Abstract
Aims
In September 1995, the indication for the oral dopamine agonist ibopamine was restricted in the Netherlands and in several other European countries to patients with NYHA-class II heart failure as a result of an interim analysis of the PRIME-II trial. This trial demonstrated an increased risk of mortality in patients with NYHA-class III/IV heart failure on ibopamine. In September 1995, we initiated an assessment of the effects of ibopamine under everyday circumstances in a cohort of users of ibopamine in all NYHA-classes.
Methods
In a nationwide retrospective cohort study all 2147 community pharmacies and drug dispensing general practitioners received a request to list all patients to whom they had dispensed ibopamine in the preceding years. All responding drug dispensing outlets (DDO) received a questionnaire on cardiovascular risk factors and mortality for the general practitioner of a random sample of these patients. DDO were also requested to send an anonymised printout of the complete medication record. On the end-date of follow-up, February 15th 1996, mortality rates were compared across categories of ibopamine use, adjusted for potential confounders. To assess medication use, drug exposure was compared in a 3 months’ period before date of death in the deceased, and before a random date in those patients who were still alive.
Results
In patients with NYHA-class III/IV heart failure, multivariate analysis indicated that current use of ibopamine was significantly associated with mortality (RR 1.37;95% CI: 1.15–1.64). In patients with NYHA-class I/II heart failure, however, multivariate analysis showed a 2.03 (95% CI: 1.10–3.72) risk of mortality in current users of ibopamine. Apart from current use of ibopamine, male gender and increased serum creatinine were also independent risk factors for mortality in all NYHA-classes. No statistically significant association was found between mortality and current use of amiodarone or use of amiodarone at baseline.
Conclusions
The increased risk of mortality in patients with NYHA-class III and IV heart failure on ibopamine seems to confirm the main finding of the recently published PRIME-II trial. However, our results indicate that also patients with NYHA-class I/II heart failure may be at an increased risk of mortality when using ibopamine. Additional research on the effects of ibopamine in these patients is warranted and the use of ibopamine in NYHA-class II heart failure patients may have to be reconsidered.
Keywords: ibopamine, mortality, risk factors
Introduction
Ibopamine is an orally administered dopamine agonist which was registered in the Netherlands in 1991 for the treatment of mild congestive heart failure (CHF) in combination with diuretics, and for moderate to severe heart failure in combination with diuretics, angiotensin converting enzyme (ACE) inhibitors or digoxin. Ibopamine has peripheral and renal vasodilatory properties, both in healthy volunteers as well as in patients with congestive heart failure [1, 2]. Other pharmacological properties of ibopamine are mild positive inotropic activity, increased diuresis and inhibition of both renin-angiotensin-aldosterone activity and noradrenaline plasma levels when elevated at baseline [1–4]. The inhibition of the neurohormonal response in patients with congestive heart failure was considered to be the main mechanism for the favourable effects of ibopamine.
In view of the increasing prevalence and incidence of congestive heart failure in western countries, ibopamine was regarded as a promising pharmacotherapeutic contribution to the treatment of patients with congestive heart failure [5, 6]. Several studies confirmed the beneficial haemodynamic and neurohormonal effects of ibopamine in patients with congestive heart failure without revealing major adverse effects, although previous experiences with new drugs such as milrinone and vesnarinone urged caution with respect to ultimate effects on survival [7–10].
Until August 1995, only limited data were available on the effects of ibopamine on mortality [11]. At that time, however, results from an interim analysis of the PRIME-II trial (Prospective Randomized studies of Ibopamine on Mortality and Efficacy) indicated a significantly increased risk of mortality in patients with moderate to severe CHF (NYHA classes III and IV) randomized to ibopamine as compared with the control group. As a consequence, the safety committee decided to terminate the PRIME-II trial. Recently, the final findings of the PRIME-II study were published [12].
In response to the findings of the interim analysis of the PRIME-II study, the indication for ibopamine in the Netherlands was restricted to heart failure NYHA-class II patients only. At that same time, the Inspectorate for Health Care in the Netherlands initiated a nationwide retrospective cohort study, which aim was to provide information on utilization of ibopamine and on mortality and associated risk factors in patients treated with ibopamine. As we were especially interested in the effects of the use of ibopamine under everyday circumstances, we included patients of all NYHA-classes in our study.
Methods
Early in September 1995, all 2147 community-based drug dispensing outlets (DDO) in the Netherlands received a request to list all patients to whom they had dispensed ibopamine in the preceding years. These 2147 DDO comprised 1516 community pharmacies and 631 drug dispensing general practitioners (GP). A reminder was sent in November 1995. The 1983 responding DDO (92%) had dispensed ibopamine to 14 024 patients. Subsequently, all GPs with a DDO who had dispensed ibopamine to a total of 1573 patients received a questionnaire for each of these patients. In addition, a random sample of 1573 patients was drawn out of the remaining 12 451 patients to whom ibopamine was dispensed by their community pharmacist. The same questionnaire was sent to the community pharmacist of the patients in this sample with the request to forward the questionnaire to the patient's GP. This procedure was necessary because we did not have the names of the GPs of the patients to whom ibopamine was dispensed by the community pharmacist. In this way, a total of 3146 questionnaires were sent to the GPs of patients to whom ibopamine was dispensed. The GPs were asked to complete the questionnaire and return it to the Inspectorate for Health Care. The questionnaire addressed on cardiovascular risk factors, such as previous myocardial infarction, angina pectoris, atrial fibrillation and diabetes mellitus, indication for ibopamine (NYHA-class), current use of ibopamine and mortality. The DDOs were also asked to return a printout of the complete medication record of these patients to the Inspectorate for Health Care. Patients were followed from the date of the first prescription until death or the end of the study period which was February 15th, 1996, whichever came first. In order to assess medication use, a drug exposure window of 3 months was defined in each patient for whom a printout of the medication record was available (n=1146). Every patient still alive at the end of the study period was assigned a random date in the printout of the medication record. Drug exposure within 3 months before the date of death in deceased patients was taken and compared with drug exposure in a similar 3 months period preceding the random date in those patients who were still alive on February 15th 1996. For every prescription, the alleged duration was calculated on the basis of the ratio of the total number of tablets or capsules and the prescribed daily number of tablets or capsules. A patient was considered to be exposed to every drug for which the date of a filled prescription fell within the 3 months drug exposure window or for which the end date of the alleged duration overlapped the date of death in deceased patients or the random date in nondeceased patients.
As mortality from all causes was the primary end point of interest, as it was in the PRIME II trial, we analysed risk factors for mortality in deceased patients and in those patients who were still alive at the end of the study period.
Continuous variables were analysed with a Student's t-test or Mann–Whitney test when non-normally distributed. The prevalence of risk factors in both deceased and nondeceased patients were analysed and expressed as a relative risk with a 95% confidence interval (95% CI). Apart from crude and stratified univariate relative risk estimates, we performed multivariate analysis on the complete dataset with a risk ratio regression model using the GENMOD procedure of the SAS statistical software package. All variables which were significantly associated with mortality in the univariate analysis were entered into the model. In a second multivariate analysis, carried out in a forward stepwise fashion with the risk ratio regression model, we introduced dummy variables to adjust for confounding which might result from missing values. In the subset for which we obtained both questionnaire data and a printout of the medication record, additional multivariate analyses were performed to correct for potential confounding by severity. Here, we made extra dichotomous variables on the basis of two indicators of the severity of CHF. These two severity indicators were based on the simultaneous use within the 3 months drug exposure window, respectively: 1) an ACE-inhibitor, loop-diuretic and digoxin and 2) an ACE-inhibitor, loop-diuretic, digoxin, and a vasodilatory agent. All tests were two-sided with rejection of the null hypothesis at a P value<0.05.
Results
More than 92% of the DDO responded to our request to list all patients to whom ibopamine was dispensed. Based on the 14 024 patients listed by the reacting DDO, the number of patients treated with ibopamine in the preceding years could be estimated at ≈15 000. The mean number of patients per community pharmacy was 9 (median:6; range 0–145), and per GP with a DDO 3 (median:1; range:0–20). Completely filled in questionnaires were obtained from the GPs on 2246 patients (71%), of which 1049 came from GPs via pharmacists (66%) and 1197 (76%) from drug dispensing GPs. Printouts of the computerised medication records were available of 1304 patients (41%). The analysis of risk factors for mortality apart from medication use was based on 2246 patients, while in a subset of 1146 patients for whom both a questionnaire and a printout of the medication record were available, the association between mortality and medication use could be studied in more detail. In Table 1, general characteristics are given of all 2246 patients on whom a questionnaire was obtained. Analyses were based on patients deceased before February 15th 1996 (n=625; mean follow-up 395 days) and those patients who were still alive on that date (n=1621; mean follow-up 679 days).
Table 1.
Description of 2246 users of ibopamine.
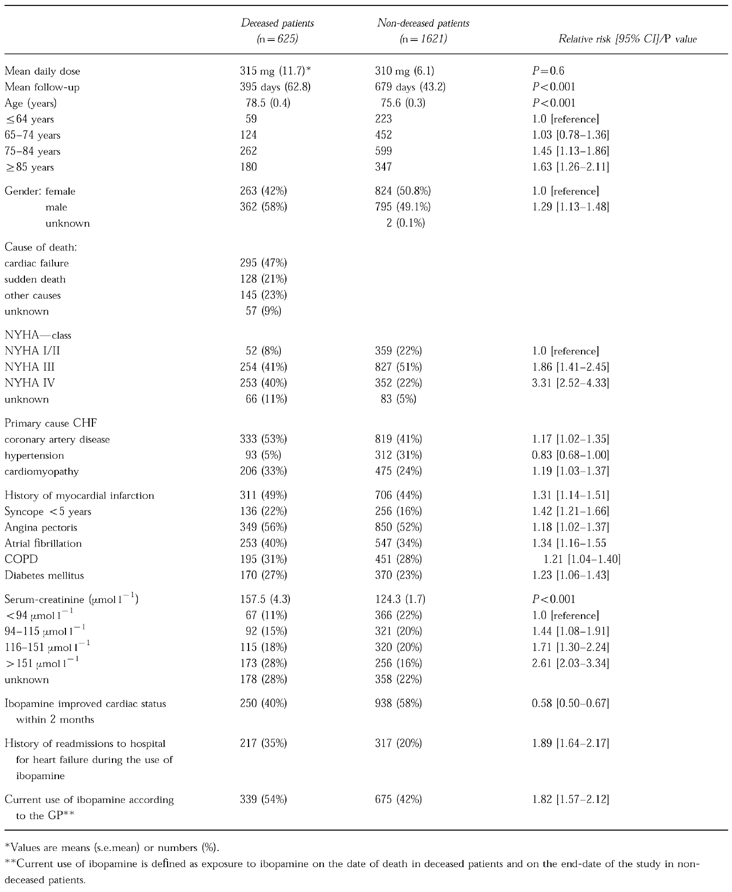
Of the patients deceased before February 15th 1996, 58% was male compared with 49% of the nondeceased (Table 1). The average age of the deceased was 78.5 years compared with 75.6 years of the nondeceased. According to the GP, 47% of the deceased died from cardiac failure and 21% from sudden death. The remaining 32% died from other causes, or the cause of death was unknown. At least 75% of all patients treated with ibopamine were in NYHA class III or IV. The risk of mortality was strongly related to NYHA-class. Compared with patients in NYHA-class I/II, patients in NYHA-class III and NYHA-class IV had a relative risk of mortality of 1.86 (95% CI: 1.41–2.45) and 3.31 (95% CI: 2.52–4.33), respectively. Coronary artery disease and idiopathic cardiomyopathy as primary causes of heart failure were both significantly more prevalent in the deceased, with a relative risk of 1.17 (95% CI: 1.02–1.35) and 1.19 (95% CI: 1.03–1.37), respectively. Several other cardiovascular risk factors were significantly more prevalent in the group of deceased patients. A history of myocardial infarction, syncope within the last 5 years, angina pectoris, atrial fibrillation, chronic obstructive pulmonary disease (COPD) and diabetes mellitus were all significantly more frequent in deceased patients. Increased serum creatinine levels were also associated with a higher risk of mortality, especially when serum creatinine levels were above 151 μmol l−1. Failure of ibopamine to improve cardiac status within 2 months after starting and a history of re-admissions to hospital for congestive heart failure were also associated with an increased risk of mortality. Current use of ibopamine was associated with a crude relative risk of 1.82 (95% CI: 1.57–2.12).
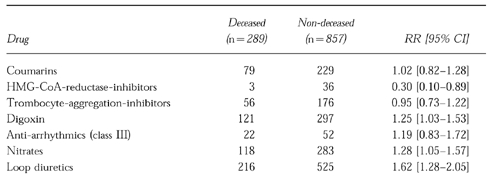
Table 2 describes drug exposure of 1146 patients of whom both a questionnaire and a printout of the medication record were available. Medication use within a drug exposure window of 3 months before decease or the random date was analysed in both deceased patients and patients who were still alive on February 15th 1996, the end of the study period. Cardiovascular drugs, such as nitrates, digoxin and loop diuretics, were all significantly more frequently used by deceased patients. β-adrenoceptor blockers and HMG-CoA-reductase-inhibitors, however, were significantly more frequently used by nondeceased patients. Apart from cardiovascular drugs, glucocorticoids, opioids, salicylates and inhalation corticoids were all significantly more frequently used by deceased patients. Other categories of drugs did not reveal significant differences between both groups. In particular, no statistically significant difference was present between deceased and nondeceased patients with respect to use of antiarrhythmics (e.g. amiodarone) during the drug exposure window.
Table 2.
Medication use in 1146 patients based on drug-prescriptions within three months before the date of death in deceased patients or random date in non-deceased patients.
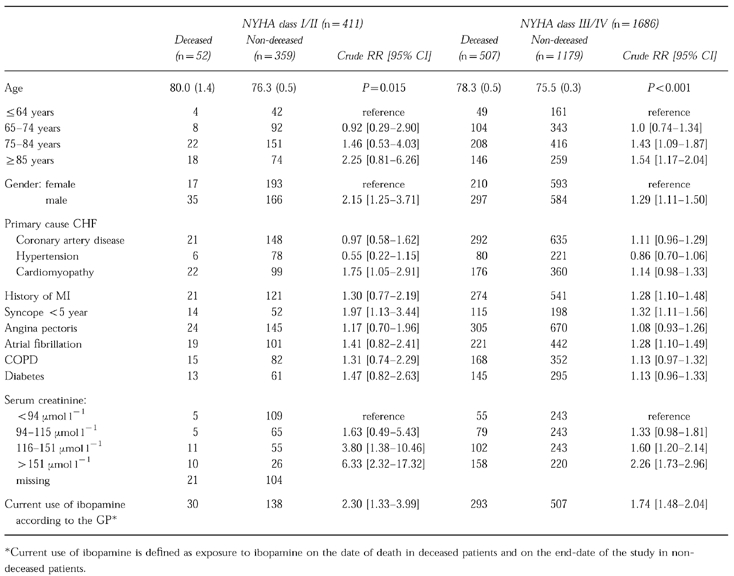
In a separate analysis stratified for NYHA-class I/II and III/IV, crude relative risk estimates of mortality in current users of ibopamine according to the GP were statistically significant in both NYHA-class I/II patients (RR 2.30 95% CI: 1.33–3.99) and NYHA-class III/IV patients (RR 1.74 95% CI: 1.48–2.04) (Table 3). Furthermore, risk of mortality increased within each stratum of increased serum creatinine level. In patients with NYHA-class I/II congestive heart failure, renal impairment increased the risk of death more than in patients with NYHA class III/IV congestive heart failure.
Table 3.
Univariate analysis of risk factors for mortality in a cohort of users of ibopamine, according to NYHA-class.
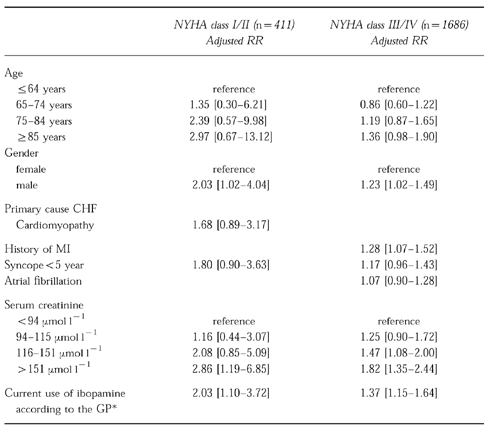
As we had a special interest in the adjusted relative risks of mortality in the pooled NYHA-classes I/II (n=411) and III/IV (n=1686), the risk ratio regression models for these groups were built separately (Table 4). In NYHA-class I/II, the univariately significant variables age (in four classes), gender, cardiomyopathy, syncope <5 years, serum creatinine (in four classes), and current use of ibopamine according to the GP were entered in the model. Only gender, serum creatinine >151 μmol l−1 and current use of ibopamine according to the GP remained statistically significantly associated with mortality. This led to an adjusted relative risk of mortality of 2.03 (95% CI: 1.10–3.72) for current use of ibopamine in patients with NYHA-class I/II CHF. in NYHA-class III/IV, the univariately significant variables age, gender, history of myocardial infarction, syncope <5 years, atrial fibrillation, serum creatinine, and current use of ibopamine according to the GP were entered in the model. After adjustment in the risk ratio model, gender, history of myocardial infarction, serum creatinine >115 μmol l−1 and current use of ibopamine according to the GP were statistically significantly associated with mortality. The adjusted relative risk of mortality was 1.37 (95% CI: 1.15–1.64) for current use of ibopamine. In a second multivariate analysis, we used dummy variables to adjust for potential confounding by missing values. This led to an adjusted relative risk of mortality of 1.97 (95% CI: 1.22–3.16) in patients with NYHA-class I/II, whereas the adjusted relative risk in patients with NYHA-class III/IV remained actually unchanged (1.38;95% CI:1.22–1.56).
Table 4.
Multivariate analysis of risk factors for mortality in a cohort of users of ibopamine, according to NYHA-class.
Discussion
In accordance with a large randomized trial (12), we found an increased risk of mortality during use of ibopamine in patients with NYHA-class III and IV at baseline. Our relative risk estimation of 1.37 was only slightly higher than the 1.26 found in the trial. Although most trials are remote from drug use under everyday circumstances, the results from our cohort study support the trial results. Importantly, we also found a statistically significantly increased risk of mortality in patients with NYHA-class I/II at baseline, patients that were not included in the PRIME-II trial.
Several other risk factors for mortality in patients with congestive heart failure could be distinguished in the overall univariate analysis (Table 1). Patients with a history of angina pectoris, myocardial infarction, atrial fibrillation, diabetes and COPD had an increased risk of mortality in our study. These findings are in accordance with several prospective follow-up studies in which patients with congestive heart failure were involved [13–18]. Consistent with our findings, use of nitrates and inhalation corticoids was more frequently encountered in deceased patients as compared with the nondeceased. Not suprisingly, a very strong association was found with opioids in the deceased, as these drugs are frequently prescribed in the terminal stage of heart failure to relieve symptoms of respiratory distress.
Ibopamine improved cardiac status within 2 months after the first prescription significantly more frequently in nondeceased patients as compared with the deceased. Hence, a rapid therapeutic response to ibopamine seems to be protective with respect to mortality, although this finding may have been subject to recall bias by its retrospective and subjective nature. A history of readmissions to hospital for congestive heart failure and current use of ibopamine were also significantly more frequent in deceased patients. As the associations remained present in the analysis stratified for NYHA-class, the severity of congestive heart failure and thereby the liability of physicians to continue treatment in the most severe patients does not seem to be the sole explanation for these associations, as also in patients with NYHA class I/II at baseline death was associated with current use of ibopamine. It should be emphasized, however, that the NYHA-classification has limitations In correctly estimating the severity of congestive heart failure. In patients with NYHA-class I/II, significantly more patients died from other causes, such as cachexia or malignancies.
Interestingly, increased serum creatinine levels were associated with the risk of death in the stratified analysis independently from NYHA-class (Table 3). In particular in patients with mild congestive heart failure (NYHA class I/II), a statistically significant association was found between various levels of renal impairment and the risk of death, also after adjustment for other risk factors. In patients with moderate to severe congestive heart failure, a similar association was found, albeit less explicit. In the recently published report on the PRIME-II trial [12], the role of renal function was not discussed. It has been suggested that impaired renal function might lead to high levels of epinine, the active metabolite of ibopamine, and that these high plasma concentrations might account for the increased mortality in patients treated with ibopamine [19].
With respect to concomitant medication, nitrates, glucocorticoids and inhalation corticoids were significantly more frequently used in deceased patients, but this finding can be explained by the higher prevalence of angina pectoris and COPD in the deceased. Loop diuretics and digoxin were significantly more frequently used by deceased patients, whereas β-adrenoceptor blockers seemed to have a protective effect with respect to mortality. These findings might be compatible with the assumption that the severity of CHF explains the increased risk of mortality during use of ibopamine. Adjustment in the multivariate analysis for severity of CHF, however, did not substantially change the risk estimate.
In a subgroup analysis of the PRIME-II trial, therapy with amiodarone at baseline was identified as an independent predictor of mortality in patients treated with ibopamine. We were able to analyse current use of amiodarone both in deceased and nondeceased patients within the drug exposure window as well as the concurrent use of amiodarone at baseline. Both at baseline as well as within the drug exposure window, no significant differences in the use of amiodarone between deceased and nondeceased patients could be observed. Therefore, our findings do not confirm the hypothesis of the investigators of the PRIME-II trial that a possible interaction between ibopamine and the antiarrhythmic drug amiodarone might contribute to the increased mortality to ibopamine.
The validity of epidemiological studies may be jeopardized by selection bias, information bias or confounding. Selection bias might explain a high mortality in the total cohort because of selective prescribing of ibopamine to high risk patients with heart failure. As we used nondeceased as a reference group of patients who had been subject to the same prescribing habits as the deceased, selection bias is not very likely. This is endorsed by the fact that the relative risk estimates of mortality during the use of ibopamine of 1.37 (GP-information) in NYHA-classes III/IV was very close to the risk estimate of 1.26 in the trial [12]. Information bias of GP might be present if they have a different recall of concurrent illnesses or drug use of deceased patients as compared to nondeceased patients. As the pharmacy data on ibopamine use showed the same trend this is also unlikely, especially as such data were gathered unbiased and before disease onset. Because filled prescriptions are not always used, however, we think that the GP will have a more reliable picture of the medicines which have actually been taken by patients in the terminal phase of their illness. Misclassification of outcome (death) is unlikely, whereas random misclassification of exposure would result in a conservative estimate of the relative risk. The calculated relative risk of mortality may have been influenced by the fact that the questionnaires were filled in after the announcement that the indication of ibopamine had been restricted to patients with NYHA class II CHF only. However, we were able to perform an additional analysis which was based on the prescription data of the medication records and restricted to only these patients with a date of death or random index date before 1 September 1995. Based on this analysis, the use of ibopamine within the 3 months drug exposure window was associated with mortality in both patients with NYHA I/II CHF as well as NYHA class III/IV CHF, with a relative risk of 4.48 (95% CI 1.11–21.20) and 2.94 (95%CI 1.99–4.33), respectively. As this analysis pertains to a period before the results of the interim-analysis of the PRIME-II trial came available, the results of this analysis can not be influenced by any negative publicity with respect to ibopamine.
Potential confounding was dealt with by adjusting for all known independent risk factors for mortality in patients with heart failure. As the analysis with dummy variables for all variables with missing values yielded similar results, these can not be regarded as a source of substantial confounding. The nonresponse of GPs to questionnaires was 34% when sent via pharmacies, and 24% when sent directly to drug dispensing GPs. Although this was significantly different, it was not associated with a difference in mortality and adjustment did not change the risk estimates. Also, there was no substantial difference in overall mortality or risk factors for those patients of whom we had only a questionnaire, and those of whom we had both a questionnaire and a medication printout. Due to negative publicity, survivors might have gradually been withdrawn from ibopamine before the end of the study period and this could have overestimated the relative risk. For this reason, we used a random end-date in the nondeceased which showed that publicity did not bias our risk estimates. Finally, we were unable to validate the information on cardiovascular risk factors provided by the GP. However, as the vast majority of patients on ibopamine are also regularly seen by a cardiologist, it is likely that most information provided by the GPs came from consultant cardiologists.
In conclusion, in our nationwide cohort study we found similar risk estimates for mortality during current use of ibopamine in patients with NYHA-classes III/IV heart failure as found in the PRIME-II trial. In addition, we also found an increased risk of mortality in patients with NYHA-classes I/II heart failure. Although definitive proof of the causal nature of this association can only come from another randomized trial, we think that additional studies on ibopamine are warranted and that its therapeutic role in NYHA class I/II patients should be reconsidered.
Acknowledgments
Financial support by the Inspectorate for Health Care in the Netherlands is gratefully acknowledged.
References
- 1.Henwood JM, Todd PA. Ibopamine. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs. 1988;36:11–31. doi: 10.2165/00003495-198836010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Spencer C, Faulds D, Fitton A. Ibopamine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in congestive heart failure. Drugs Aging. 1993;3:556–584. doi: 10.2165/00002512-199303060-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Sendon J. Ibopamine in chronic congestive heart failure: hemodynamic and neurohumoral effects. Am J Med. 1991;90(5B):43S–49S. doi: 10.1016/0002-9343(91)90273-z. [DOI] [PubMed] [Google Scholar]
- 4.Lieverse AG, van Veldhuisen DJ, Smit AJ, et al. Renal and systemic hemodynamic effects of ibopamine in patients with mild to moderate congestive heart failure. J Cardiovasc Pharmacol. 1995;25:361–367. doi: 10.1097/00005344-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Cowie MR, Mosterd A, Wood DA, et al. Epidemiology of heart failure. Eur Heart J. 1997;18(2):208–225. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 6.Man in′t Veld AJ. Ibopamine in the treatment of heart failure. Am J Med. 1991;90(5B):50S–54S. doi: 10.1016/0002-9343(91)90274-2. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Res Group. N Engl J Med. 1991;325(21):1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 8.Feldman AM, Bristow MR, Parmley WW, et al. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone Study Group. N Engl J Med. 1993;329(3):149–155. doi: 10.1056/NEJM199307153290301. [DOI] [PubMed] [Google Scholar]
- 9.Condorelli M, Bonaduce A, Montemurro A, et al. The long-term efficacy of ibopamine in treating patients with severe heart failure: a multicenter investigation. J Cardiovasc Pharmacol. 1989;14(Suppl 8):S92. [PubMed] [Google Scholar]
- 10.Caponnetto S, Terrachini V, Canale C, Bruzzone F, Licciardello L, Marchetti GV. Absence of proarrhythmic effects of ibopamine in patients with congestive heart failure. J Cardiovasc Pharmacol. 1989;14(Suppl 8):S104, S110. [PubMed] [Google Scholar]
- 11.Rolandi E, Sabino F, Cantoni V, Ghirardi P, Marchetti GV, Cicchetti V. Long-term therapy of chronic congestive heart failure with ibopamine: a multicenter trial. J Cardiovasc Pharmacol. 1989;14(Suppl 8):S93, S103. [PubMed] [Google Scholar]
- 12.Hampton J, van Veldhuisen D, Kleber F, et al. Randomised study on effect of ibopamine on survival in patients with advanced severe heart failure. Lancet. 1997;349:971–977. doi: 10.1016/s0140-6736(96)10488-8. [DOI] [PubMed] [Google Scholar]
- 13.Cohn JN, Johnson GR, Shabetai R, et al. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 Suppl: VI):5–16. [PubMed] [Google Scholar]
- 14.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study 390 patients. Circulation. 1991;84:40–48. doi: 10.1161/01.cir.84.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Middlekauff HR, Stevenson WG, Stevenson LW, Saxon LA. Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope. J Am Coll Cardiol. 1993;21:110–116. doi: 10.1016/0735-1097(93)90724-f. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann T, Meinertz T, Kasper W, et al. Mode of death in idiopathic dilated cardiomyopathy: a multivariate analysis of prognostic determinants. Am Heart J. 1988;116(6 Part; 1):1455–1463. doi: 10.1016/0002-8703(88)90728-4. [DOI] [PubMed] [Google Scholar]
- 17.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 18.Madsen BK, Hansen JF, Stokholm KH, Brons J, Husum D, Mortensen LS. Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–310. doi: 10.1093/oxfordjournals.eurheartj.a060495. [DOI] [PubMed] [Google Scholar]
- 19.Girbes AJG, Zijlstra JG. Ibopamine and survival in severe congestive heart failure: PRIME II. Lancet. 1997;350:147–148. doi: 10.1016/s0140-6736(05)61858-2. [DOI] [PubMed] [Google Scholar]


