s0140-6736(95)90699-1. [DOI] [PubMed] [Google Scholar]
- 6.Schapiro JM, Winters MA, Stewart F, et al . The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Noble S, Faulds D. Saquinavir. A review of its pharmacology and clinical potential in the management of HIV infection. Drugs. 1996;52:93–112. doi: 10.2165/00003495-199652010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Farrar G, Mitchell AM, Hooper H, Stewart F, Malcolm SL. Prediction of potential drug interactions of saquinavir (Ro 31–8959) from in vitrodata. Br J Clin Pharmacol. 1994;38 [Google Scholar]
- 9.De Waziers I, Cugenenc PH, Yang CS, Leroux JP, Beaune PH. Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990;253:387–394. [PubMed] [Google Scholar]
- 10.Kivistö KT, Bookjans G, Fromm MF, Griese EU, Münzel P, Kroemer HK. Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue. Br J Clin Pharmacol. 1996;42:387–389. doi: 10.1046/j.1365-2125.1996.42615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahonen J, Olkkola KT, Neuvonen PJ. Effect of itraconazole and terbinafine on the pharmacokinetics and pharmacodynamics of midazolam in healthy volunteers. Br J Clin Pharmacol. 1995;40:270–272. [PMC free article] [PubMed] [Google Scholar]
- 12.Aranko K, Luurila H, Backmann JT, Neuvonen PJ, Olkkola KT. The effect of erythromycin on the pharmacokinetics and pharmacodynamics of zoplicone. Br J Clin Pharmacol. 1994;38:363–367. doi: 10.1111/j.1365-2125.1994.tb04367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey DG, Bend JR, Arnold JMO, Tran LT, Spence JD. Erythromycine felodipine interaction: magnitude, mechanism, and comparison with grapefruit juice. Clin Pharmacol Ther. 1996;60:25–33. doi: 10.1016/S0009-9236(96)90163-0. [DOI] [PubMed] [Google Scholar]
- 14.Edwards DJ, Bernier SM. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci. 1996;59:1025–1030. doi: 10.1016/0024-3205(96)00417-1. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DG, Arnold JMO, Munoz C, Spence JD. Grapefruit juice-felodipine interaction: mechanism, predictability, and effect of naringin. Clin Pharmacol Ther. 1993;53:637–642. doi: 10.1038/clpt.1993.84. [DOI] [PubMed] [Google Scholar]
- 16.Edwards DJ, Bellevue FH, Woster PM. Identification of 6′,7′-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–1290. [PubMed] [Google Scholar]
- 17.Yee GC, Stanley DL, Pessa LJ, et al. Effect of grapefruit juice on blood cyclosprin concentration. Lancet. 1995;345:955–956. doi: 10.1016/s0140-6736(95)90700-9. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DG, Spence JD, Munoz C, Arnold JMO. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 19.Kupferschmidt HHT, Ha HR, Ziegler WH, Meier PJ, Krähenbühl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 20.Ha HR, Follath F, Bloemhard Y, Krähenbühl S. Determination of saquinavir in human plasma by high-performance liquid chromatography. J Chromatogr B. 1997;694:427–433. doi: 10.1016/s0378-4347(97)00165-5. [DOI] [PubMed] [Google Scholar]
- 21.Pirttiaho HI, Sotaniemi EA, Pelkonen RO, Pitkänen U. Hepatic blood flow and drug metabolism in patients on enzyme-inducing anticonvulsants. Eur J Clin Pharmacol. 1982;22:441–445. doi: 10.1007/BF00542550. [DOI] [PubMed] [Google Scholar]
- 22.Dixon WJ, Mood AM. The statistical sign test. J Am Statist Assoc. 1946;41:557–566. doi: 10.1080/01621459.1946.10501898. [DOI] [PubMed] [Google Scholar]
- 23.Muirhead GJ, Shaw T, Williams PEO, Madigan MJ, Mitchell AM, Houston AC. Pharmacokinetics of the HIV-proteinase inhibitor, Ro 318959, after single and multiple oral doses in healthy volunteers. Br J Clin Pharmacol. 1992;34:170P–171P. [Google Scholar]
- 24.Williams PEO, Muirhead GJ, Madigan MJ, Mitchell AM, Shaw T. Disposition and bioavailability of the HIV-proteinase inhibitor, Ro31–8959, after single doses in healthy volunteers. Br J Clin Pharmacol. 1992;34:155P–156P. [Google Scholar]
- 25.Wu CY, Benet LZ, Hebert MF, Gupta SK, Rowland M, Gomez DY, Wacher VJ. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: Studies with cyclosporine. Clin Pharmacol Ther. 1995;58:492–497. doi: 10.1016/0009-9236(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 26.Thummel KE, O’Shea D, Paine MF, et al . Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 27.Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 28.Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11:F29–33. doi: 10.1097/00002030-199704000-00001. [DOI] [PubMed] [Google Scholar]